Risk factors and molecular characterization of acute sporadic symptomatic hepatitis E virus infection in Thailand
*
1Division of Gastroenterology, Department of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
2Center of Excellence in Clinical Virology, Chulalongkorn University, Bangkok 10330, Thailand
3Department of Biochemistry, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
Risk factors and molecular characterization of acute sporadic symptomatic hepatitis E virus infection in Thailand
Kittiyod Poovorawan1, Salyavit Jitmitrapab1, Sombat Treeprasertsuk1, Thanunrat Thongmee2, Apiradee Theamboonlers2, Pisit Tangkijvanich3, Piyawat Komolmit1, Yong Poovorawan2*
1Division of Gastroenterology, Department of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
2Center of Excellence in Clinical Virology, Chulalongkorn University, Bangkok 10330, Thailand
3Department of Biochemistry, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
Objective: To report clinical outcomes and viral genotypes of acute symptomatic hepatitis E virus (HEV) infection in Thailand. Methods: Forty patients with acute symptomatic HEV infection were recruited during 2009-2013. Clinical, demographic and laboratory data were collected. Diagnosis was accomplished by detection of anti-HEV IgM and/or HEV RNA in the serum or stool. HEV genotypes were classified by direct sequencing of RT-PCR products and phylogenetic analysis.
Results: The high risk group, comprising immune-compromised, liver cirrhosis and very elderly (>80 years) patients (17 cases), had higher levels of serum alkaline phosphatase at presentation compared with the low risk group. Two fatal cases resulted from acute hepatitis E in the high risk group. Initial clinical presentation did not show statistically significant differences. In six cases (6/40), the virus could be detected in serum or stool by RT-PCR and sequencing. Upon molecular characterization, the viruses were classified as HEV genotype 3f and were in the same cluster as Thai swine HEV. Conclusions: Our data showed that acute HEV infection has various clinical presentations and outcomes. Higher levels of serum alkaline phosphatase were observed in high risk patients. All isolated viruses were identified as HEV genotype 3f possibly originating from swine.
ARTICLE INFO
Article history:
Received 10 May 2014
Received in revised form 15 June 2014
Accepted 15 August 2014
Available online 20 September 2014
Hepatitis E
Hepatitis E virus
Genotype 3f
Thailand
Risk factors
1. Introduction
Hepatitis E virus (HEV), a member of the genus Hepevirus in the family Hepeviridae is a small RNA virus[1]. HEV infection occurs in mammals, including humans, domestic pigs, wild boar, deer and rodents. HEV has been classified into four major genotypes by molecular characterization and HEV genotype 3 has been identified almost worldwide[2]. HEV is an emerging pathogen worldwide including Asia and the Far East[4]. In Thailand, seroprevalence of HEV ranges from 9% to 22% in adult subjects and increases with age[4]. This prevalence is comparable with the data from east Asia[5]. In occupational risk groups such as pig and poultry farmers who have worked in farms for more than 2 years, HEV prevalence amounted to 27.9%[6]. HEV has been known to be transmitted via contaminated water in endemic areas and recent data have demonstrated animal to human transmission in almost every part of the world[7-9].
In developed countries, hepatitis E infection is more common than previously recognized and might be more common than hepatitis A[10]. The majority of patients with acute HEV infection remain asymptomatic[11].Clinical manifestations of acute hepatitis E can range from asymptomatic to fulminant with ensuing liver failure[12]. The risk of acute or subacute liver failure from acute HEV infection is exacerbated by pre-existing chronic liver disease[13]. In case of liver cirrhosis, HEV infection can induce rapid liver decompensation and death[14]. In contrast to generally healthy individuals, HEV infection can become chronic in post organ transplant patients[15]. Extrahepatic presentations, for example, as previously reported neurological symptoms such as aseptic meningitis and neuralgic amyotrophy are unusual[16].
Acute HEV infection can be detected by laboratory tests for anti-HEV IgM with sensitivities and specificities of close to 90% and 100%, respectively[17]. False positives of anti-HEV IgM can appear in some conditions including acute hepatitis A[18]. Molecular techniques appear to be complementary for diagnosing HEV infection during early acute infection and in immunosuppressed patients[19].
Clinical data, outcomes and risk factors of patients with acute HEV infection in Thailand have not been well researched. Our study has aimed at reporting clinical outcomes, distribution of disease and genotypes of acute symptomatic HEV infection in Thailand.
2. Materials and methods
This study was conducted on acute HEV patients who presented at or were referred to King Chulalongkorn Memorial Hospital during 2009 to 2013. The research protocol was approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University.
2.1. Patients
Forty patients with acute symptomatic HEV infection, who attended or were hospitalized at King Chulalongkorn Memorial Hospital during 2009-2013 were recruited. Acute HEV infection was diagnosed when the clinical presentation was consistent with acute hepatitis and serum was positive for HEV-IgM. All patients were assessed for other viral hepatitis and chronic liver diseases. Clinical, demographic and laboratory data were collected from the hospital records. Patients were divided into high and low risk groups. The high risk group comprised very elderly patients (>80 years), liver cirrhosis patients or patients who were immunecompromised due to previous organ transplantation, chemotherapy or HIV infection. None of these risk factors applied to patients in the low risk group. This study did not include acute HEV infection during pregnancy. A case of HEV infection with neurological complications has been reported elsewhere[16].
2.2. Clinical assessment
Initial demographic data, clinical presentation and laboratory data were obtained. Duration of hospitalization and clinical outcome were analyzed.
2.3. Laboratory methods
Enzyme linked immunosorbent assay (ELISA) was used to determine anti-HEV IgM antibodies (DIA.PRO Diagnostic Bioprobes Srl, Sesto San Giovammi, Milano, Italy) according to the manufacturer’s specifications.
2.4. HEV-RNA detection and genotyping
The serum and/or stool samples obtained in this study were tested for HEV-RNA by one step-semi-nested reverse transcription polymerase chain reaction (RT-PCR). Briefly, the cDNA was amplified by nested PCR using three primers comprising an inner sense forward primer HE5-1 (nt 19-37) 5’-TCG ATG CCA TGG AGG CCC A-3’, and two outer antisense primers HE5-4 (nt 541-60) 5’-CAT AGC CTC SGC RAC ATC AG-3’and HE5-5 (nt 541-560) 5’-CAT YGC CTC SGC AAC ATC GG-3’ for the first round of PCR amplification and HE5-2 (nt 105-123) 5’-GCC YTK GCG AAT GCT GTG G-3’, HE5-3 (nt 450-469) 5’-TCR AAR CAG TAR GTG CGG TC-3’ and HE5-6 (nt450-469) 5’-TYA AAA CAG TAG GTT CGA TC-3’ for the second round. Each reaction mixture comprised 1.5 µL and 0.5 µL of template for the primary and secondary PCR, respectively, combined with 10.0 µL of 2.5× Master Mix (Perfect Taq Plus, Hamburg, Germany), five primers with each primer at a final concentration of 0.5 µmol/L, and nuclease free water to a final volume of 25.0 µL. Both amplification reactions were carried out in a Mastercycler Personal (Eppendorf, Hamburg, Germany). After an initial denaturation step at 95 ℃ for 3 min, amplification was performed for 35 cycles including denaturation (94 ℃ for 45 s), annealing (50 ℃ for 45 s) and extension (72 ℃ for 78 s), followed by a final extension step at 72 ℃ for 10 min. A total of 25 µL of amplified product was subjected to 2% agarose gel (Agarose molecular grade; Promega, WI) electrophoresis at 100 V for 1 h and then stained with ethidium bromide. The expected amplified product (approximately 365 bp in length) was visualized on a UV transilluminator (Gel Doc 1000, BIORAD, CA).
2.5. HEV sequencing
The cDNA of all samples were amplified by semi-nested PCR with the above primers to determine subtype. The band of interest was excised and purified using the GelElute Extraction kit (5 prime, Hamburg, Germany) according to the manufacturer’s specifications for whole genomesequencing. The purified DNA served as templates for DNA sequencing performed by FirstBASE Laboratories SDNBHD (Selangor Darul Ehsan, Malaysia). The nucleotide sequences were analyzed in both directions using forward and reverse primers to confirm the consistency of the sequencing result and ensure that variations of nucleotide sequences were not due to sequencing errors. The genotype was determined by BLAST/FASTA (http://www.ncbi.nlm.nih.gov) analysis. The sequences were edited and assembled by using programs CHROMAS LITE v.2.0 (www.technelysium.com. au) and SeqMan (DNASTAR, Madison, WI). Multiple protein translation and sequence alignments were generated with BioEdit version 7.0.1 (http://www.mbio.ncsu.edu/BioEdit/ bioedit.html).
2.6. Phylogenetic analysis of HEV
All sequences were multiple aligned using Clustal X Version 1.8 and the nucleotide identities with reference sequences were analyzed using the BioEdit Sequence Alignment Editor version 5.0.9. Phylogenetic trees were constructed using the Neighbor-Joining Method of the MEGA (Molecular Evolutionary Genetics Analysis) version 3.1 software. To investigate the relationship between HEV strains, an un-rooted tree topology based on multiple alignments of the ORF1 and ORF2 nucleotide sequences and those of known genotypes from Genbank (genotypes 1, 2, 3a, 3b, 3c, 3d, 3e, 3f and 4) was established by the neighborjoining method, and calculation was done with MEGA 3.1 (http://www.megasoftware.net). Consistency of branching was tested by bootstrap analysis with 1 000 re-samplings of the data using MEGA 3.1. Multiple protein translation and sequence alignments were generated with BioEdit version 7.0.1 (http://www.mbio.ncsu.edu/BioEdit/bioedit.html).
2.7. Statistical analysis
Continuous variables were compared between groups using unpaired t-test and one-way ANOVA. Categorical variables were compared between groups usingchi-square/Fisher’s exact test. All statistical analyses were performed using SPSS version 16.
3. Results
From 2009 to 2013, 40 patients were diagnosed with acute HEV infection. They comprised 32 males and 8 females, and their age ranged from 27 to 92 years with a median age of 49 years. Seven patients were immune-compromised. Five very elderly patients were above the age of 80 years and five patients had liver cirrhosis. Eight cases (20%) have a history of exposure to raw pork (Table 1). Residence and region of exposure of acute HEV cases were in various regions of Thailand (Figure 1). In this study, 16 of 40 patients were classified as high risk group. Clinical presentations were not statistically different between the high risk and low risk group(Table 2). Laboratory data were not statistically different between the high risk and low risk group except for higher levels of serum alkaline phosphatase at presentation in the high risk group, (221±119) vs. (156±57) U/L, P=0.01) (Table 3). Mean duration of hospitalization was longer in the high risk group than the low risk group (31.8 vs. 10.6 d, P=0.13).
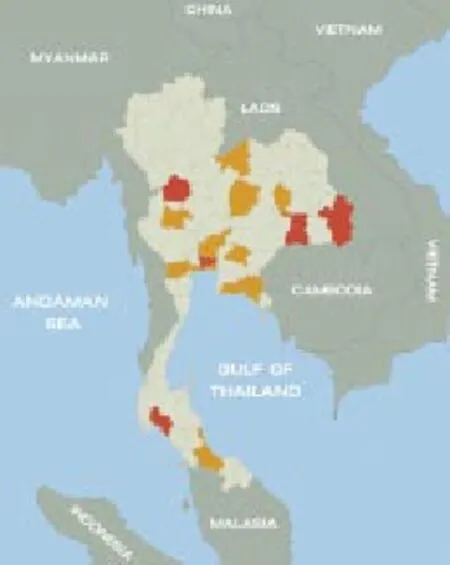
Figure 1. Regions of exposure (red) and residence (yellow) of acute HEV cases in Thailand.Regions of confirmed relevant exposure included Nonthaburi, Ubonratchathani, Kamphaengphet, Krabi, Surin and Bangkok; regions of residence included Ayutthaya, Ratchaburi, Rayong, Prachinburi, Uthaithani, Chanthaburi, Loei, Samutprakarn, Samut Sakhon, Mahasarakham, Chaiyaphum, Saraburi and Songkhla.
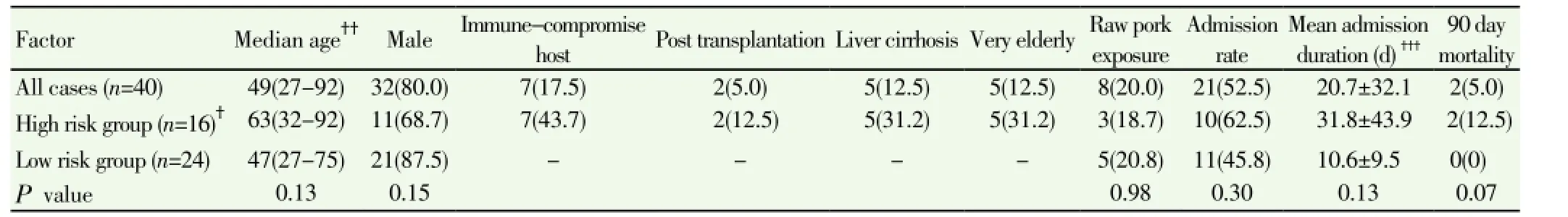
Table 1 Demographic data, risk exposure and clinical outcome of acute hepatitis E patients classified by patient risk [n(%)].
Overall mortality rate from acute hepatitis E in Thailand was 5% (2/40). The 90-day mortality rate in patients at high risk was 12.5% (2/16) compared to 0% in the low risk group. Two fatal cases from acute hepatitis E were observed in a very elderly patient and a cirrhosis patient. Cause of death was multi-organ dysfunction related to acute liver failure after acute hepatitis E infection.
In six cases (6/40), the virus could be detected in serum or stool by RT-PCR and sequencing. All sequences were submitted to the GenBank database under accession numbers FJ653660 for complete genome, JX679072-6 for ORF1; GU947815 and JX841316 for ORF2. Phylogenetic analysis of ORF1 and ORF2 sequences in comparison with the known genotypes showed that the viruses were classified as HEV genotype 3f, and they were closely related to swine HEV strains from Thailand (Figure 2).
4. Discussion
This study has reported clinical manifestations of patients who were diagnosed with acute sporadic symptomatic HEV infection and treated at the tertiary hospital between 2009 and 2013. These cases originated from various regions of Thailand which confirmed the sporadic nature of HEV genotype 3. We found that middle-aged and elderly individuals were at higher risk of infection, which is consistent with a previous report[20], and the majority of patients diagnosed with symptomatic acute HEV hepatitis were male. Acute HEV infection presented with various clinical symptoms, with the most pronounced one being jaundice. These can mimic other viral hepatitis and various causes of hepatitis including drug induced liver injury[21]. None of the HEV patients in this study had a history of epidemic jaundice.
Acute hepatitis E in Thailand presented with clinical symptoms of acute viral hepatitis and tended to be more severe in high risk patients including elderly, cirrhotic and immune compromised patients. This study established that levels of serum alkaline phosphatase at presentation were more pronounced in the high risk group. Multiple conditions such as pregnancy, alcoholic liver disease and pre-existing chronic liver disease were related to high mortality from acute HEV infection[22,23].
Extra-hepatic manifestations, such as acute pancreatitis, hematological abnormalities, autoimmune phenomena, and neurological syndromes have been reported, although the pathogenesis of these manifestations has remained unclear[24]. One patient from our study developed extrahepatic neurological complications of acute hepatitis E[16]. Our data have shown that acute HEV infection can have various clinical presentations and outcomes.
Longer duration of hospitalization was observed in elderly, cirrhotic and immune-compromised patients. In this study, the 90-day mortality rate in high risk patients was 12.5% compared with 0% in the low risk group. Previously reported mortality rates in HEV epidemics have ranged from 0.2% to 4.0%, potentially increasing to 10%-25% in pregnantwomen[22].
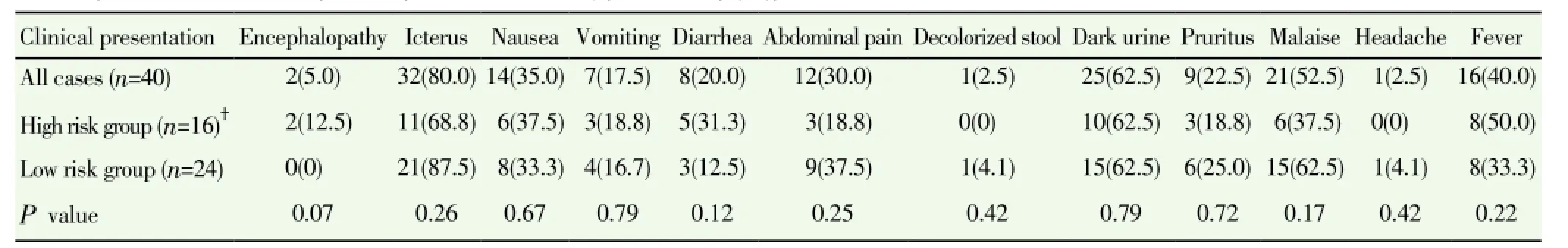
Table 2 Clinical presentation of acute hepatitis E patients classified by patient risk [n(%)].
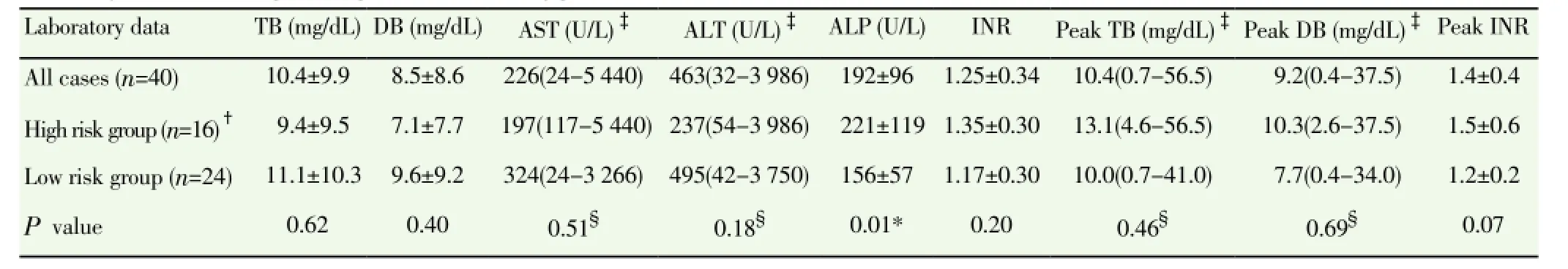
Table 3 Laboratory data of acute hepatitis E patients classified by patient risk.

Figure 2. Phylogenetic tree of the ORF1 (A) and ORF2 (B).The samples from this study were indicated at the respective branches. Symbol ▲ strains were isolated from swine; • strains were isolated from human.
HEV genotype 3 infection has been recognized as zoonotic but the definitive primary source of infection has as yet proven difficult to discover[25]. Eight of our patients had a history of raw pork intake (8/40, 20%). Evidence of pigs as a reservoir for human HEV disease has previously been reported with up to 21.1% seroprevalence[8]. HEV-RNA was detected in six cases from sera or stool. Characterization and phylogenetic analysis of ORF1 and ORF2 sequences of Thai strains showed that they clustered within HEV genotype 3f. One case from our series harbored HEV resembling swine HEV that has been previously reported[8]. Our HEV sequences exhibited the same genotype (3f) closely related to the swine HEV Thailand strains[26]. Infection of the Thai population may have originated from raw pork or contaminated water and food on pig farms.
In conclusion, acute HEV infection in Thailand can be found sporadically and has various clinical presentations and outcomes. Higher mortality rate and longer duration of hospitalization were observed in very elderly, cirrhotic and immune-compromised patients. HEV genotype 3f was detected in all cases and the infection may have originated from swine.
Conflicts of interest statement
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was supported by the Chulalongkorn University Research unit of Hepatitis and Liver Cancer, the Higher Education Research Promotion and National Research University Project of Thailand (HR1155A-55; HR1162A-55), Thailand Research Fund (DPG5480002; BRG5580005), Office of the Commission on Higher Education, Center of Excellence in Clinical Virology, Chulalongkorn University, Chulalongkorn University Centenary Academic Development Project (CU56-HR01) and King Chulalongkorn Memorial Hospital. We would like to express our gratitude to the entire staff of the Gastroenterology unit, department of Medicine, Center of Excellence in Clinical Virology, Department of Biochemistry, Faculty of Medicine, Chulalongkorn University and Hospital, Thai Red Cross Society.
[1] Purcell RH, Emerson SU. Hepatitis E: An emerging awareness of an old disease. J Hepatol 2008; 48: 494-503.
[2] Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: Genetic diversity, subtypes and zoonosis. Rev Med Virol 2006; 16: 5-36.
[3] Jeong SH. Current status of hepatitis e virus infection in Korea. Gut Liver 2011; 5: 427-431.
[4] Poovorawan Y, Theamboonlers A, Chumdermpadetsuk S, Komolmit P. Prevalence of hepatitis E virus infection in Thailand. Ann Trop Med Parasitol 1996; 90: 189-196.
[5] Park HK, Jeong SH, Kim JW, Woo BH, Lee DH, Kim HY, et al. Seroprevalence of anti-hepatitis E virus (HEV) in a Korean population: Comparison of two commercial anti-HEV assays. BMC Infect Dis 2012; 12: 142.
[6] Pourpongporn P, Samransurp K, Rojanasang P, Wiwattanakul S, Srisurapanon S. The prevalence of anti-hepatitis E in occupational risk groups. J Med Assoc Thai 2009; 92: S38-42.
[7] Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis 2010; 202: 825-834.
[8] Suwannakarn K, Tongmee C, Theamboonlers A, Komolmit P, Poovorawan Y. Swine as the possible source of hepatitis E virus transmission to humans in Thailand. Arch Virol 2010; 155: 1697-1699.
[9] Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003; 362: 371-373.
[10] Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: An emerging infection in developed countries. Lancet Infect Dis 2008; 8: 698-709.
[11] Clayson ET, Shrestha MP, Vaughn DW, Snitbhan R, Shrestha KB, Longer CF, et al. Rates of hepatitis E virus infection and disease among adolescents and adults in Kathmandu, Nepal. J Infect Dis 1997; 176: 763-766.
[12] Peron JM, Bureau C, Poirson H, Mansuy JM, Alric L, Selves J, et al. Fulminant liver failure from acute autochthonous hepatitis E in France: Description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat 2007; 14: 298-303.
[13] Dalton HR, Hazeldine S, Banks M, Ijaz S, Bendall R. Locally acquired hepatitis E in chronic liver disease. Lancet 2007; 369: 1260.
[14] Kumar Acharya S, Kumar Sharma P, Singh R, Kumar Mohanty S, Madan K, Kumar Jha J, et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol 2007; 46: 387-394.
[15] Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008; 358: 811-817.
[16] Rianthavorn P, Thongmee C, Limpaphayom N, Komolmit P, Theamboonlers A, Poovorawan Y. The entire genome sequence of hepatitis E virus genotype 3 isolated from a patient with neuralgic amyotrophy. Scand J Infect Dis 2010; 42: 395-400.
[17] Legrand-Abravanel F, Thevenet I, Mansuy JM, Saune K, Vischi F, Peron JM, et al. Good performance of immunoglobulin M assays in diagnosing genotype 3 hepatitis E virus infections. Clin Vaccine Immunol 2009; 16: 772-774.
[18] Kim HS, Jeong SH, Jang JH, Myung HJ, Kim JW, Bang SM, et al. Coinfection of hepatitis A virus genotype IA and IIIA complicated with autoimmune hemolytic anemia, prolonged cholestasis, and false-positive immunoglobulin M anti-hepatitis E virus: A case report. Korean J Hepatol 2011; 17: 323-327.
[19] Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med 2009; 361: 1025-1027.
[20] Aggarwal R, Kumar R, Pal R, Naik S, Semwal SN, Naik SR. Role of travel as a risk factor for hepatitis E virus infection in a disease-endemic area. Indian J Gastroenterol 2002; 21: 14-18.
[21] Dalton HR, Fellows HJ, Stableforth W, Fellows HJ, Stableforth W, Bendall R, et al. The role of hepatitis E virus testing in druginduced liver injury. Aliment Pharmacol Ther 2007; 26: 1429-1435.
[22] Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, et al. Hepatitis E. Lancet 2012; 379: 2477-2488.
[23] Dalton HR, Bendall RP, Rashid M, Ellis V, Ali R, Ramnarace R, et al. Host risk factors and autochthonous hepatitis E infection. Eur J Gastroenterol Hepatol 2011; 23: 1200-1205.
[24] Aggarwal R. Clinical presentation of hepatitis E. Virus Res 2011; 161: 15-22.
[25] Lockwood GL, Fernandez-Barredo S, Bendall R, Banks M, Ijaz S, Dalton HR. Hepatitis E autochthonous infection in chronic liver disease. Eur J Gastroenterol Hepatol 2008; 20: 800-803.
[26] Keawcharoen J, Thongmee T, Panyathong R, Joiphaeng P, Tuanthap S, Oraveerakul K, et al. Hepatitis E virus genotype 3f sequences from pigs in Thailand, 2011-2012. Virus Genes 2012; 46: 369-370.
ment heading
10.1016/S1995-7645(14)60121-8
*Corresponding author: Prof. Yong Poovorawan, Center of Excellence in Clinical Virology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand.
Fax: +662-256-4929
E-mail: Yong.P@chula.ac.th
Foundation project: This work was supported by the Chulalongkorn University Research unit of Hepatitis and Liver Cancer, the Higher Education Research Promotion and National Research University Project of Thailand (HR1155A-55; HR1162A-55), Thailand Research Fund (DPG5480002; BRG5580005), Office of the Commission on Higher Education, Center of Excellence in Clinical Virology, Chulalongkorn University, Chulalongkorn University Centenary Academic Development Project (CU56-HR01) and King Chulalongkorn Memorial Hospital.
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Sonic Hedgehog signaling pathway in primary liver cancer cells
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Protection effect of Emodin pretreatment on intestinal I - RI damage of intestinal mucosa in ratsa
- Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
