Antimicrobial chemical constituents from endophytic fungus Phoma sp.
,3*
1Department of Chemistry, University of Paderborn, Warburger Strasse 100, 33098 Paderborn, Germany
2UoN Chair of Oman’s Medicinal Plants and Marine Natural Products, University of Nizwa, P.O Box 33, Postal Code 616, Birkat Al Mauz, Nizwa, Sultanate of Oman
3Department of Chemistry, CIIT, Abbottabad Campus, Abbottabad-22060, Pakistan
4Department of Chemistry and Polymer Science, University of Stellenbosch, P/Bag X1 Matieland 7602, South Africa
5Department of Chemistry, Quaid-i-Azam University, 45320 Islamabad, Pakistan
6Department of Chemistry, The Islamia University of Bahawalpur, Baghdad Campus-ul-Jadid, Bahawalpur, Pakistan
7Institute of Microbiology, University of Braunschweig, Spielmannstraße 7, 38106 Braunschweig, Germany
Antimicrobial chemical constituents from endophytic fungus Phoma sp.
Hidayat Hussain1,2*, Ines Kock1, Ahmed Al-Harrasi2, Ahmed Al-Rawahi2, Ghulam Abbas2,3, Ivan R. Green4, Afzal Shah5, Amin Badshah5, Muhammad Saleem6, Siegfried Draeger7, Barbara Schulz7, Karsten Krohn1*
1Department of Chemistry, University of Paderborn, Warburger Strasse 100, 33098 Paderborn, Germany
2UoN Chair of Oman’s Medicinal Plants and Marine Natural Products, University of Nizwa, P.O Box 33, Postal Code 616, Birkat Al Mauz, Nizwa, Sultanate of Oman
3Department of Chemistry, CIIT, Abbottabad Campus, Abbottabad-22060, Pakistan
4Department of Chemistry and Polymer Science, University of Stellenbosch, P/Bag X1 Matieland 7602, South Africa
5Department of Chemistry, Quaid-i-Azam University, 45320 Islamabad, Pakistan
6Department of Chemistry, The Islamia University of Bahawalpur, Baghdad Campus-ul-Jadid, Bahawalpur, Pakistan
7Institute of Microbiology, University of Braunschweig, Spielmannstraße 7, 38106 Braunschweig, Germany
Objective: To evaluate the antimicrobial potential of different extracts of the endophytic fungusPhomasp. and the tentative identification of their active constituents. Methods: The extract and compounds were screened for antimicrobial activity using the Agar Well Diffusion Method. Four compounds were purified using column chromatography and their structures were assigned using1H and13C NMR spectra, DEPT, 2D COSY, HMQC and HMBC experiments. Results: The ethyl acetate fraction ofPhomasp. showed good antifungal, antibacterial, and algicidal properties. One new dihydrofuran derivative, named phomafuranol (1), together with three known compounds, phomalacton (2), (3R)-5-hydroxymellein (3) and emodin (4) were isolated from the ethyl acetate fraction ofPhomasp. Preliminary studies indicated that phomalacton (2) displayed strong antibacterial, good antifungal and antialgal activities. Similarly (3R)-5-hydroxymellein (3) and emodin (4) showed good antifungal, antibacterial and algicidal properties. Conclusions: Antimicrobial activities of the ethyl acetate fraction of the endophytic fungus Phoma sp. and isolated compounds clearly demonstrate that Phoma sp. and its active compounds represent a great potential for the food, cosmetic and pharmaceutical industries.
ARTICLE INFO
Article history:
Received 10 May 2014
Received in revised form 15 June 2014
Accepted 15 July 2014
Available online 20 September 2014
Natural product
1. Introduction
In recent times the number of people reported with health problems caused by different forms of cancers, drugresistant bacteria, parasitic protozoans and fungi is on the increase and is thus a cause for concern[1]. Fungi have the potential to produce biologically active secondary metabolites and consequently fungal derived natural products have primarily served as lead compounds for the development of anticancer, antifungal and antibacterial agents. It is equally true that their natural products have also been used in a host of different applications. Interestingly, most currently “new” secondary metabolites reported in the literature have been isolated from fungi[1-6]. Examples of fungal secondary metabolites include the penicillins and cephalosporins. Penicillins were discovered by Fleming in 1928 and were originally isolated from the fungus Penicillium notatum (P. notatum) and are still considered to be extremely important antibiotics. Penicillin G was initially used on a large scale during World War II to treat soldiers wounded on battlefields tostave off infections[7]. On the other hand cephalosporin C showed activity towards typhoid fever. Interestingly, the 4th generation cephalosporins that are currently used to treat patients allergic to penicillins as well as their broad spectrum of activity and excellent safety profiles, make them one of the most widely prescribed class of antimicrobials in the world[7].
We have reported on the isolation of secondary metabolites of diverse structures isolated from endophytic fungi and these include: xanthones, dihydroxanthones, biaryl ethers (phomosines), octadrides, pyrenocine, pestalotheols, chromanone derivatives, isocoumarins, anthraquinones, epoxycyclohexenes and steroids[1-6]. Some of the secondary metabolites isolated by our group showed significant in vitro antimicrobial activities. Continuing our work on the characterization of structurally novel and/or biologically active metabolites from fungal endophyte cultures, we found that the EtOAc extract of the culture of Phoma sp. isolated from Fucus serratus (F. serratus) showed good antifungal activity and good antibacterial and antialgal activities. Fractionation of the ethyl acetate extract led to the isolation and structural determination of one new dihydrofuran derivative, named phomafuranol (1), together with three known compounds viz., phomalacton (2), (3R)-5-hydroxymellein (3), and emodin (4). Details of the isolation, structure elucidation, and biological activity of these compounds are reported herein.
2. Materials and methods
2.1. General experimental procedures
Column chromatography was performed with commercial silica gel (Merck, 0.040-0.063 mm) and Sephadex LH-20 (Amersham Biosciences). Analytical and preparative thin-layer chromatography (TLC) were performed with precoated silica gel plates Merck G60 F-254 or G50 UV-254. Optical rotation was recorded with a Perkin-Elmer 241 MC polarimeter at the sodium D-line. IR spectra were recorded with a Nicolet-510P spectrophotometer.1H and13C NMR spectra were recorded with a Bruker Avance 500 (500 MHz for1H and 125 MHz for13C) spectrometer. MS and HRMS were recorded in the EI mode with MAT 8200 and Micromass LCT mass spectrometers. Microbiological methods and culture conditions are as described previously[8,9].
2.2. Culture, extraction, and isolation
The marine endophytic fungus Phoma sp. was isolated from the plant F. serratus internal strain 6282. It was cultivated at room temperature for 28 days[8,9] on biomalt solid agar medium. The culture media were then extracted with ethyl acetate to afford 2.2 g of a residue after removal of the solvent under reduced pressure. The extract was separated into six fractions by column chromatography (CC) on silica gel, using gradients of dichloromethane/ethyl acetate (85:15, 50:50, 0:100). The six fractions were further fractionated by silica gel column chromatography (CC) and preparative TLC with n-hexane/ethyl acetate (9:1, 8:3, and 6:4) to give pure phomafuranol (1) (3.5 mg), together with three known compounds, phomalacton (2) (40 mg), (3R)-5-hydroxymellein (3) (3.6 mg) and emodin (4) (3.6 mg).
2.3.Phomafuranol (1)
Colorless solid; Mp: 105-107 ℃;[α]D25= -12.9 (c 0.17, CH2Cl2); IR (KBr): 3 350, 1 630, 1 555, 1 450, 1 250 cm-1;1HNMR (200 MHz, CDCl3): δ = 1.78 (d, J8,7= 6.6 Hz, 3H, H-8), 4.22 (br. dd, J3,2= 5.9 Hz, 1H, H-2), 5.03 (br. d, J3,2= 5.9 Hz, 1H, H-3), 5.54 (ddd, J6,7= 15.3 Hz, J6,2= 7.3 Hz, J6,3= 1.4 Hz, 1H, H-6), 5.91 (dq, J7,6= 15.3 Hz, J7,8= 6.6 Hz, 1H, H-7), 6.22 (m, 1H, H-4), 7.46 (d, J5,4= 5.8 Hz, 1H, H-5);13C-NMR (50 MHz, CDCl3): δ = 18.3 (C-8), 73.9 (C-2), 86.3 (C-3), 123.3 (C-4), 127.7 (C-6), 132.2 (C-7), 153.8 (C-5); CIMS (iso-Butan, 150 ℃): m/z (%) = 127[M+H+] (2.2); HREIMS: m/z 126.068 0 (Calcd. 126.068 1 for C7H10O2).
2.4. Agar diffusion test for biological activity
Phomalacton, (3R)-5-hydroxymellein, and emodin were dissolved in acetone at a concentration of 1 mg/mL. Fifty microliters of the solutions (50 µg) was pipetted onto a sterile filter disk (Schleicher & Schuell, 9 mm), which was placed onto an appropriate agar growth medium for the respective test organism and subsequently sprayed with a suspension of the test organism[9]. The test organisms were the tram-positive bacterium Bacillus megaterium (B. megaterium) (both grown on NB medium), the fungi Microbotryum violaceum (M. violaceum), and the alga Chlorella fusca (C. fusca) (all grown on MPY medium). Reference substances were penicillin, nystatin, actidione, and tetracycline. Commencing at the middle of the filter disk, the radius of the zone of inhibition was measured inmillimeters. These microorgansims were chosen because (a) they are nonpathogenic and (b) they had in the past proved to be accurate initial test organisms for antibacterial, antifungal, and antialgal/herbicidal activities.
3. Results
The fungus Phoma sp. that had been cultivated on biomalt agar medium for 4 weeks at 21 ℃ was subsequently extracted with ethyl acetate. The crude extract was fractionated on a silica gel column to yield a crude mixture containing compounds phomafuranol, phomalacton, (3R)-5-hydroxymellein, and emodin (Figure 1). Further silica column chromatography gave the pure compounds.
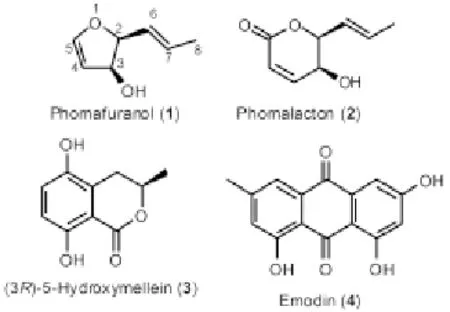
Figure 1. Structures of compounds phomafuranol, phomalacton, (3R)-5-hydroxymellein, and emodin isolated from Phoma sp.
The molecular formula of phomafuranol was assigned C7H10O2 on the basis of HREIMS with a molecular peak at m/z = 126.068 0 and1H and13C NMR spectral analyses (see Experimental). IR absorption bands at 3 400 and 1 620 cm-1indicated the presence of an hydroxyl group and C-C double bond, respectively. The NMR spectrum (see Experimental) exhibits two trans olefinic signals[δH 5.54 (ddd, J6,7= 15.3 Hz, J6,2= 7.3 Hz, J6,3= 1.4 Hz, 1H, H-6); δC127.7 (C-6), δH5.91 (dq,J7,6= 15.3 Hz,J7,8= 6.6 Hz, 1H, H-7); δC132.2 (C-7)] and one olefinic methyl signal[δH1.78 (d, J8,7= 6.6 Hz, 3H, H-8); δC18.3 (C-8)]. Moreover, the 1H NMR spectrum also exhibited two olefinic methine proton signals at δ 6.22 (d,J4,5= 5.8 Hz, 1H, H-4) and 7.46 (d,J5,4= 5.8 Hz, 1H, H-5) for those in the furan ring. This is further confirmed from signal at δ 123.3 (C-4) and the downfield signal at δ 153.8 (C-5) in the13C NMR spectrum. Additionally1H NMR spectrum showed two oxygenated methine proton signals at δ 4.22 (br. dd, J3,2= 5.9 Hz, 1H, H-2) and 5.03 (br. d, J3,2= 5.9 Hz, 1H, H-3) and this is also evident from signals at δ 73.9 (C-2) and 86.3 (C-3) in the13C NMR spectrum. The13C NMR spectrum of phomafuranol displayed signals for seven carbon atoms, and the DEPT spectrum indicates the presence of one methyl group and six methine carbons. The1H-1H and1H-13C connectivity’s were determined from the1H-1H-COSY and HMQC spectra. The correlations in the1H1H-COSY NMR spectrum were also consistent with one dihydrofuran unit and one 1-propene, and the interconnections between these units were determined through the relevant HMBC correlations (Figure 2).
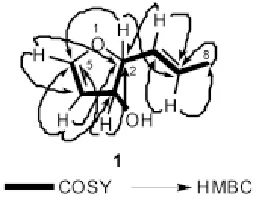
Figure 2. Key COSY and HMBC correlations for microdiplanol (1).
The HMBC correlations (Figure 2) between CH3-8 and C-6, C-7, and C-8; H-7 and C-2, C-6, and C-8; H-6 and C-2, C-7, and C-8 indicate the presence of 1-propene moiety in phomafuranol. Moreover HMBC correlations H-2 to C-3, C-4, C-6, and C-7; H-3 to C-2, C-4, and C-5; H-4 to C-2, C-3, and C-5; H-5 to C-2, C-3, and C-4 indicated the presence of dihydrofuran ring and also confirm the attachment of 1-propene side chain to C-2. The structure of phomafuranol was definitely determined by 2D NMR experiments, giving pertinent COSY and HMBC correlations (Figure 2). The relative stereochemistry between C-2 and C-3 in phomafuranol was determined by comparison of the1H-NMR coupling constants of phomafuranol with those recorded for synthetic dihydrofurans[10,11]. The coupling constant between H-2 and H-3 (J=5.9 Hz) indicated that H-2 to H-3 must be cis to each other and that the dihedral angle between H-3/H-4 approaches an angle precluding observed coupling between them and thus confirms the assignment of the cis configuration of the H-2 and H-3. Consequently, the structure was established to be (2S,3S,E)-2-(prop-1’-enyl)-2,3-dihydrofuran-3-ol, named phomafuranol, after the producing organism, Phoma sp.
The antibacterial, fungicidal, and algicidal properties of three selected compounds phomalacton, (3R)-5-hydroxymellein, and emodin are compiled in Table 1 and are compared to a number of standard antibiotics and the solvent acetone.

Table 1 Biological activities of pure metabolites 2-4 against microbial test organisms in agar diffusion assay.
4. Discussion
Fungal derived natural products have primarily served as lead structures for the development of antibacterial, anticancer and antifungal agents. In the present study, antimicrobial activity is observed for three pure compounds. These three compoundsviz., phomalacton, (3R)-5-hydroxymellein, and emodin were isolated from endophytic fungus Phoma sp.
The isolated compounds viz., phomalacton, (3R)-5-hydroxymellein, and emodin were tested in an agar diffusion assay for their antifungal, antibacterial, and algicidal properties towards Microbatryum violaceum, Bacillus megaterium, and Chlorella fusca. All the tested compounds viz., phomalacton, (3R)-5-hydroxymellein, and emodin have good antifungal, antibacterial, and algicidal properties towardsMicrobotryum violaceum,Bacillus megaterium, and Cchlorella fusca. It is interesting to note that phomalacton showed strong antibacterial activity towards Gram-positive bacterium B. megaterium.
Assuming that the metabolites produced in culture are also synthesized in planta, they could, for example, play a role in inhibiting competitive microorganisms within the endophyte’s natural habitat. Additionally, due to the fact that a broad range of microorganisms are inhibited, it would also be interesting to learn whether phomalacton, (3R)-5-hydroxymellein, and emodin are generally cytotoxic.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We thank BASF AG and the BMBF (Bundesministerium für Bildung und Forschung, project no. 03F0360A).
[1] Qin S, Krohn K, Hussain H, Schulz B, Draeger S. Pestalotheols EH. antimicrobial metabolites from endophytic fungus Ascocarpe sp. Eur J Org Chem 2011; 5163-5166.
[2] Hussain H, Krohn K, Ahmed I, Draeger S, Schulz B, Pietro S, et al. Four new pyrenocines from an endophytic fungus, Phomopsis sp. Eur J Org Chem 2012; 1783-1789.
[3] Hussain H, Krohn K, Floerke U, Schulz B, Draeger S, Pescitelli G, et al. Absolute configuration of globosuxanthone A and secondary metabolites from Microdiplodia sp. Eur J Org Chem 2007; 292-295.
[4] Hussain H, Krohn K, Floerke U, Schulz B, Draeger S, Pescitelli G, et al. Absolute configuration of hypothemycin and new 5’-O-methylhypothemycin from Phoma sp.- A novel solid state CD/TDDFT approach to 14 membered conformationally flexible system. Tetrahedron Asymmetry 2007; 18: 925-930.
[5] Hussain H, Krohn K, Schulz B, Draeger S. Bioactive constituents of two endophytic fungi” Bio Chem Syst Ecol 2007; 35: 898-900.
[6] Hussain H, Krohn K, Schulz B, Draeger S. Exochromone: Structurally unique chromone sdimer with antifungal and algicidal agent from Exophiala sp. Heterocycles 2007; 74: 331-337.
[7] Neff SA. Chemical investigations of secondary metabolites from selected fungi and from peanut seeds challenged by Aspergillus caelatus. Ph. D Thesis. The University of Iowa, USA.
[8] Höller U, Wright AD, Matthée GF, König GM, Draeger S, Aust HJ, et al. Fungi from marine sponges: diversity, biological activity and secondary metabolites. Mycol Res 2000; 104: 1354-1365.
[9] Schulz B, Sucker J, Aust HJ, Krohn K, Ludewig K, Jones PG, et al. Biologically active secondary metabolites of endophytic Pezicula species. Mycol Res 1995; 99: 1007-1015.
[10] Paquette LA, Lanter JC, Johnston JN. Single stereodifferentiation associated with carbon atom insertion during the oxonium ion-initiated Pinacol rearrangement of dihydrofuranyl and dihydropyranyl carbinols. J Org Chem 1997; 62: 1702-1712.
[11] Le Ménez P, Fargeas V, Berque I, Poisson J, Ardisson J. Sequential homoaldolization-Cuprate rearrangement in a stereoselective synthesis of stannyl dienes: Application to the synthesis of the western C10-C15subunit of Tylosin aglycon. J Org Chem 1995; 60: 3592-3599.
ment heading
10.1016/S1995-7645(14)60119-X
*Corresponding author: Hidayat Hussain and Karsten Krohn, Department of Chemistry, University of Paderborn, Warburger Strasse 100, 33098 Paderborn, Germany.
E-mail: Hidayat110@gmail.com (H. Hussain); K.Krohn@upb.de (K. Krohn)
Tel: +495251602172
Fax: +495251603245
Foundation project: It is supported by the BMBF (Bundesministerium für Bildung und Forschung, project no. 03F0360A).
Endrophytic fungi Phoma sp.
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Sonic Hedgehog signaling pathway in primary liver cancer cells
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Protection effect of Emodin pretreatment on intestinal I - RI damage of intestinal mucosa in ratsa
- Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
