Detection of respiratory pathogens Mycoplasma hyorhinis and Mycoplasma hyopneumoniae from clinically infected porcine using nested PCR in Jiangsu Province,China
Joyce Wanjiru MAINGI,XIONG Qi-yan,WEI Yan-na,MA Qing-hong,JI Yan,William KIMARU,HUA Li-zhong,WANG Jia,SHAO Guo-qing,BAO En-dong
(1.College of Veterinary Medicine,Nanjing Agricultural University,Nanjing210014,China;2.Institute of Veterinary Medicine,Jiangsu Academy of Agricultural Sciences/Key Laboratory of Veterinary Biological Engineering and Technology,National Center for Engineering Research of Veterinary Bio-products,Ministry of Agriculture,Nanjing210014,China)
M.hyopneumoniae(Mhp)is the primary etiological agent that causes enzootic pneumonia in pigs[1]whileM.hyorhinis(Mhr)has often been reported as a secondary pathogen in porcine pneumonias[1-5].Enzootic pneumonia is a chronic respiratory disease marked by chronic coughing and minimal performance in grow-finishing pigs and results to great economic losses to the swine industry[1,6-8].Since isolation ofM.hyopneumoniaeandM.hyorhinisin suitable media is tedious and impractical for daily routine diagnostics,Nested-PCR(nPCR)technique is currently used for improving the diagnosis[9].
AlthoughM.hyorhinisis part of normal flora of the upper respiratory tract of pigs,it has been described as one of the etiological agents of arthritis,pleuritis,pericarditis and peritonitis and some strains are capable of causing pneumonia and microscopic lesions in sucking pigs[10-11].Manifestation of clinical signs may vary and most infections caused byM.hyorhinisare subclinical.Variations in virulence ofM.hyorhinisstrains,climatic changes,concomitant pathogens and the host immune system are some of the mechanisms that dictate its systemic nature[4,8,12].Co-infection ofM.hyopneumoniaeandM.hyorhinisis common and the main source of infection is from sick animals or asymptomatic carriers through direct contact or by airborne transmission by aerosol from coughing pigs.The pathogens have been detected from the air samples of infected barns[13].Transmission between farms is probable up to a distance of 3-9.2 kilometers frominfected farm[3-4,13-14]. Enzootic pneumonia caused byM.hyopneumoniaeleads to high morbidity rates due to poor growth and poor feed conversion whileM.hyorhinismay cross-react withM.hyopneumoniaehence confusing diagnosis[1,5,15].
The purpose of this study was to establish the field status of piglets during the early stages of stocking in the production houses.To investigate the prevalence of the two pathogens,the detection rate using nPCR of a total of 399nasal swabs from each piglet between 1-5weeks of age was assessed.Clinical samples were taken from each unvaccinated piglet;115nasal swabs from the Jiangquhai porcine lean(JQHPL)strain that is a new meat-type strain developed in China in the last few years from the parent lines Duroc,Fengjing,and Jiangquhai pigs[16]and 284nasal swabs from other Western commercial pigs breeds introduced in China such as Duroc,Landrace,and Yorkshire.Nested PCR as a diagnostic tool is shown to be suitable for determining infection dynamics since it is effective,specific and allows rapid detection of the pathogens in either living or dead animals.
Materials and methods
Animals
The study was conducted on pig farms practicing all-in all-out production system located in Jiangsu Province,China.The pigs had not received any previous vaccination or sensitive antibiotics against mycoplasma.When the sucking piglets were 7days old,they were creep fed.During the experiment,none of the suckling piglet received any vaccinations or other immunologically active drugs againstM.hyopneumoniaeandM.hyorhinis,other than the antibiotic concentration that could be present in the commercial creep feed.All of the piglets were divided intofive groups and clinical samples were taken from each and grouped according to different ages among 7,14,21,28,and 35 days and tested for the presence ofM.hyopneumoniaeandM.hyorhinis.
Sample preparation
Samples were taken from different age groups at different weekly intervals.The method of nasal sampling used in this study was previously described by Fablet et al.(2010)[17]with slight alter-ations.Both nasal cavities of each pig were swabbed with sterilized cotton swabs inserted deep into the nostrils to reach deep into the turbinates.After the animal had sneezed severally,the nasal swabs were removed and placed in 1.5mL sterilized phosphate buffered saline(PBS)and incubated at 4 ℃ overnight.After centrifugation at 18 500×g for 30min at 4℃,target DNA extraction from the precipitate was done using TIANamp Bacteria DNA Kit,Tiangen Biotech(Beijing)Co.,Ltd.following manufacturer’s instructions.To avoid false positive results due to contamination,positive and negative controls were included during DNA extraction and amplification process.
Nested PCR
For the amplification of DNA,PCR assay was carried out in a thermal cycler using 25μL reaction mixture containing 2.5μL 10×PCR buffer(Mg2+ Free),1.5μL MgCl2(25mM),2μL dNTPs,0.5μL of each forward and reverse primer,5μL DNA,0.2μL Taq(5U/μL),and 13.8μL of DNAase-free deionized water under the amplification conditions indicated in Table 1.
The size of the amplified DNA fragment of 346base pairs(bp)forM.hyorhinisand 427bp forM.hyopneumoniaein 10μL of the reaction mixture was visualized on a 1%agarose gel stained with ethidium bromide(0.5mg/mL)and run for 30min at 120v(Figure 1).Water and respective DNA of pure cultures were used as negative and positive controls respectively.
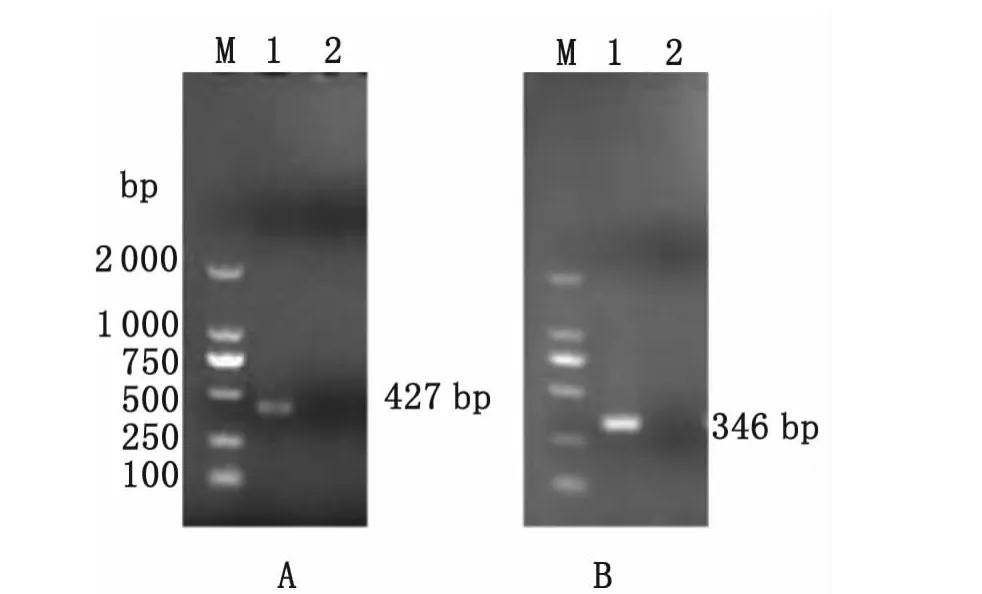
Fig.1 Nested PCR amplified products on 1%agarose gel
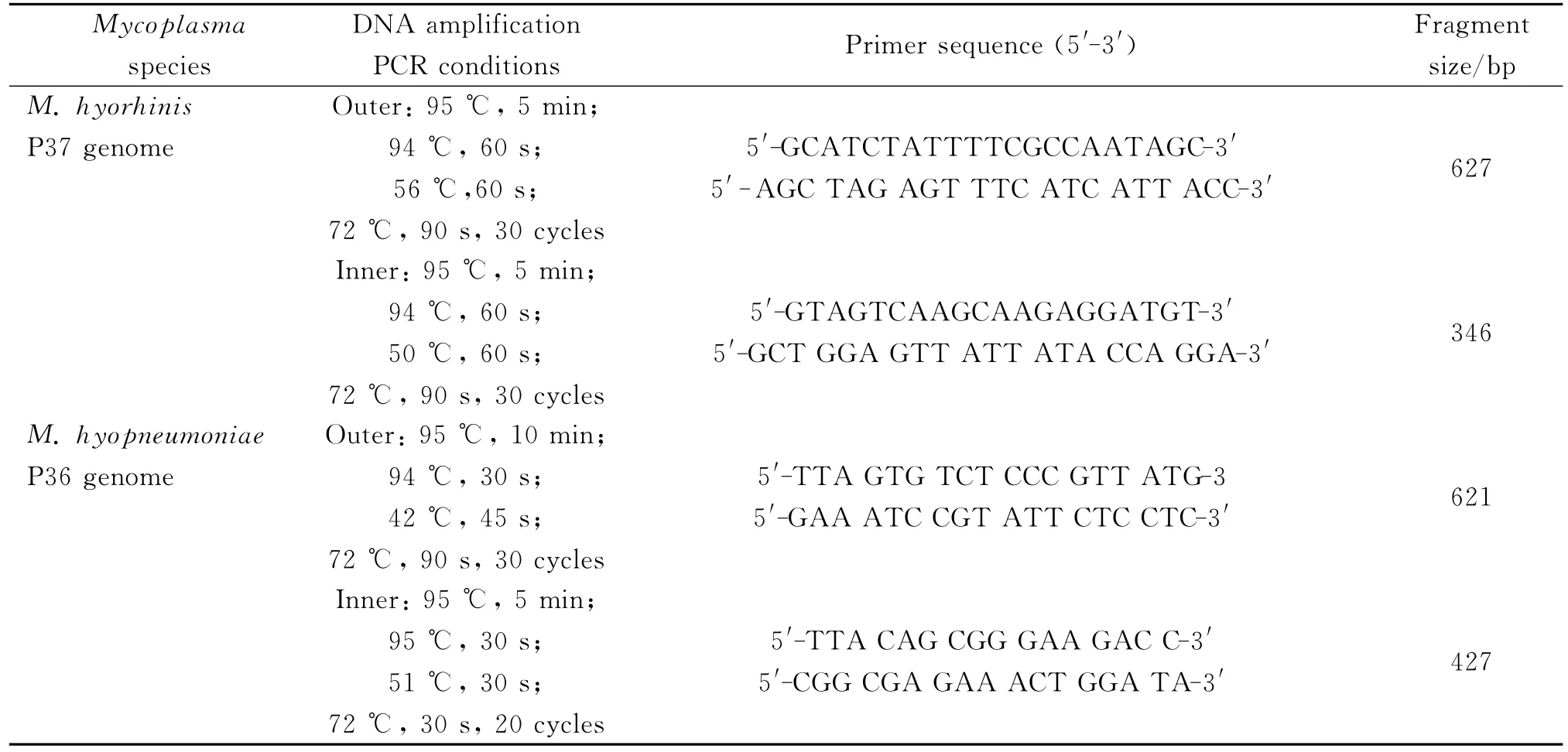
Tab.1 Oligonucleotide primers and amplification condition for the nested PCR of M.hyopneumoniae and M.hyorhinis
Statistical analysis
The data was analyzed using the statistical package SPSS 16.0,(SPSS Inc.,IL,USA).Chi-Square test was used and a statistical significance level was set at 0.05.Variables were regarded as statistically significant whenP-value was less than 0.05.
Results
Clinical samples were grouped on weekly basis according to different age between 7and 35days and detected for the presence ofM.hyopneumoniaeandM.hyorhinisby nPCR.A pig was defined as positive forM.hyopneumoniaeandM.hyorhiniswhen the nasal swab taken had a positive PCR outcome.From this study,the prevalence rate from different herds in Jiangsu Province in China was 70.9%(283/399)forM.hyorhinisand 13.5%(54/399)forM.hyopneumoniaerespectively.Thirty samples(7.5%,30/399)were positive for co-infection of bothM.hyopneumoniaeandM.hyorhinis(Table 2).
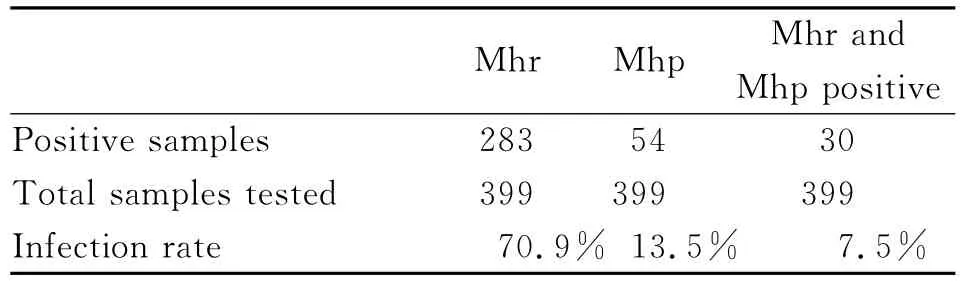
Tab.2 Detection of M.hyopneumoniae and M.hyorhinisin the nasal swabs samples by nested PCR and the infection rate
In general,the rate ofM.hyorhinisinfection demonstrated a positive trend,which increased as the animals became older.Nevertheless,between 14 and 21days of age,the infection rate slightly declined and then increased again between 21and 35 days.The 35days old piglets had the highest infection rate ofM.hyorhinis.On the other hand,M.hyopneumoniaeinfection trend did not change much and piglets appeared to be infected byM.hyopneumoniaemuch later,when they were more than 35days old.From the current study,piglets were infected withM.hyorhinisearlier as opposed toM.hyopneumoniae(Figure 2).
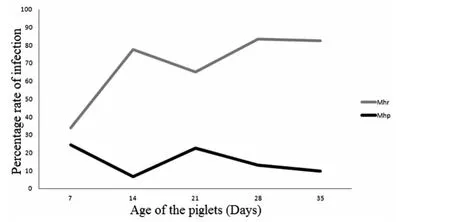
Fig.2 Infection pattern of M.hyorhinis(Mhr)and M.hyopneumoniae(Mhp)in relation to the age of piglets as detected in the nasal swabs by nested PCR
The infection rate of a specific breed of JQHPL was also studied in the present work.The 91.3%and 11.3%of 115nasal swabs samples from JQHPL were positive while 62.7%and 14.4%of 284samples from the other breeds were positive forM.hyorhinisandM.hyopneumoniaeinfections respectively(Table 3).There was statistically stronger evidence of a relationship(P<0.001)between the infection rate ofM.hyorhinisand the breed type.JQHPL appeared to be more prone toM.hyorhinisinfection as compared to the other breeds.However,there appeared to be no supported relationship between the type of the breed andM.hyopneumoniaeinfection(P>0.05).

Tab.3 Summaries of the number of positive samples tested for M.hyorhinis and M.hyopneumoniae for each breed
Discussion
Accuracy of mycoplasma detection has previously been assessed using different type of the diagnostic assays and samples.Different samples ranging from nasal swabs,ear swabs,tonsil,tracheal washings,sputum,synovial fluid,blood,lungs,and other biopsy samples have been used in different instances to detect porcine mycoplasma species[9,11,18-20].Currently, molecular techniques such as PCR assays have been useful in detecting and identifying the fastidious mycoplasmas by amplifying a specific gene.Nested PCR assays,employing two sets of primers,have been commonly used by diagnostic laboratories due to its sensitivity and specificity[1,9].PCR technology is ideally suitable for diagnosis because it can be automated,is rapid and specific,does not rely on viable pathogens and can be used to detect samples from live or dead animals[1,3,18,21].However,the presence of bacterial DNA in the nasal swabs does not guarantee the infection,but it definitely shows that the animals were exposed to the pathogens[22].Some animals that are exposed to the pathogen may seroconvert and have a reduced performance even if they do not clinically exhibit the symptoms of the disease[23].The prevalence of the two respiratory pathogens may vary between endemically infected herds.The results may reveal the pathogen load of the herds but not necessarily the shedding off.However,testing nasal swabs in a particular herd may be useful to establish the time of exposure pri-or to implementation of a vaccination program,other than relying solely on the assessment of the clinical symptoms.
Our findings suggest that the prevalence ofM.hyorhinisin different herds in Jiangsu Province in China is 70.9%while that ofM.hyopneumoniaeis 13.5%.The 7.5%of the nasal swabs samples were positive of bothM.hyopneumoniaeandM.hyorhinisinfections.A separate but similar study conducted by Lobo et al.(2011)[10]in Cuba reveals that the presence ofM.hyorhinisfrom different regions of the country was 66%while coinfection of bothM.hyorhinisandM.hyopneumoniaewas found from 19.84%of the lung samples tested.The results are similar to a study on Korean slaughtered pigs(19%)[20].Kawashiwa et al.(2007)investigated the prevalence swine pathogens in Japan from different postmortem tissues using various diagnostic tools including PCR.Similar to our study,their findings indicated thatM.hyopneumoniaewas present in 14.0%of the samples and 56.5%of the samples were positive forM.hyorhinis.In a different study in Thailand,the overall prevalence ofM.hyopneumoniae,andM.hyorhiniswere 40.3%,and 64.6%respectively in the lung samples[19].Clavijo et al.(2012)reported a high prevalence ofM.hyorhinis(98%)in the nasal cavities of nursery pigs and 85%in postweaning pigs in Minnesota[28].
For the detection ofM.hyopneumoniae,Ruiz et al.(2003)[24]reported a prevalence of 20%using nPCR in the nasal swabs of pre-weaned piglets and 18%in sows in Minnesota.Between 5.5%and 13.2%of piglets were infected in a breeding unit at 19days of age.This study is consistent with our findings that 13.5%infection rates was found in pigs less than five weeks of age.However,a lower percentage has been reported in the nasal swabs from piglets by nPCR in Spain,to be between 1.5%and 3.8%in farrowing units and up to 12.8%in tonsillar swabs of three weeks old piglets in nurseries[25-26].From the nasal swabs samples,Vicca et al.(2002)[6]observed that the prevalence ofM.hyopneumoniaeis 16%at 6weeks while Calsamiglia and Pijoan(2000)[27]reported that the prevalence ofM.hyopneumoniaevaried between 24%and 56%from non-vaccinated sows and is lower in 17days old piglets(between 7.7%and 9.6%).
The current study shows that older pigs have a higher rate ofM.hyorhinisinfection as compared to younger pigs from 7to 35days.Probably,maternal antibodies againstM.hyorhinispresent soon after birth confers maternal immunity againstM.hyorhinisinfection.As the pig grows,the antibodies decreases,and it may become prone toM.hyorhinisinfection gradually.In our study,a high number of pigs are colonized afterwards when they are over three weeks of age.No obvious increase ofM.hyopneumoniaeinfection rate was observed from 7to 35days in our study.The infection ofM.hyopneumoniaeappears later and lower than the infection ofM.hyorhinis.
Conclusion
The present study indicated that the prevalence forM.hyorhinisis 70.9%and 13.5%forM.hyopneumoniaeinfection in Jiangsu Province,China.The co-infection rate of the two porcine pathogens was 7.5%.The rate ofM.hyorhinisinfection increased as the animals became older.The 35-days-old piglets had the highest infection rate ofM.hyorhinis.The infection trend ofM.hyopneumoniaedid not change when the piglets were 7to 35days old.Piglets are infected byM.hyorhinisearlier as compared toM.hyopneumoniaeinfection.JQHPL appears to be more prone toM.hyorhinisinfection but notM.hyopneumoniaeinfection.Determining the dynamics of infection in the field would facilitate the execution of better control measures against the porcine pathogens and lower the economic burden in commercialized pig farms.
[1]Silva F,Castro L,Silva Junior A,et al.Detection ofMycoplasmahyopneumoniaein lungs and nasal swabs of pigs by nested PCR[J].Arquivo Brasileiro de Medicina Veterinária e Zootecnia,2009,61:149-155.DOI:10.1590/S0102-09352009000100021
[2]Kawashima K,Katsuda K,Tsunemitsu H.Epidemiological investigation of the prevalence and features of postweaning multisystemic wasting syndrome in Japan[J].J Vet Diagn Invest,2007,19:60-68.
[3]Charlebois A,Marois-Crehan C,Helie P,et al.Genetic diversity ofMycoplasmahyopneumoniaeisolates of abattoir pigs[J].Vet Microbiol,2014,168:348-356.DOI:10.1016/j.vetmic.2013.11.006
[4]Sibila M,Pieters M,Molitor T,et al.Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection[J].Vet J,2009,181:221-231.DOI:10.1016/j.tvjl.2008.02.020
[5]Siqueira FM,Thompson CE,Virginio VG,et al.New insights on the biology of swine respiratory tract mycoplasmas from a comparative genome analysis[J].BMC Genomics,2013,14:175.DOI:10.1186/1471-2164-14-175
[6]Vicca J,Maes D,Thermote L,et al.Patterns ofMycoplasma hyopneumoniaeinfections in Belgian farrow tofinish pig herds with diverging disease course[J].J Vet Med B,2002,49:349-353.
[7]Simionatto S,Marchioro SB,Maes D,et al.Mycoplasmahyopneumoniae:from disease to vaccine development[J].Vet Microbiol,2013,165:234-242.DOI:10.1016/j.vetmic.2013.04.019
[8]Wilson S,Van Brussel L,Saunders G,et al.Vaccination of piglets up to 1week of age with a single-doseMycoplasmahyopneumoniaevaccine induces protective immunity within 2weeks against virulent challenge in the presence of maternally derived antibodies[J].Clin Vaccine Immunol,2013,20:720-724.DOI:10.1128/CVI.00078-13
[9]Yamaguti M,Muller E,Piffer A,et al.Detection ofMycoplasmahyopneumoniaeby polymerase chain reaction in swine presenting respiratory problems[J].Braz J Microbiol,2008,39:471-476.
[10]Lobo E,Poveda C,Suarez A,et al.Mycoplasmashyorhinisin different regions of cuba:diagnosis[J].Braz J Microbiol,2011,42:721-725.DOI:10.1590/S1517-838220110002000039
[11]Neto JG,Gauger P,Strait E,et al.Diagnostic notes peer reviewedMycoplasma-associated arthritis:Critical points for diagnosis[J].J Swine Health Prod,2012,20:82-86.
[12]Dee S,Otake S,Deen J.Use of a production region model to assess the efficacy of various air filtration systems for preventing airborne transmission of porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae:Results from a 2-year study[J].Virus Res,2010,154:177-184.DOI:10.1016/j.virusres.2010.07.022
[13]Desrosiers R.A review of some aspects of the epidemiology,diagnosis,and control ofMycoplasmahyponeumoniaeinfections[J].J Swine Health Prod,2001,9:233-238.
[14]Tamiozzo P,Lucchesi PMA,Ambrogi A.Monitoring forMycoplasmahyopneumoniaebefore and after a partial depopulation program using a typing scheme based on the polyserine repeat motif of p146[J].J Swine Health Prod,2013,21:309-312.
[15]Nicholas R.The veterinary significance of mycoplasmas[M].InMycoplasmaprotocols.New York:Humana Press.1998:17-23.
[16]Fulin J.Study on breeding meat type line of Jiangquhai.Experiment of breeding basic female strain[J].Jiangsu Agr Res,1999.
[17]Fablet C,Marois C,Kobisch M,et al.Estimation of the sensitivity of four sampling methods forMycoplasmahyopneumoniaedetection in live pigs using a Bayesian approach[J].Vet Microbiol,2010,143:238-245.DOI:10.1016/j.vetmic.2009.12.001
[18]Kang I,Kim D,Han K,et al.Optimized protocol for multiplex nested polymerase chain reaction to detect and differentiateHaemophilusparasuis,Streptococcussuis,andMycoplasma hyorhinisin formalin-fixed,paraffin-embedded tissues from pigs with polyserositis[J].Canad J Vet Res,2012,76:195.
[19]Makhanon M,Tummaruk P,Thongkamkoon P,et al.Com-parison of detection procedures ofMycoplasmahyopneumoniae,Mycoplasmahyosynoviae,andMycoplasmahyorhinisin lungs,tonsils,and synovial fluid of slaughtered pigs and their distributions in Thailand[J].Trop Anim Health Prod,2012,44:313-318.DOI:10.1007/s11250-011-0022-z
[20]Barate AK,Lee HY,Jeong HW,et al.An improved multiplex PCR for diagnosis and differentiation ofMycoplasmahyopneumoniaeand Mycoplasma hyorhinis[J].Korean J Vet Res,2012,52:39-43.
[21]Calsamiglia M,Pijoan C,Trigo A.Application of a nested polymerase chain reaction assay to detectMycoplasmahyopneumoniaefrom nasal swabs[J].J Vet Diagn Investig,1999,11:246-251.
[22]Villarreal I,Vranckx K,Duchateau L,et al.EarlyMycoplasmahyopneumoniae infections in European suckling pigs in herds with respiratory problems:detection rate and risk factors[J].Vet Med,2010,55:318-324.
[23]Kobisch M,Friis N.Swine mycoplasmoses[J].Revue scientifique et technique(Int Office Epizootics),1996,15:1569-1605.
[24]Ruiz AR,Utrera V,Pijoan C.Effect ofMycoplasmahyopneumoniaesow vaccination on piglet colonization at weaning[J].J Swine Health Prod,2003,11:131-136.
[25]Sibila M,Bernal R,Torrents D,et al.Effect of sow vaccination againstMycoplasmahyopneumoniaeon sow and piglet colonization and seroconversion,and pig lung lesions at slaughter[J].Vet Microbiol,2008,127:165-170.DOI:10.1016/j.vetmic.2007.07.027
[26]Sibila M,Nofrarias M,Lopez-Soria S,et al.Exploratory field study onMycoplasmahyopneumoniaeinfection in suckling pigs[J].Vet Microbiol,2007,121:352-356.DOI:10.1016/j.vetmic.2006.12.028
[27]Calsamiglia M,Pijoan C.Colonisation state and colostral immunity toMycoplasmahyopneumoniaeof different parity sows[J].Vet Record,2000,146:530-532.
[28]Clavijo M,Bruner L,Olson S,et al.Dynamics of infection ofMycoplasmahyorhinisin two commercial swine herds[J].Vet Cont Educ,2012,39:91-93.Received:2014-05-21;Revision accepted:2014-07-28

