Phototaxis effect of Oncomelania hupensis hupensis and O.h.robertsoni--a comparative study
GONG Zhen-qiang,LI Chuan-chang,TAO Bo,WANG Yan,ZHOU Xian-min,3,ZOU Jie-xin
(1.Department of Parasitology,College of Basic Medicine,Nanchang University,Nanchang330031,China;2.Schistosomiasis Control Station of Xingzi County,Xingzi 332800,China;3.Key Laboratory of Poyang Lake Environment and Resource Utilization,
Ministry of Education,Nanchang University,Nanchang330047,China)
Light acts as an important ecological factor in nature with an obvious impact on animal behavior.It has a wide range of ecological functions,directly or indirectly affecting animal feeding,growth,reproduction and other physiological activities[1-3].It has been found that all of the studied species of pulmonate gastropods exhibit paired,simple camera-type eyes that operate with advanced fixed focal-length optics[4].The pulmonate gastropods use their eyes primarily for the following two types of visual tasks:(1)discriminating objects and possible enemies in their environment and(2)monitoring environmental brightness levels to orient towards dark places.The first type of visual task is characteristic of aquatic snails and is served by image-forming eyes,while the second type is typical of terrestrial snails and slugs and is best served by a blurred image.Attention has been focused on visual ecological adaptations,specific visual needs and the evolutionary history of gastropods[4].From the groups of research topics mentioned above concerning insects,the phototaxis effect of insects has also clearly been determined.
Oncomelaniahupensis(Gredler,1881),the sole intermediate host ofSchistosomajaponicum,is a type of amphibious snail[5-6].Although light is important in the life cycle ofS.japonicumfor the eggs to reach fresh water,hatch into ciliated miracidia,invade the snail,develop into cercaria and escape from the snail[7-9],little research has been conducted on snail phototaxis.Field observations have shown that,regarding the sensitivity ofO.hupensisto light,in April,June and September,snail activity is the highest in the evening and morning.Over the course of the entire day,snail activity reaches maximum levels at 6AMand midnight(12AM),followed by 6PM.The least amount of activity is observed at noon(12PM),and light affects snail feeding,spawning and egg hatching activities[10].In research on the photo-taxis ofO.hupensis,it has been discovered that at an illumination intensity of less than 2 000Lux,as the illumination intensity is enhanced,phototaxis increases.The phototaxis index reaches maximum levels under a light intensity of 2 000Lux,and when the illumination intensity is greater than 2 000Lux,as the illumination intensity is enhanced,the phototaxis index declines.Snails exhibit different phototaxis reactions to different colors of light.Four colors of light that influence the size of snails via phototaxis effects are(in the order of sequence)green>blue>yellow>red[11].The above-mentioned research findings are very useful.However,we believe that these studies do not fully reflect the physiological characteristics of the phototaxis reaction ofO.hupensis.Almost no research has addressedS.japonicuminfections and the influence of phototaxis onO.hupensis.O.hupensishas been found in China,Japan,the Philippines and on the Indonesian island of Sulawesi[12].Four subspecies ofO.hupensishave been identified in mainland China:O.h.hupensis,O.h.robertsoni,O.h.tangiandO.h.guangxiensis,which are distributed throughout many regions[13-15].The same subspecies can be distributed in different regions[16].Different subspecies of the intermediate host ofS.japonicumcercaria display an inconsistent carrier rate[17],asO.h.hupensispresents a markedly higher carrier rate thanO.h.robertsoni.No study has been reported ex-amining the differences in phototaxis expression between these twoO.hupensissubspecies and the same subspecies of snail from different regions.It is also important to understand the differences in phototaxis betweenO.hupensisinfected withS.japonicumcercariae and uninfected snails.
To accomplish these research objectives and further our understanding of the physiological characteristics of the phototaxis reaction ofO.hupensis,we assessed(under laboratory conditions)the differences in phototaxis between the two subspecies ofO.hupensis(O.h.hupensisandO.h.robertsoni)and the four related geographic strains of male and female snails,which were either infected withS.japonicumor uninfected.
Materials and methods
LiveO.h.hupensisandO.hrobertsonisnails were collected from four different regions in China:Xingzi County and Yushan County in Jiangxi Province and Tianquan County and Pengshan County in Sichuan Province(Table 1).The organisms were then transported to the laboratory and placed in a RXZ-type multi-segment,programmed artificial climate chamber during the experimental feeding session.Male and female snails were differentiated under a stereomicroscope,and the cercariae escaping method was used to screen infected and uninfected snails.

Tab.1 Specimens of Oncomelania hupensis
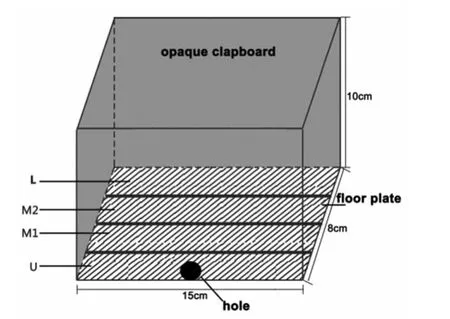
Fig.1 Experimental apparatus lateral view
The device used for the phototaxis experiment was made in our laboratory and consisted of an opaque,middle strip composed of a paper box and a floor plate.The landscape of the floor plate was divided intofour regions,as follows:U(0-2cm),M1(2-4cm),M2(4-6cm)and L(6-8cm).The outermost side of the U end was close to the holes for the light source(Figure 1).
A white light source was placed externally to supply illumination,with the light intensity being regulated in a continuous fashion.A photometer was placed at the site of the small holes to measure the light intensity.
Phototaxis index
Before the experiment,the base of the floor plate was covered with coarse paper and moistened with dechlorinated water,using an appropriate amount of water so as not to affect the crawling action of the snails.The temperature and humidity were measured at the small holes and were regulated at 25±1℃and 60%-70%,respectively.A white light source was used to supply illumination,with the light intensity being regulated at a variable frequency;the source passed through the holes,creating apoint of light to attract the snails.For each experiment,20adult snails of 5-7 screws were selected and placed on the central line between the M1and M2regions of the floor plate,and apparatus was subsequently illuminated.Within a specific time frame,the number of snails in each territory was recorded.The tested snails were not used again within 24hours of the experiment.
According to the literature,phototaxis can be represented using the phototaxis index(IP).The formula for the IP[18]was as follows:IP=(U-L)/(U+M1+M2+L).U,M1,M2,and L are the numbers of animals in each compartment.This formula has carried on the quantitative phototaxis of snails.
Determination of a suitable time frame for the snail phototaxis experiment
According to a method described in the literature[11],the phototaxis experiment was carried out under a light intensity of 2 000Lux.A combination of uninfected male and female snails was selected from Pengshan,Sichuan,as the experimental group.The data were recorded at the 5,10,15… minutes after the start of the experiment,and a graph was plotted showing the changing trend of the phototaxis index ofO.hupensisunder a light intensity of 2 000Lux to determine a suitable time frame for the snail phototaxis experiment.
Determination of a suitable light intensity for the phototaxis experiment
According to the determined experimental time,a combination of uninfected male and female snails was selected from Pengshan,Sichuan,as the experimental group.Starting with a light intensity of 0Lux,at every unit of 250or 500Lux,the light intensity was progressively increased during the phototaxis experiment.Under the selected experimental time and different light intensities,a graph was plotted showing the changing trend of the phototaxis index ofO.hupensisto obtain the most suitable light intensity for the phototaxis experiment.
Snail phototaxis experiment
The determined experimental time and light intensity were applied.O.hupensisfrom the four geographical strains were separated into uninfected males and females,with a total of 8groups being used for the phototaxis experiment.
Applying the determined experimental time and light intensity,a combination ofS.japonicum-infected male and female snails were selected as the experimental group for the phototaxis experiment.Only the infected snails derived from the Xingzi County,Jiangxi Province,and the strain presented sufficient numbers to complete this experiment,and the infected and uninfected snails from this strain were therefore used for the contrasting experiment.
Statistical analyses
SPSS20.0statistical analysis software was employed for the statistical analysis of the experimental data.The mean and standard deviation of the phototaxis indexes of the 8groups of snails were calculated(Table 2).
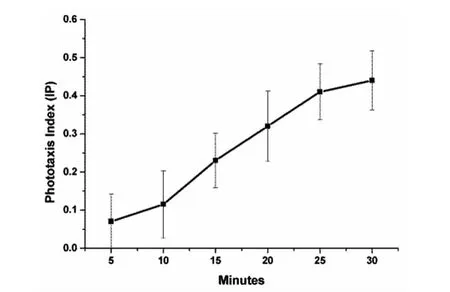
Fig.2 Phototaxis index of Oncomelania hupensis in different time points
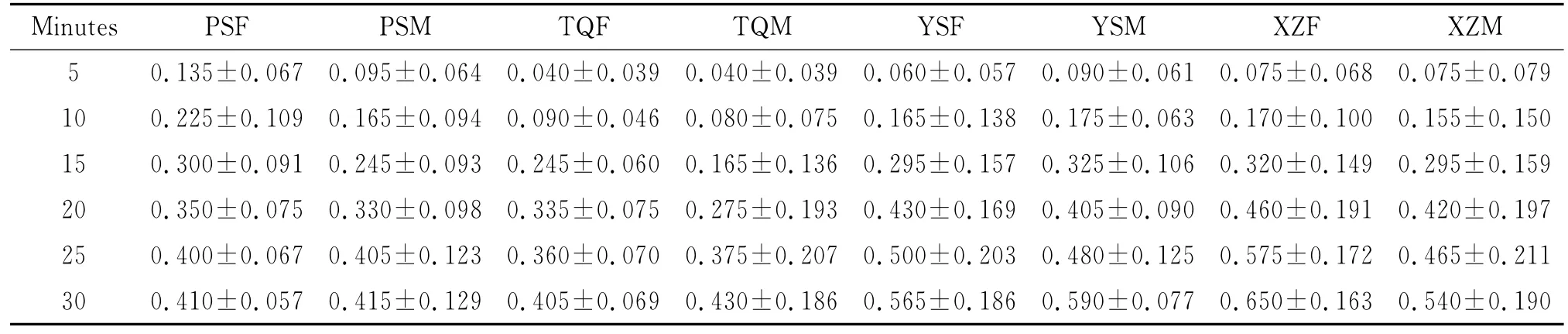
Tab.2 Mean±SD of phototaxis index of Oncomelania hupensis over minutes
The statistical method of Repeated Measures ANOVA was used to compare the phototaxis indexes of the 8groups of male and female snails from the four regions.At the final time point of the observations(30minutes),at-test was applied for pair-wise comparison of the phototaxis index values between the two subspecies,between the same subspecies from different geographic strains and between theS.japonicum-infected and uninfected male and female snails from Xingzi County,Jiangxi Province,China.
Results
Firstly,the time required for theO.hupensisphototaxis test was determined.This part of the experiment showed that the phototaxis index ofO.hupensisgradually increased over time under a light intensity of 2 000Lux.When the experiment was carried out for 30minutes,some of the snails crawled all the way to the small light holes,thereby making it impossible to continue measuring the phototaxis index.Therefore,a time period of 30 minutes was considered a suitable experimental time frame(Figure 2).
An additional experiment was carried out to determine the most suitable light intensity.These results showed that when the light intensity was less than 1 500Lux,as the light intensity was increased,the phototaxis index gradually increased,thereby resulting in a distinct phototaxis profile.When the light intensity was greater than 1 500 Lux,and the phototaxis index gradually decreased with an increase in light intensity.Therefore,1 500Lux was considered as the most suitable light intensity for this experiment(Figure 3).
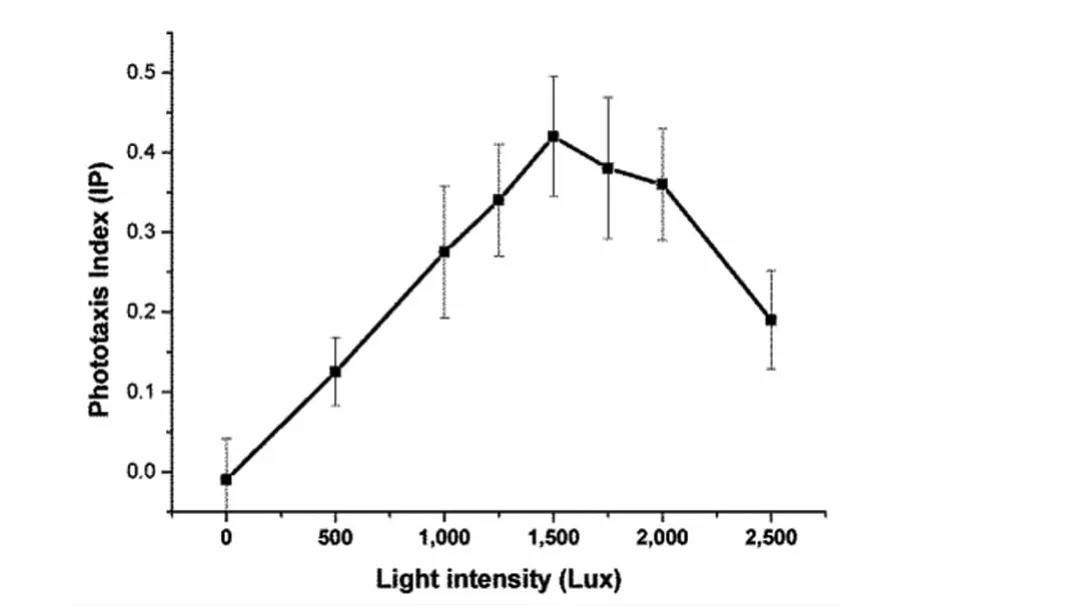
Fig.3 Phototaxis index of Oncomelania hupensis under different light intensities
In the snail phototaxis experiments,all 8 groups of uninfected male and female snails from the four geographical strains showed distinct phototaxis profiles.According to the statistical analysis,as time increased,the phototaxis index increased significantly(F=362.644,P<0.001,n=10).Although no significant difference in the phototaxis index was observed between the male and female snails(F=0.902,P=0.345,n=10),after combining each group of male and female snails,a significant difference in the phototaxis index was found(at the 30thminute)between the snails from the four different geographical regions(F=5.125,P=0.003,n=20).Individual comparison of the phototaxis index between the two subspecies ofO.hupensisshowed that the phototaxis index ofO.h.hupensis(0.586±0.160)was significantly greater than that ofO.h.robertsoni(0.415 ±0.117)(t=5.467,P<0.01,n=40).In contrast,no difference in the phototaxis index was observed between the snails of theO.h.hupensissubspecies from the Xingzi strain(0.595±0.181)and the Yushan stain(0.577±0.139)(t=0.343,P=0.734,n=20).Within theO.h.robertsonisubspecies,no significant difference was found between the Pengshan strain(0.412±0.097)and the Tianquan strain(0.418±0.137)(t=0.133,P=0.895,n=20;Figure 4).
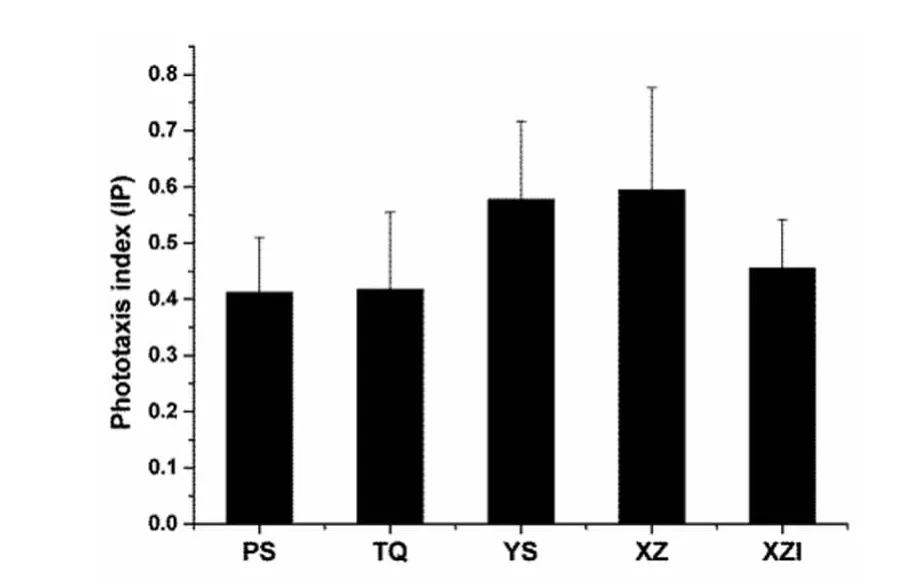
Fig.4 Charts of the variety which the phototaxis index of Oncomelania hupensis that from different groups strain at the 30th minute
Finally,the phototaxis indexes obtained forS.japonicum-infected and uninfected snails were compared.This part of the experiment showed that,at the 30thminute,the phototaxis index ofS.japonicum-infected snails from the Xingzi,Jiangxi(0.455±0.086,n=10)was signifi-cantly lower than that of uninfected snails(0.595±0.181,n=20)(t=2.863,P=0.008).
Discussion
Researchers who have studied the phototaxis ofO.h.hupensisfrom Jiangsu Province have reported that the index reaches maximum levels at 2 000Lux[11].Our experimental results revealed a maximum phototaxis index at 1 500Lux.Although the conclusion arising from the previous study and our experimental results are not completely consistent,the observed changing trends in the phototaxis index are consistent.We believe that this inconsistency may be due to the difference in experimental equipment,the differences in the geographical areas from which the snails were obtained and the time frame of experimental observations.
Previous studies have indicated that femaleHelicoverpaarmigeradisplay a stronger phototaxis reaction and can adapt to higher light intensities than corresponding males[19].The conclusion of this research clearly indicates a difference in phototaxis between some male and female insects,thus indicating that snails,which are mollusks,are generally less sensitive to light than insects.Moreover,different geographic strains of the same subspecies displayed no difference in phototaxis,which demonstrates that the sensitivity of snails to light is quite low.
O.hupensisis an amphibious animal that requires certain natural environmental conditions for survival and reproduction.Moisture,temperature,vegetation and soil are necessary ecological factors for survival and breeding.DifferentO.hupensissubspecies exhibit unique habitat types.Beginning the year 1881,Gredler collected samples from Hubei identified asO.hupensis,and after more than one hundred years of repeated comprehensive investigations,the distribution range ofO.hupensishas been found to include mainland China,starting in the south from Yulin City in Guangxi Province(22°25′N),extending north to Baoying Town in Jiangsu Province(33°15′N),east to Nanhui District in Shanghai(121°53′E)and west to Yunlong Town in Yunnan Province(99°05′E)as well as throughout the Yangtze River Basin in China and in 12provinces to the south(municipalities and autonomous regions)[20].Although there is controversy among both domestic and foreign researchers regarding the subspecies classifications for different geographic populations ofO.hupensis,scholars agree that theO.hupensissnails distributed within the territory of Sichuan in Yunnan Province belong toO.h.robertsoni,while those distributed throughout the middle and lower Yangtze region,on the banks of lakes and rivers,belong toO.h.hupensis.In China,O.h.robertsoniis only found in the central and southern portions of the Sichuan Basin and in the northwest area of Yunnan,at an altitude between 400and 1 000m,with the highest records occurring at 2 400m.Among the various subspecies of these snails,O.h.robertsoniexhibits the highest vertical distribution above sea level.Its habitat is primarily terrestrial,although it has also been found in streams,irrigation ditches,hillside marshes and paddy fields.O.h.hupensisprimarily occurs in the waters of river branches,irrigation canals or swamps and in marshlands of surrounding lakes and rivers in the middle and lower reaches of the Yangtze River.In contrast,O.h.hupensisis the subspecies with the lowest elevational distribution,primarily being found at altitudes below 50m.These snails mainly live in a more humid environment and are affected by changes in temperature and water levels.There is a relationship between the phototaxis of an animal and its vision or visual sensitivity.Whether vertebrate or invertebrate,all animals exhibit the basic structure of visual pigments.Light energy transforms into neural potential,thereby enabling animals to be sensitive to light.Animals express phototaxis because visual pigments strongly absorb light[21].Therefore,there are differences in the expression of phototaxis between different subspecies of snails,suggesting the existence of different photosensitive systems between these subspecies.These differences may be innate and would potentially lead to changes in their breeding environments.
The four geographical strains used in this study belong to two subspecies.One of these subspecies wasO.h.hupensis,obtained from Yushan(altitude:241m)and Xingzi(altitude:27m)in Jiangxi Province.These two locations are endemic forS.japonicum,although the varying geographical environments determine differences in the type of endemic area[22],as they are differentiated into marshland and mountainous types.However,no difference in the phototaxis responses of these snails was observed.Although the environmental conditions of the geographical distributions ofO.h.hupensisfrom Yushan,Jiangxi andO.h.robertsoniare quite similar,as both are found in mountainous areas,differences were observed in the phototaxis responses of these snails.There-fore,we believe that geographical factors such as altitude and the breeding environment do not affect the phototaxis reaction ofO.hupensis.Instead,we hypothesize that genetic factors in the different subspecies may be responsible for the variations in phototaxis.
Several surveys have indicated thatO.h.hupensisexhibits a higher rate ofS.japonicuminfection thanO.h.robertsoni[23].Is it possible that snails showing elevated phototaxis are more easily infected withS.japonicum?If so,then should the phototaxis of already infected snails be higher?Based on the comparison of the phototaxis indexes ofS.japonicum-infected and uninfected snails derived from the same location,we can rule out this possibility,as the results showed that snails infected withS.japonicumlarvae display a lower phototaxis index.After being infected byS.japonicum,we hypothesize that the physical condition of the snails becomes impaired,causing their crawling speed to slow.It is also possible that after being infected withS.japonicum,the phototaxis system of the snails becomes damaged.However,tofully understand this aspect of snail phototaxis,these hypotheses will require further investigation.
[1]Muntz WR.The development of phototaxis in the frog(Rana temporaria)[J].J Exper Biol,1963,40:371-379.
[2]Rimet M.Polyphasic phototaxis ofDaphniapulexde Geer[J].J de physiologie,1960,52:769-781.
[3]Stahl N,Mayer AM.Experimental differentiation between phototaxis and motility inChlamydomonassnowiae[J].Science,1963,141(3587):1282-1284.DOI:10.1126/science.141.3587.1282
[4]Vakoliuk IA,Zhukov VV.Photoreception inLymnaeastagnalisbased on phototaxis data[J].Zhurnal evoliutsionnoi biokhimii i fiziologii,2000,36(5):419-423.
[5]Mao CP,Li L,Wu CC.Studies on the emergence of cercariae ofSchistosomajaponicumfrom their Chinese snail host,Oncomelaniahupensis[J].Am J Trop Med Hyg,1949,29(6):937-944.
[6]Sturrock RF.The schistosomes and their intermediate hosts[M].In Schistosomiasis.Edited by Mahmoud AAF.London:Imperial College Press;2001:7-83.
[7]Yasuraoka D.The behavior ofOncomelanianosophora,the intermediate host ofSchistosomajaponicum,to light in water[J].Jpn J Med Sci Biol,1955,8(4-5):323-329.
[8]Ye XP,Fu YL,Wu ZX,et al.The effects of temperature,light and water upon the hatching of the ova ofSchistosomajaponicum[J].Southeast Asian J Trop Med Public Health,1997,28(3):575-580.
[9]Sulieman Y,Pengsakul T,Guo Y.Development and effects ofSchistosomajaponicum(Trematoda)on its intermediate host,Oncomelaniahupensis(Gastropoda)[J].Iranian J Parasitol,2013,8(2):212-218.
[10]Zhou XN,Zhang Y,Hong QB,et al.Science onOncomelania Snail[M].InOncomelaniahupensis.Beijing;Science Press.2005:150-154.(in Chinese)周晓农,张仪,洪青标,等.实用钉螺学(M).北京.科学出版社,2005,150-154.
[11]Shen YX,Zhuge HX,Liang YS,et al.The phototaxis ofOncomelaniahupensisto intensity and color of light[J].Chin J Zoonoses,2010,26(10):939-941.(in Chinese)申云侠,诸葛洪祥,梁幼生,等.光照强度和光色对钉螺趋光性的影响.中国人兽病共患病学报.2010,26(10):939-941.
[12]Zhao QP,Jiang MS,Littlewood DT,et al.Distinct genetic diversity ofOncomelaniahupensis,intermediate host ofSchistosomajaponicumin mainland China as revealed by ITS sequences[J].PLoS Negl Trop Dis,2010,4(3):e611.DOI:10.1371/journal.pntd.0000611
[13]Zhou YB,Yang MX,Zhao GM,et al.Oncomelaniahupensis(Gastropoda:Rissooidea),intermediate host ofSchistosoma japonicumin China:genetics and molecular phylogeny based on amplified fragment length polymorphisms[J].Malacologia,2007,49(2):367-382.
[14]Zhou YB,Zhao GM,Jiang QW.Genetic variability ofSchistosomaJapontcum(Katsorada,1904)intermediate hostsOncomelaniahupensis(Gredler,1881)(Gastropoda:Rissooidea);proceedings of the Annales Zoologici,F,2008[C].BioOne.
[15]Lo CT,Lee KM.Schistosomajaponicum,zoophilic strain,inOncomelaniahupensischiuiandO.h.formosana:miracidial penetration and comparative histology[J].J Parasitol,1995,81(5):708-713.
[16]Li SZ,Wang YX,Yang K,et al.Landscape genetics:the correlation of spatial and genetic distances ofOncomelaniahupensis,the intermediate host snail ofSchistosomajaponicumin mainland China[J].Geospatial Health,2009,3(2):221-231.
[17]Rachford FW.Oncomelaniahupensisquadrasifrom Mindoro(Victoria),Leyte(Palo),and Mindanao(Davao del Norte)of the Philippines:susceptibility to infection with Philippine isolates ofSchistosomajaponicum[J].J Parasitol,1977,63(6):1129-1130.
[18]Yuan L,Michels E,De Meester L.Changes in phototactic behavior ofDaphniamagnaclone C1 242in response to copper,cadmium and pentachlorophenol[J].J Environ Sci,2003,15(6):841-847.
[19]Wei G,Zhang Q,Zhou M,et al.Studies on the phototaxis ofHelicoverpaarmigera[J].Acta Biophysica Sinica,2000,16:89-95.
[20]Liang YS,Wang W,Li HJ,et al.The South-to-North Water Diversion Project:effect of the water diversion pattern on transmission ofOncomelaniahupensis,the intermediate host ofSchistosomajaponicumin China[J].Parasit Vectors,2012,5:52.DOI:10.1186/1756-3305-5-52
[21]Wasserman GS.Invertebrate color vision and the tuned-receptor paradigm[J].Science,1973,180(4083):268-275.
[22]Zhou YB,Yang MX,Yihuo WL,et al.Effect of habitat fragmentation on the schistosome-transmitting snailOncomelania hupensisin a mountainous area of China[J].Transac Royal Soc Trop Med Hyg,2011,105(4):189-196.DOI:10.1016/j.trstmh.2010.12.006
[23]Hong QB,Zhou XN,Sun LP,et al.Susceptibility ofOncomelaniahupensistoSchistosomajaponicum[J].Chin J Schistosomiasis Ctrl,1995,7(2):83-86.(in Chinese)洪青标,周晓农,孙乐平,等.不同地区不同环境类型钉螺对日本血吸虫易感性的测定[J].中国血吸虫病防治杂志,1995,02,83-86.
- 中国人兽共患病学报的其它文章
- Detection of respiratory pathogens Mycoplasma hyorhinis and Mycoplasma hyopneumoniae from clinically infected porcine using nested PCR in Jiangsu Province,China
- 结核分枝杆菌NrdF1、PE_PGRS35、Rv1985c和Rv1986的原核表达及检测牛结核病抗体之应用
- CD14对生殖支原体LAMPs激活NF-κB的影响
- 禽流感病毒特异性NP单克隆抗体的鉴定及禽流感特异性检测方法的建立
- 家养野猪脑心肌炎病毒的分离、鉴定及全基因组序列分析
- 恒河猴H5N1禽流感病毒性肺炎模型建立及其发病机制

