Antimalarial activity and toxicity of Garcinia mangostana Linn.
Chulabhorn International College of Medicine, Thammasat University, Pathumthani, Thailand
Antimalarial activity and toxicity of Garcinia mangostana Linn.
Ratchanu Bunyong, Wanna Chaijaroenkul, Tullayakorn Plengsuriyakarn, Kesara Na-Bangchang*
Chulabhorn International College of Medicine, Thammasat University, Pathumthani, Thailand
Objective: To investigate the antimalarial activity and toxicity of the crude ethanolic extract of its pericarp bothin vitroandin vivo. Methods: The antimalarial activity ofGarcinia mangostana(G. mangostana) Linn. extract against 3D7 and K1Plasmodium falciparum(P. falciparum)clone were assessed using SYBR greenⅠ-based assay. A 4-day suppressive test ofPlasmodium berghei(P. berghei) infected mouse was performed to investigatein vivoantimalarial activity. Results: The in vitro antimalarial activity was selective (SI > 5) and classified as weak and good to moderate activity against both 3D7 and K1 P. falciparum clones with median IC50(range) values of 11.12 (10.94-11.29) and 7.54 (6.80-7.68) µg/mL, respectively. The extract was considered nontoxic to mice. The maximum tolerated doses for acute and subacute toxicity in mice were 5 000 and 2 000 mg/kg, respectively. Median (range) parasite density on day 4 of the negative control group (25% Tween-80), mice treated with 250, 500, 1 000, and 2 000 mg/kg body weight of the extract, and 10 mg/kg body weight of chloroquine for 14 d were 12.8 (12.2-13.7), 11.4 (9.49-13.8), 11.6 (9.9-12.5), 11.7 (10.6-12.8), 10.9 (9.4-11.6) and 0 (0-0)% respectively. Parasite density on day 4 in the control group treated with Tween-80 was higher than the groups treated with chloroquine and all dose levels of the extract. Conclusions:G. mangostanaLinn. showed weak antimalarial activity of the extract bothin vitroandin vivocould be due to limitation of absorption of the active compounds.
ARTICLE INFO
Article history:
Received 10 May 2014
Received in revised form 15 June 2014
Accepted 15 August 2014
Available online September 2014
Garcinia mangostana Linn. Plasmodium falciparum
1. Introduction
Malaria remains one of the most important infectious diseases in the world. It constitutes a public health problem in more than 90 countries inhabited by about 40% of the world’s population. Statistics of the World Health Organization in 2012 showed an estimate of about 274 million malaria cases annually with approximately 1.1 million deaths, mostly in children under five years old[1]. Of the five species of human malarial parasites, Plasmodium falciparum (P. falciparum) is remarkable for its high case fatality rate and resistance developed to existing antimalarial drugs. The emergence and spread of multidrug resistant P. falciparum continue to be a major public health problem in the management and control of malaria in several endemic countries. This situation was particularly threatening until the introduction of artemisinin-based combination therapies (ACTs)[2]. It is being debated whether P. falciparum has developed resistance to artesunatemefloquine combination, one of the most commonly used ACTs. The increase in fifty percent inhibitory concentration (IC50) and a delay in parasite clearance have been observed following treatment with this combination[3-5]. The observed delayed parasite clearance suggests that some parasites can survive the treatment for a longer period of time than expected, although they are eventually killed by the drugs. This raises the possibility of a unique survival mechanism different from a classical drug-resistance phenotype. New effective and affordable antimalarial drugs are therefore urgently needed. Plants are important sources of medicines including antimalarial drugs as in the cases of quinine and artemisinins[6]. The aim of the study was to assess the antimalarial activity (in vitro and in vivo) and toxicity of the crude ethanolic extract of Garcinia mangostana (G. mangostana) Linn. or mangosteen. This fruit pericarp has been used in traditional medicine in several Asian countries for several purposes including treatment of skin infections and wounds[7,8].
2. Materials and methods
2.1. Plant materials and preparation of the crude ethanolic extract of G. mangostana Linn.
G. mangostana Linn. fruits were purchased from Talard-Tai Market in Pathumthani Province of Thailand. The fruit was rinsed thoroughly with tap water to remove extraneous contaminants and the pericarp was cut into small pieces, dried at 45 ℃ and ground into powder with pestle and mortar. Extraction was carried out by macerating the powder plant materials (100 g) in a flask containing 500 mL of 95% ethanol (at 25-30 ℃) for 7 d. The extraction solvent was separated, filtered (through Whatman no.1 filter paper) and evaporated under reduced pressure by rotary evaporation. The ethanolic extract yield of 400 g dried weight of mangosteen pericarp powder was 10.55% (w/w).
2.2. Determination of fingerprint of the crude ethanolic extract of G. mangostana Linn.
Fingerprint of the crude ethanolic extract ofG. mangostanaLinn. pericarp was analyzed by HPLC using α- mangostin as a chemical marker[9]. The HPLC system consists of a solvent delivery system (Spectra System P4000 Quaternary Solvent Delivery/Controller: Thermo Fisher Scientific, California, USA), equipped with solvent degasser (SpectraSystem SCM1000 Solvent Degasser: Thermo Fisher Scientific, California, USA), an auto sampler (SpectraSystem AS3500: Thermo Fisher Scientific, California, USA), and a UV detector (SpectraSystem UV/Vis3000: Thermo Fisher Scientific, California, USA). The optimal wave length was set at 254 nm. The separation was carried out on a reversedphase column (Thermo Hypersil Gold C18, 250 mm×2.1 mm i.d., 5 µm: Thermo Scientific, California, USA). The elution solvent consisted of water (A) and acetonitrile (B) running at a flow rate of 1.0 mL/min. Total running time was 37 min and the gradient mode was as follow: 55% B to 85% B for 0-30 min, constant at 85% B for 5 min, 85% B to 55% B for 1 min, and constant at 55% B for 4 min. The chromatographic analysis was operated at 25 ℃. An aliquot of 20 µL of sample or standard solution was injected into the column.
2.3. In vitro experiment
2.3.1. Parasite culture
P. falciparum chloroquine-resistant K1 and chloroquinesensitive 3D7 clones were kindly provided by the Malaria Research Unit, Institute of Health Research, Chulalongkorn University, Thailand. Both clones were maintained in continuous culture in O+ human erythrocytes suspended in RPMI 1640 (Gibco, California, USA) culture medium supplemented with 10% human B serum and 25 mM HEPES (at 37 ℃ under a gas mixture of 5% CO2, 5% O2and 90% N2) according to the standard method described by Trager and Jensen[10]. The level of parasitemia in the culture was maintained between 2 and 10%. Synchronization of the parasite culture to ring stage P. falciparum was performed using 5% sorbitol[11].
2.3.2. Assessment of antimalarial activity in vitro
Antimalarial activity of the crude ethanolic extract of G. mangostana Linn. pericarp was investigated in vitro using SYBR Green I assay[12,13]. Highly synchronous ring stage parasite was used in each assay. An aliquot of parasite inoculum (50 µL) with 2% parasitemia and 1% hematocrit was added into each well of microtiter plate. The extract (dissolved in dimethyl sulfoxide or DMSO and diluted with RPMI 1640 to final concentration of 1%) was added to the malaria culture at eight final concentrations of 0.78, 1.56, 3.13, 6.25, 12.5, 25, 50 and 100 g/mL. Chloroquine (3.89-498.15 nM) and artesunate (0.39-50.0 nM) were used as standard antimalarial drugs. The experiment was repeated three times (triplicate each). IC50value (drug concentration that inhibits the parasite growth by 50%) was used as an indicator of antimalarial activity and was determined from a log-dose response curve plotted using the CalcusynTMversion 1.1 (BioSoft, Cambridge, UK).
2.3.3. In vitro assay for cytotoxic activity
Normal human epithelial cell (HRE) was purchased from Promocell Co. Ltd. (Heidelberg, Germany) and cultured in renal epithelial cell growth medium 2 supplemented with SupplementPack (Promocell Co. Ltd., Heidelberg, Germany). The cell was incubated at 37 ℃ in 5% CO2atmosphere with 95% humidity and was seeded in a 96-well plate at a density of 104cells/well in 100 µL culture medium. Following 24 h incubation and attachment, cells were treated with varying concentrations of each plant extract. The concentration range of the extract used was 1.95-250.00 µg/mL. After 48 h incubation, following washing and incubation with MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromidesolution, 20 µL of 5 mg/mL] at 37 ℃ for 3 h, cells were lyzed with DMSO. The yellow MTT dye was reduced by succinic dehydrogenase in the mitochondria of viable cells to purple formazan crystals. Absorbance was measured at 570 nm using a microplate reader (Varioskan, Thermo, USA). Experiments were repeated three times, triplicate for each experiment). The IC50values were determined using CalcySyn™ software (BioSoft, Cambridge, UK). The selectivity index (SI) was calculated from the IC50ratio of each extract obtained from HRE cell and K1 or 3D7 clone.
2.4. In vivo experiments
2.4.1. Animals
Imprinting control region (ICR) mice (5-7 weeks of age, weighting 20-40 g) of both genders were used in the study. All were obtained from the National Laboratory Animal Centre, Thailand. Animal experiments were carried out in accordance with the OECD Guideline for Chemicals. Theanimals were housed under standard conditions and fed with a stock diet and water ad labitum. Approval of the study protocol was obtained from the Ethics Committee for Animal Research, Thammasat University, Thailand.
2.4.2. Toxicity tests
The ethanolic extract of G. mangostana Linn. (pericarp) was weighted and resuspended with 25% Tween-80 to obtain the desired concentrations. ICR mice were fasting 2 h before feeding with the extract. Animals were divided into four groups of six (3 males and 3 females for each group). For the acute toxicity test, mice in each group were fed with the extract at a single oral dose of 5 000 mg/kg body weight; control group received a single oral dose of 25% Tween-80 (1 mL). For the subacute toxicity test, mice in each group were fed with the extract at a daily oral dose of 2 000 mg/kg body weight for 14 d; control group received a daily oral dose 1 mL of 25% Tween-80 for 14 d. General behavior of each mouse was observed continuously for 1 h after each dose, intermittently every 4 h, and thereafter over a period of 24 h[14]. Animals were observed for up to 14 d for the acute toxicity test and 28 d for the subacute toxicity test for any sign of toxicity (behavioral change related to central nervous, cardiovascular and gastrointestinal systems as well as complete blood count, liver and kidney function tests), body weight change and water and food consumption. At the end of the observational period, all animals were sacrificed under ether anesthesia and vital organs (heart, lung, liver, spleen and kidney) were removed from all animals for gross and histopathological examination.
2.4.3. Assessment of antimalarial activity of plumbagin in Plasmodium berghei (P. berghei)-infected mouse model (4-day suppressive test)
The in vivo antimalarial activity of the ethanolic extract of G. mangostana Linn. was evaluated using a 4-day suppressive test in P. berghei-infected mouse model[15]. P. berghei (ANKA) strain used in the experiment was obtained from the National Center for Genetic Engineering and Biotechnology (BIOTEC), Thailand. The parasite had been maintained by serial blood passage in mice, and blood stage stored at -196 ℃ until use.
ICR mice were divided into six groups (3 males and 3 females for each group). The donor mice were infected with 200 µL of P. berghei parasite inoculum. The parasitized blood of each donor mouse was collected from the tail vein and diluted with 0.9% sodium chloride. Mice were infected with saline suspension of 1×107parasitized erythrocytes (0.2 mL) by intraperitoneal injection (Day 0). Four hours after infection, animals were treated with the ethanolic extract of G. mangostana Linn. at oral daily doses of 250, 500, 1 000 or 2 000 mg/kg body weight for four consecutive days (test group 1, 2, 3 and 4, respectively). Positive and negative control groups were fed with the antimalarial chloroquine at oral daily doses of 10 mg/kg body weight and 25% Tween-80, respectively. On day 4 (96 h post infection), parasitemia of individual mouse was determined under light microscope by examination of giemsa-stained thin blood smears prepared from mouse tail blood[16]. The mean parasitemia in each group of mice was used to calculate the % suppression for each dose using the formula:

The antimalarial activity of the ethanolic extract of G. mangostana Linn. was determined from the ratio of percentage of parasite reduction in treated and negative control groups[17]. Results are expressed as median (range) values. Comparison of difference in quantitative variables between more than two and two groups was performed using Kruskal Wallis and Mann-WhitneyUtests (SPSS version 12.0, SPSS Inc., California, USA). Statistical significance level was set at α<0.05 for all tests.
3. Results
3.1. In vitro antimalarial activities of G. mangostana Linn.The antimalarial activity of the ethanolic extract of G. mangostana Linn. against 3D7 chloroquine-sensitive and K1 chloroquine-resistant P. falciparum clones were categorized as weak and good to moderate with median IC50(range) values of 11.12 (10.94-11.29) and 7.54 (6.80-7.68) g/mL, respectively. The corresponding IC50values for chloroquine and artesunate were 10.5 vs. 128.7 and 2.1 vs 1.91 nM, respectively.
Cytotoxicity assay was performed using normal human HRE cell line in order to evaluate the selectivity of antimalarial activity of the crude ethanolic extract of G. mangostana Linn. The median (range) IC50value was 67.3 (58.00-71.60) µg/mL. The antimalarial activity of the extract against both clones were found to be selective, with SI values of 6.04 and 8.90, respectively.
3.2. Toxicity tests
The toxicity of the ethanolic extract ofG. mangostanaLinn. when given as a single oral dose (acute toxicity) and 14-day daily doses (subacute toxicity) in mice was investigated in order to define optimal dose to be used for evaluation of its in vivo antimalarial activity in malarial mouse model. Results indicated virtually no toxicity of G. mangostana Linn. at a maximum single oral dose of 5 000 mg/kg body weight (acute toxicity, Figure 1) and daily oral doses of 2 000 mg/kg body weight for 14 d (subacute toxicity, Figure 2). All mice survived and there was neither sign of toxicity nor significant change in water and food consumption and body weights of mice in both groups during the 14 d observation period. The gross examination of vital organs,i.e., heart, lung, liver, spleen and kidney in both treated and control groups were similar either in size and cell morphology.
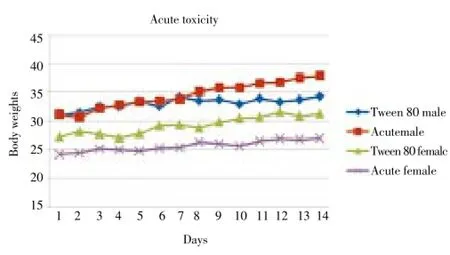
Figure 1. Median body weights of male and female mice (n=3 each) during the first 14 d following the administration of a single oral dose of 5 000 mg/kg body weight of the crude ethanolic extract of G. mangostana Linn. pericarp compared with 25% Tween 80 (negative control) in acute toxicity test.
Figure 2. Median body weights of male and female mice (n=3 each) during the first 14 d following the administration of daily oral doses of 2 000 mg/kg body weight of the crude ethanolic extract of G. mangostana Linn. pericarp for 28 d, compared with 25% Tween 80 (negative control) in subacute toxicity test.
3.3. Antimalarial activity of G. mangostana Linn. in P. berghei-infected mouse model (4-day suppressive test)
Results of the 4-day suppressive antimalarial test of the ethanolic extract of G. mangostana Linn. and chloroquine in mice infected with P. berghei ANKA strain are summarized in Table 1. Median (range) parasite density on day 4 of the negative control group (25% Tween-80), mice treated with 250, 500, 1 000 and 2 000 mg/kg body weight of the extract, and 10 mg/kg body weight of chloroquine for 14 d were 12.8 (12.2-13.7), 11.4 (9.49-13.8), 11.6 (9.9-12.5), 11.7 (10.6-12.8), 10.9 (9.4-11.6) and 0 (0-0)% respectively. Parasite density on day 4 in the control group treated with Tween-80 was higher than the groups treated with chloroquine and all dose levels of the extract. Chloroquine exhibited the most potent antimalarial activity with respect to its activity on reduction of parasitemia on day 4 and prolongation of survival time. Parasite density (%) on day 4 following chloroquine treatment (0%) was significantly lower than 25% Tween 80 (negative control) and the extract at all dose levels. In addition, parasite suppression (%) on day 4 of mice treated with chloroquine (100%) was significantly higher than the negative control group (0%) and the groups treated with the extract at the doses of 2 000 (14.4%), 1 000 (8.5%), 500 (9.3%) and 250 (10.5%) mg/kg body weight. The survival time in the group treated with chloroquine was also significantly shorter than the negative control and the groups treated with the extract at all dose levels. Parasitemia of mice treated with chloroquine started to rise on day 5 (0.20%) until day 8 (24.11%) of treatment, while that was rising between 19%-29% in mice treated with the extract (Table 2).
4. Discussion
The in vitro antimalarial activity against K1 and 3D7 P. falciparum clones of the crude ethanolic extract of G. mangostana Linn. pericarp was categorized into good to moderate and weak to antimalarial acivities with median IC50values of 7.54, and 11.12 µg/mL, respectively. These findings were in agreement with that reported by Thiengsusuk and colleagues, where the IC50of the extract against K1 and 3D7 P. falciparum clones were reported as3.6 and 11.8 µg/mL, respectively[18]. G. mangostana Linn. pericarp has been shown to contain phenolic compounds such as condensed tannins, anthocyanins and xanthone derivatives[19-21]. Over 68 xanthone-type compounds have been isolated including the major constituent α-mangostin, as well as β-mangostin, γ-mangostin, garcinone E, gartanin and 9 hydroxycalabaxanthone[22]. Among them, α-mangostin appears to be the most potent compound against both chlrooquine-resistant and chloroquinesensitiveP. falciparumclones with IC50ranging from 3.7 to 20 µM[23]. Both α-mangostin and β-mangostin were shown to exert inhibitory effects on the growth of D6 P. falciparum clone (chloroquine-sensitive) with mean IC50of 5.1 and 7.0 µM, respectively[24]. A series of oxygenated xanthones was synthesized and their antimalarial activities were evaluated using a 4-day suppressive assay against ANKAP. bergheistain in BALB/c mice at repeated doses of 20 mg/kg body weight/d. The most active compound was 1, 3, 6, 8-tetrahydroxyxanthone, which reduced the percentage of erythrocytes infected by 70.5%, followed by norlichexanthone (44.3%) and its isomer 1,3,8-trihydroxy-6-methylxanthone (37.0%). Di-C-allyl-dihydroxyxanthone showed notable activity but relatively weaker (33.4%) and 1,3-dihydroxyxanthone exhibited very weak activity (15.1%)[25]. Result of the in vivo antimalarial activity based on the standard 4-day suppressive test shown in the present study was also in accordance with the in vitro test showing moderate antimalarial activity of the ethanolic extract at the minimum dose of 250 mg/kg body weight/day. The mechanism of inhibition of P. falciparum growth by mangostin and derivatives is still unclear. However, it has been suggested that hydroxyxanthones exert their antiplasmodium activity through formation of soluble complexes with heme and thereby inhibiting parasite hemozoin formation, a process by which the malaria parasite protects itself against toxic effects of heme released following digestion of hemoglobin[24,26]. The extract was well tolerated when given to mice either at a single or repeated doses of up to 5 000 and 2 000 mg/kg body weight, respectively. Jujan and colleagues also reported no toxic effect of this plant in rats following a single oral dose of 5 000 mg/kg body weight[27]. In addition, a 28-day oral toxicity test at the dose of 1 000 mg/kg body weight/day did not produce any significant dose-related changes on hematological parameters, serum biochemistry or histopathology of liver, kidney, lung, heart, spleen, adrenal grand, thymus, stomach and duodenum, small intestine, ovary, uterus, testis, epididymis muscle and nerve, thoracic spine eye, and brain[27]. In other studies, the crude extract (200 mg/kg body weight) and α-Mangostin were shown to increase serum glutamic oxaloacetic and serum glutamic pyruvic transaminase in mice and rats[28].
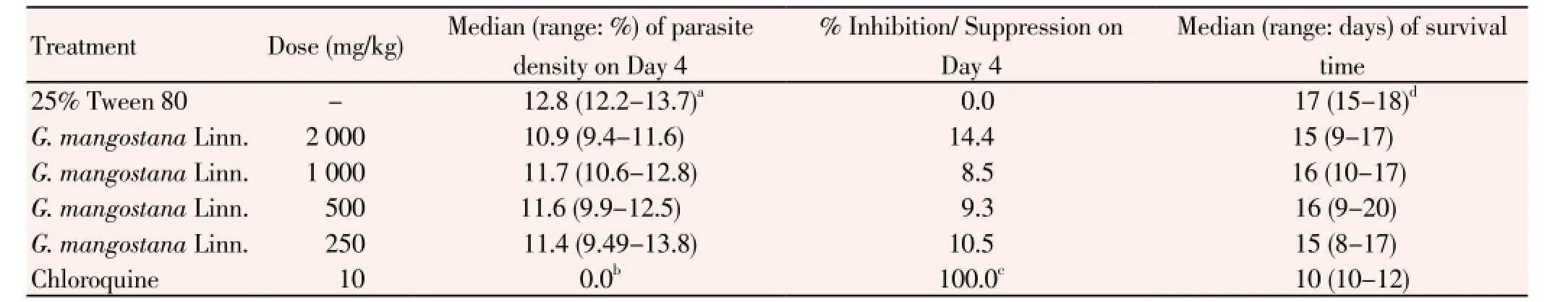
Table 1 In vivo antimalarial activities of the ethanolic extract of G. mangostana Linn. pericarp against P. berghei ANKA strain.
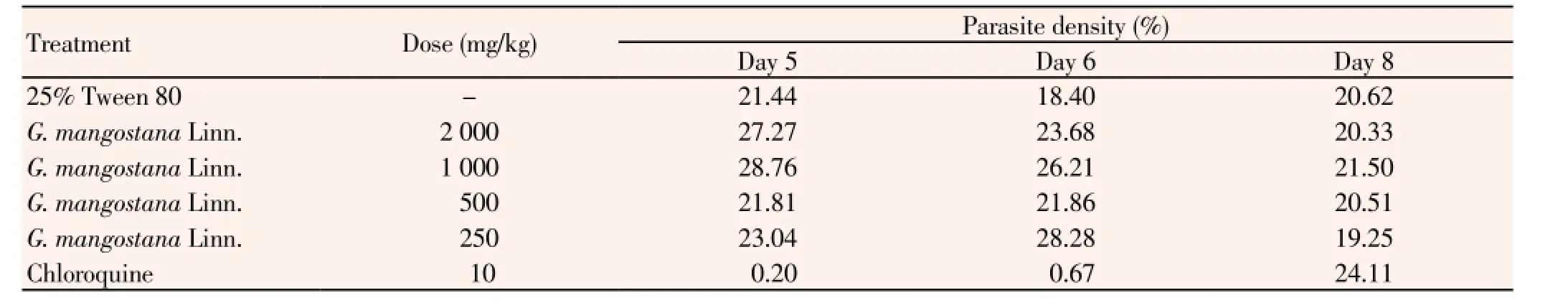
Table 2 In vivo antimalarial activity (represented by % parasite density on Day 5, 6 and 8) of the crude ethanolic extract of G. mangostana Linn. pericarp against ANKA strain P. berghei.
It was noted however that the survival time of mice treated with the extract at all dose levels including chloroquine observed in the present study were significantly shorter than the negative control. For chloroquine, this observation could be due to an increase in malaria disease severity as a consequence of rapid rise of parasitemia after day 5. It is possible that the antimalarial activity of the crude ethanolic extract of G. mangostana Linn. could be compromised by other inactive components. Furthermore, permeability of the active ingredients-and β-mangostin across intestinal membrane (Caco-2 cell) was shown to be extremely low (unpublished observation) and the oral bioavailability of α-mangostin was also reported to be markedly low in rats[29]. Evidence of extensive first-pass metabolism of α -mangostin[30] was provided in rats with an initial low plasma concentration followed by a rapid drop following oral dose administration. This pharmacokinetic factor would have led to an inadequate plasma concentration of this active moiety to suppress the parasite. Chemical derivatization of -and/or β-mangostin, or preparation of modified formulation such as liposomal formulation is required to improve their systemic bioavailability.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The study was supported by The National Research Council of Thailand, (Grant No.034/2556), Thammasat University and the Commission on Higher Education, Ministry of Education of Thailand (NRU Project).
[1] WHO. World malaria report 2012. Geneva, Switzerland: WHO Press; 2012.
[2] Davis TM, Karunajeewa HA, Ilett KF. Artemisinin-based combination therapies for uncomplicated malaria. Med J Aust 2005; 182(4): 181-185.
[3] Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009; 361(5): 455-467.
[4] Na-Bangchang K, Muhamad P, Ruaengweerayut R, Chaijaroenkul W, Karbwang J. Identification of resistance of Plasmodium falciparum to artesunate-mefloquine combination in an area along the Thai-Myanmar border: integration of clinico-parasitological response, systemic drug exposure, and in vitro parasite sensitivity. Malar J 2013; 12: 263.
[5] Na-Bangchang K, Ruengweerayut R, Mahamad P, Ruengweerayut K, Chaijaroenkul W. Declining in efficacy of a three-day combination regimen of mefloquine-artesunate in a multi-drug resistance area along the Thai-Myanmar border. Malar J 2010; 9: 273.
[6] Wright CW, Phillipson JD. Natural products and the development of selective antiprotozoal drugs. Phytother Res 1990; 4(4): 127-139.
[7] Ho CK, Huang YL, Chen CC. Garcinone E. A xanthone derivative, has potent cytotoxic effect against hepatocellular carcinoma cell lines. Planta Med 2002; 68(11): 975-979.
[8] Kaomongkolgit R, Jamdee K, Chaisomboon N. Antifungal activity of alpha-mangostin against Candida albicans. J Oral Sci 2009; 51(3): 401-406.
[9] Yodhnu S, Sirikatitham A, Wattanapiromsakul C. Validation of LC for the determination of alpha-mangostin in mangosteen peel extract: a tool for quality assessment of Garcinia mangostana L. J Chromatogr Sci 2009; 47(3): 185-189.
[10] Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976; 193(4254): 673-675.
[11] Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 1979; 65(3): 418-420.
[12] Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, et al. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother 2004; 48(5): 1807-1810.
[13] Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 2004; 48(5): 1803-1806.
[14] Twaij HA, Kery A, Al-Khazraji NK. Some pharmacological, toxicological and phytochemical investigations on Centaurea phyllocephala. J Ethnopharmacol 1983; 9(2-3): 299-314.
[15] Peters W. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol 1975; 69(2): 155-171.
[16] Esume CO, Emudainohwo JOT, Opajobi AO, Osifo IM, Onyemekeih UR. An investigation into the anti-malaria property of ethanolic extract of the leaves of gongronema latifolium on artesunate sensitive P. berghei infected albino mice. Continental J Trop Med 2011; 5(1): 10-14.
[17] Tona L, Mesia K, Ngimbi NP, Chrimwami B, Okond’Ahoka, Cimanga K, et al. In-vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri. Ann Trop Med Parasitol 2001; 95(1): 47-57.
[18] Thiengsusuk A, Chaijaroenkul W, Na-Bangchang K. Antimalarial activities of medicinal plants and herbal formulations used in Thai traditional medicine. Parasitol Res 2013; 112(4): 1475-1481.
[19] Fu C, Loo AE, Chia FP, Huang D. Oligomeric proanthocyanidins from mangosteen pericarps. J Agric Food Chem 2007; 55(19): 7689-7694.
[20] Maisuthisakul P, Gordon MH, Pongsawatmanit R, Suttajit M. Enhancing the oxidative stability of rice crackers by addition of the ethanolic extract of phytochemicals from Cratoxylum formosum Dyer. Asia Pac J Clin Nutr 2007; 16 (Suppl 1): 37-42.
[21] Chin YW, Jung HA, Chai H, Keller WJ, Kinghorn AD. Xanthones with quinone reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen). Phytochemistry 2008; 69(3): 754-758.
[22] Walker EB. HPLC analysis of selected xanthones in mangosteen fruit. J Sep Sci 2007; 30(9): 1229-1234.
[23] Mahabusarakam W, Kuaha K, Wilairat P, Taylor WC. Prenylated xanthones as potential antiplasmodial substances. Planta Med 2006; 72(10): 912-916.
[24] Riscoe M, Kelly JX, Winter R. Xanthones as antimalarial agents: discovery, mode of action, and optimization. Curr Med Chem 2005; 12(21): 2539-2549.
[25] Fotie J, Nkengfack AE, Rukunga G, Tolo F, Peter MG, Heydenreich M, et al. In-vivo antimalarial activity of some oxygenated xanthones. Ann Trop Med Parasitol 2003; 97(7): 683-688.
[26] Ignatushchenko MV, Winter RW, Bachinger HP, Hinrichs DJ, Riscoe MK. Xanthones as antimalarial agents; studies of a possible mode of action. FEBS Lett 1997; 409(1):67-73.
[27] Jujun P, Pootakham K, Pongpaibul Y, Duangrat C, Tharavichitkul P. Acute and repeated dose 28-day oral toxicity study of Garcinia mangostana Linn. rind extract. CMU J Nat Sci 2008; 7: 199-208.
[28] Sornprasit A, Sripiyarattanakul K, Chuay-Yim P, Tanakittiham P. Preliminary toxicological study of mangostin. Songklanakarin J Sci Technol 1987; 9: 51-7.
[29] Bumrungpert A, Kalpravidh RW, Suksamrarn S, Chaivisuthangkura A, Chitchumroonchokchai C, Failla ML. Bioaccessibility, biotransformation, and transport of alphamangostin from Garcinia mangostana (Mangosteen) using simulated digestion and Caco-2 human intestinal cells. Mol Nutr Food Res 2009; 53(Suppl 1): S54-S61.
[30] Li L, Brunner I, Han AR, Hamburger M, Kinghorn AD, Frye R, et al. Pharmacokinetics of alpha-mangostin in rats after intravenous and oral application. Mol Nutr Food Res 2011; 55(Suppl 1): S67-S74.
ment heading
10.1016/S1995-7645(14)60118-8
*Corresponding author: Prof. Dr. Kesara Na-Bangchang, Chulabhorn International College of Medicine, Thammasat University (Rangsit Campus), Pathumtani 12121, Thailand.
Tel: +662-564-4440-79 Ext.1803
Fax: +662-564-4398
E-mail: kesaratmu@yahoo.com
Foundation project: The study was supported by The National Research Council of Thailand, (Grant No.034/2556), Thammasat University and the Commission on Higher Education, Ministry of Education of Thailand (NRU Project).
Plasmodium berghei
Antimalarial activity
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Sonic Hedgehog signaling pathway in primary liver cancer cells
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Protection effect of Emodin pretreatment on intestinal I - RI damage of intestinal mucosa in ratsa
- Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
