Hesperidin as a preventive resistance agent in MCF-7 breast cancer cells line resistance to doxorubicin
Rifki Febriansah, Dyaningtyas Dewi P.P., Sarmoko,3, Nunuk Aries Nurulita,4, Edy Meiyanto, Agung Endro Nugroho*
1Cancer Chemoprevention Research Center, Faculty of Pharmacy, Universitas Gadjah Mada Yogyakarta, Indonesia
2Pharmacy study programme, Faculty of Medicine and Health Sciences, Universitas Muhammadiyah Yogyakarta, Yogyakarta, Indonesia
3Faculty of Pharmacy, Universitas Jenderal Soedirman, Purwokerto, Indonesia
4Faculty of Pharmacy, Universitas Muhammadiyah Purwokerto, Purwokerto, Indonesia
Hesperidin as a preventive resistance agent in MCF-7 breast cancer cells line resistance to doxorubicin
Rifki Febriansah1,2, Dyaningtyas Dewi P.P.1, Sarmoko1,3, Nunuk Aries Nurulita1,4, Edy Meiyanto1, Agung Endro Nugroho1*
1Cancer Chemoprevention Research Center, Faculty of Pharmacy, Universitas Gadjah Mada Yogyakarta, Indonesia
2Pharmacy study programme, Faculty of Medicine and Health Sciences, Universitas Muhammadiyah Yogyakarta, Yogyakarta, Indonesia
3Faculty of Pharmacy, Universitas Jenderal Soedirman, Purwokerto, Indonesia
4Faculty of Pharmacy, Universitas Muhammadiyah Purwokerto, Purwokerto, Indonesia
PEER REVIEW
Peer reviewer
Takuji Tanaka, MD, PhD, FIAC, Principle Consultant, Clin-ToxPath (C-Top) Consulting, Visiting Professor, Asahi University, 5-1-2 Minami-uzura, Gifu 500-8285, Japan.
Tel: +81-76-273-4399
Fax: +81-76-2273-4392
E-mail: takutt@toukaisaibou.co.jp
Comments
This is an interesting and valuable research work. The authors demonstrated that hesperidin has cytotoxic effect on MCF-7/Dox cells with IC50of 11 µmol/L. Hesperidin did not increase the apoptotic induction combined with doxorubicin. Co-chemotherapy application of doxorubicin and hesperidin on MCF-7/ Dox cells showed synergism effect through inhibition of Pgp expression. Details on Page 232
Objective:To evaluate of hesperidin to overcome resistance of doxorubicin in MCF-7 resistant doxorubicin cells (MCF-7/Dox) in cytotoxicity apoptosis and P-glycoprotein (Pgp) expression in combination with doxorubicin.
Hesperidin, Doxorubicin, MCF-7/Dox cells line, Apoptosis, Pgp expression
1. Introduction
Drug resistance is one of the problem in cancer therapy, especially in breast cancer. Breast cancer is the first ranked cases of cancer in women worldwide. In developing countries, breast cancer is the second leading cause of death after cervical cancer. In Indonesia, breast cancer patients as much as 12.10%, are the second largest number after cervical cancer (19.18%). The high mortality rate indicates the treatment with chemotherapy has not overcome the cancer. Strategies and the development of breast cancer treatment should be pursued. Problems in the chemotherapy of breast cancer becomes larger, since the emergence of breast cancer resistance to chemotherapyagents[1].
Breast cancer cell resistance to chemotherapeutic agents is caused by various factors, but it is predominantly due to increased Akt activity and expression of multi-drug resistance 1 (MDR1) gene, the gene encoding P-glycoprotein (Pgp) after administration of doxorubicin[2,3]. Because of these problems, the development of breast cancer chemotherapy directed to the combination of doxorubicin with other compounds (co-chemotherapy) that can increase the effectiveness of doxorubicin[4]. One of the proteins that regulate the proliferation and survival genes is NF-êB[5].
Hesperidin, a citrus flavonoid showed strong toxic effect in Caco-2, CEM/ADR5000 and CCRF-CEM cancer cell lines with IC50, 195, 230 and 95 µmol/L, respectively[6]. Hesperidin also showed antiproliferative effect in MCF-7 cells transfected with green fluoresens protein (GFP)/alpha-tubulin (MCF-7-GFP-Tubulin)[7]. It is also reported that hesperidin protective effect in Benzo(a)pirene induced testicular toxicity paradigm and repaired the function of lactate dehydrogenase, superoxide dismutase, and glutathione-S-transferase enzyme[8]. Previous study has reported that hesperidin could induce apoptosis in human colon cancer cells through Caspase-3 (CASP3) activation. Hesperidin down-regulated the protein expression of pro-CASP3, and upregulated the level of active CASP3[9].
This study was conducted to determine the effect of hesperidin performed on MCF-7, MCF-7/Dox cells. Cytotoxicity effect of hesperidin, apoptosis induction and Pgp expression observations made on single and combination with doxorubicin. The results of this study is expected to be used as a reference for further research in order to explore hesperidin as an alternative to co-chemotherapy agent in breast cancer therapy.
2. Materials and methods
2.1. Chemical and reagent
Hesperidin, dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) were purchased from Sigma Chemical Co., St Louis, MO, USA; rabbit anti-Pgp and horseradish peroxidase-conjugated goat antimouse or anti-rabbit secondary antibodies, CA; Dulbecco’s modified Eagle medium (DMEM) high glucose medium and fetal bovine serum from Gibco, Grand Island, NY; 96-well plates from Iwaki.
2.2. Cell culture and cytotoxicity assay
MCF-7 and MCF-7/Dox human breast cancer cell line was a generous gift from the laboratory of Prof. M Kawaichi (NAIST, Japan). Cells maintained in DMEM high glucose medium supplemented with 10% fetal bovine serum, 1% penicillinstreptomycin at 37 °C in 5% CO2incubator. To study the cytotoxic effect of hesperidin, confluent cell cultures were trypsinized and seeded in 96-well plates at a density of 1× 104cells per well in growth medium. After 24 h, cells were treated with various concentrations of hesperidin (dissolved in DMSO as a stock solution). The DMSO concentration in the final cell treatment solutions was less than 0.1%. After 24 h of treatments, cells were washed with Phosphate Buffer Solution (PBS) and 100 µL of MTT solution (0.5 mg/mL in DMEM medium) was added. Four hours later, the precipitated formazan was dissolved in 100 µL of sodium dodecyl sulfonate stopper reagent. Cell viability was determined by measuring the absorbance at 595 nm using microplate reader (Biorad). In this study, the drug concentration required to inhibit cell growth by 50% (IC50) was determined from a plot of percent cell viability from control untreated cells versus treated cells.
2.3. Apoptotic assay
Apoptosis was detected using acrydine orange-etidium bromide staining (acridine orange/ethidium bromide double staining). MCF-7/Dox cells (5×104cells/well) were seeded on cover slips in 24-well plates until 50%-60% confluent. Cells were incubated with hesperidin alone, doxorubicin alone and their combination for 24 h. Culture medium was removed and cells were washed with PBS. Coverslips were placed into object-glass and added with 10 µL 1X working solution acrydine orange (Sigma)-etidium bromide (Sigma), observed using fluoroscence microscopy (Zeiss MC 80). Apoptotic cells which had lost their membrane integrity appeared orange and showed morphological features of apoptosis. Cells were identified as apoptotic on the basis of specific morphological criteria, including condensation and fragmentation of chromatin, and formation of apoptotic bodies.
2.4. Immunocytochemistry assay
MCF-7 and MCF-7/Dox cells were plated at 5×104cells/ well and cultured in 24-wells plate at cover slip until 80% confluent. At time 0, medium was replaced by fresh complete medium with hesperidin 3.5 µmol/L and doxorubicin 230 nmol/L and placed in CO25% incubator at 37 °C for 18 h. Then, cells were harvested and were washed with PBS and fixed with cold methanol for 10 min at freezer -40 °C. Cells washed and blocked in hydrogen peroxide blocking solution for 10 min at room temperature. After that, cells washed with PBS and incubated with prediluted blocking serum for 10 min at room temperature. Cells were stained for 1 h at room temperature with primary Pgp antibody. After washing three times in PBS, secondary antibody were applied for 15-30 min, 1:2 in PBS and added 5% AB serum then washed with PBS three times. The slide was incubated with streptavidinbiotin complex for 15 min, 1:2 in PBS and added 5% AB serumand washed three times in PBS. Slides were incubated in 3,3 diaminobenzidin solution for 3-8 min and washed with aquadest. Cells were counterstained for 3-4 min with Mayer-Haematoksilin. After incubation, cover slip washed with aquadest and then immersed with xylol and alcohol. Protein expression was assessed under light microscope. Positive expression will give a dark brown colour in cell membrane and cells with no expression will give purple colour.
3. Results
3.1. Effect of hesperidin and its combination with doxorubicin on cell viability
Cell viability assay was done to determine the IC50of hesperidin alone and its combination with doxorubicinon MCF-7/Dox cells. All of these compounds showed growth inhibitory effect in dose dependent manner. Hesperidin and doxorubicin had the IC50values of 11 µmol/L (Figure 1) and 700 nmol/L, respectively. The combination of hesperidin and doxorubicin increased the viability cells higher than hesperidin alone (Figure 2). This result showed that the combination resulted antagonist effect.
3.2. Effect of hesperidin and doxorubicin on apoptosis induction
All of hesperidin or doxorubicin alone were capable of inducing apoptosis at inhibitory concentration, but when they were combined does not showed apoptotic induction (Figure 3). The green fluorescence indicates the viable cells while the orange-red fluorescence indicates the death cells. Apoptotic cells show the occurrence of chromatin condensation and the orange-red apoptotic bodies. Combination of hesperidin and doxorubicin appeared does not showed apoptotic induction.
3.3. Effect of hesperidin and doxorubicin toward Pgp expression on MCF-7/Dox cells line
To confirm the mechanism of hesperidin and its combination with doxorubicin induced apoptosis, this research observed the effect of hesperidin, doxorubicin and their combination on Pgp expression using immunocytochemistry method. Interestingly, the expression of Pgp on the hesperidin and doxorubicin-treated cells was decreased compare to the control cells. The decreasing level of Pgp expression on the combination treated cells was higher than on the hesperidin or doxorubicin single treated cells (Figure 4). Moreover, the increasing level of Pgp expression on the both single compared with combination treated cells showed significantly different, but still higher than the control cells. These data showed that the combination more potent to reduce the Pgp expression on the MCF-7/Dox cells than single treatment.

Figure 1. The effect of hesperidin on viability of MCF-7/Dox cells.Tests were carried out by incubating 5×103MCF-7/Dox cells in 96-well plates for 24 h to adapt, then treated hesperidin concentrations of 1-100 µmol/L, then incubated again for 24 h. Profiles of cell viability expressed mean±SD of 3 experiments (A). Obvious morphological changes and cell populations in the treatment of hesperidin concentrations of 10 (C), 50 (D), and 100 µmol/L (E) compared with controls (B). Black arrow indicates a normal living cells, whereas white arrows indicate the cell morphology changes. Cell morphology observations conducted with an inverted microscope with a magnification of 100×. IC50of 11 µmol/L obtained from the linear regression calculation of cell viability vs log concentrations with P<0.05.
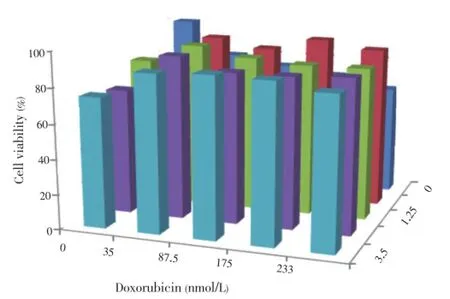
Figure 2. Combination effect of hesperidin-doksorubisin on viability of MCF-7/Dox cells.Tests were carried out by incubating 5×103MCF-7/Dox cells in 96-well plates for 24 h to adapt, then given a single treatment and combination of hesperidin 0.5, 1.25, 2.5 and 3.5 µmol/L, and doxorubicin 35, 87.5, 175 and 233 nmol/L, and then incubated again for 24 h. Profiles of cell viability expressed mean±SD of 3 experiments. Hesperidin is not able to enhance doxorubicin effects in reducing cell viability (A). Changes in cell morphology seen in the treatment of hesperidin least 3.5 µmol/L (C), treatment of 233 nmol/L doxorubicin (D), and the treatment of hesperidin combination of 3.5 µmol/L and 233 nµmol/L doxorubicin (E) compared with controls (B). Black arrow indicates a normal living cells, whereas white arrows indicate the cell morphology changes. Cell morphology observations conducted with an inverted microscope with a magnification of 100×. Doxorubicin combination treatment of cell viability 3.5 µmol/L and 233 nmol/L hesperidin did not differ significantly to 233 nmol/L doxorubicin single (P<0.05).

Figure 3. Apoptosis induction of hesperidin, doxorubicin and its combination on MCF-7/Dox cells in 24-well plates .A: all cells seen alive in the control; B: incidence of apoptosis treated with 230 nmol/L doxorubicin; C: incidence of apoptosis treated with 3.5 µmol/L hesperidin; D: Incidence of apoptosis treated with combination of 3.5 µmol/L hesperidin and 230 nmol/L doxorubicin not increased significantly; Arrows: the place where the incidence of apoptotic cells seen any fragmentation of the cell nucleus. Observations made under a fluorescent microscope with a magnification of 100×.

Figure 4. The effect of hesperidin, doxorubicin and its combination on Pgp expression on MCF-7/Dox cells.Tests were carried out by incubating 5×104MCF-7/Dox cells on coverslips in 24 wells plates for 24 h to adapt, then were subjected to 230 nmol/L doxorubicin, hesperidin 3.5 µmol/L and a combination of both. Tues incubated for 18 h and Pgp staining done as mentioned in the research procedure. Control cells without primary anti-Pgp antibody did not showed the brown color on the cell membrane (A). Control cells with antibodies showed expression of Pgp viewed from the cell membrane of brown color (B). In the doxorubicin group showed expression of the increasingly intense brown color on the cell membrane (C). Hesperidin and combination with doxorubicin (D and E) showed a decrease in the intensity of brown color when compared with doxorubicin single treatment group.
4. Discussion
The aim of this research was to investigate the biological activity of hesperidin to overcome cancer cell resistance because of doxorubicin chemotherapy drugs. MTT assay showed that hesperidin able to increase of MCF-7/Dox cells sensitivity to doxorubicin with IC50value of 11 µmol/ L. It was lower than the MCF-7 (ori) of 200 µmol/L[10]. The combination of hesperidin with doxorubicin was interesting in overcoming resistance through its action in suppressing the Pgp expression.
One of the mechanisms of cancer cell resistance to anticancer agents associated with MDR1 gene expression and the over-expression of Pgp protein, which could pumps the drugs out of cells. The MCF-7/Dox cells resistant to doxorubicin is experiencing over-expression of Pgp. Pgp encoded by the MDR1 gene and protein products transported through endosom with early transport to the plasma membrane. The MCF-7/Dox that overexpression of Pgp has been successfully made in this study with the method of induction by low concentration of doxorubicin.
Hesperidin was able to increase the sensitivity of cells with decreased the IC50value to MCF-7/Dox of 11 µmol/ L compared to MCF-7 cells. These results were consistent with several studies which showed that polyphenols such as flavonols quersetin increase the sensitivity of cells that are resistant to daunorubicin[11]. Several other flavonoids like silymarin were able to reverse the function of Pgp[12]. Flavonoid compounds also able to modulate Pgp expression so that the amount of intracellular doxorubicin increase and provide a greater cytotoxic effect.
Doxorubicin is a chemotherapy agent that is widely used in the treatment of various epithelial cancers. In the previous study showed that doxorubicin has a high potential as anticancer agents, Fitriasariet al. (2010) in MCF-7 cells and Junediet al. (2010) in T47D cells showed that doxorubicin could inhibit cell growth with IC50values were 460 nmol/L and 15 nmol/L, respectively[13-14]. Mechanism of doxorubicin is formed intercalation with DNA. It will directly affect the transcription and replication. Doxorubicin is able to form a tripartite complex with topoisomerase II and DNA.
From the research, it resulted that doxorubicin has IC50value of 700 nmol/L in MCF-7/Dox cells. On the combination of hesperidin with doxorubicin, it did not show a strong cytotoxic effect and combination index had high value (additive to antagonist effect). This is probably due to flavonoids such as hesperidin which are antioxidants will inhibit the cytotoxic activity of doxorubicin. Research about hesperidin as an antioxidant has been widely reported previously[15]. Mechanism of hesperidin as antioxidant was to inhibit the peroxidation of linoleic acid induced by Fe2+and autooxidation in membranes cerebral and inhibits the production of reactive oxygen species including hydroxyl radicals and nitric oxide (NO)[16]. In contrast with the anticancer activity of doxorubicin, one of the mechanisms of doxorubicin in inhibiting the progression of cancer is to stimulate the production of reactive oxygen species and NO that would undermine the stability of DNA and eventuallywill cause death of cancer cells. The combination of hesperidin with doxorubicin in this study proved to be potent and synergistic in inhibiting cancer cell growth. This is probably due to a mechanism that is opposite from the second agent. This is an interesting research findings, in which a natural compound in combination with chemotherapeutic agents did not always produce a synergistic effect. To explore the mechanism of action of this combination, it is necessary to observe the mechanism of cell death and see that the modulation of protein expression plays a role against the resistance of cancer cells, namely Pgp.
The results of apoptosis by double staining method in the treatment of hesperidin (3.5 µmol/L) showed that only a few occurrence of the phenomenon of apoptosis. Similar results are also shown in the treatment of hesperidin combination with doxorubicin, while a single doxorubicin could increase the occurrence of apoptosis. This is consistent with the results of combination between hesperidin and doxorubicin at low concentrations showed no cytotoxic effects, even to additive and antagonistic. This is in line with research of Sakataet al. (2003) which showed that hesperidin can reduce the occurrence of cell apoptosis through its ability to reduce the overproduction of NO as a result of an inhibitory effect on the expression and activity of inducible nitric oxide synthase[17,18].
From the results of Pgp observation was known that the combination of hesperidin with doxorubicin to inhibit the expression of Pgp in MCF-7/Dox cells. This was shown by a decrease in the intensity of brown color on the cell membrane compared to doxorubicin single tratment. This is consistent with previous studies that flavonoids including hesperidin can be located as a substrate of Pgp on adenosine triphosphate binding site, so that the expression of Pgp will be decreased[19-23]. So the prospect of hesperidin has developed further as Pgp expression-suppressing agents.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We gratefully thanks to DP2M DIKTI (Directorate of higher Education) Ministry of Education Indonesia trough HKI research grant 2011 for financial support of this study.
Comments
Background
Drug resistance is one of the problems in cancer therapy, especially in breast cancer. Strategies and the development of breast cancer treatment should be pursued. Problems in the chemotherapy of breast cancer become larger, since the emergence of breast cancer resistance to chemotherapy agents.
Research frontiers
Hesperidin has antiproliferative effect on MCF-7 cells. This study aimed to determine the effect of hesperidin on MCF-7 and MCF-7/Dox cells. The study demonstrated cytotoxicity of hesperidin, apoptosis induction and Pgp expression, when given singly and combination with doxorubicin. The results will be basic research in order to explore hesperidin as an alternative to co-chemotherapy agent in breast cancer therapy.
Related reports
Hesperidin showed strong toxic effect in Caco-2, CEM/ ADR5000 and CCRF-CEM cancer cell lines with dengan IC50, 195, 230 and 95 µmol/L, respectively. Hesperidin protects benzo(a)pirene-induced testicular toxicity by repairing the function of lactate dehydrogenase, superoxide dismutase, dan glutathione-S-transferase enzymes. Also, hesperidin is able to induce apoptosis in human colon cancer cell lines by CASP3 activation.
Innovations and breakthroughs
Hesperidin possesses a variety of biological properties, including cancer chemoprevention. In thisin vitrostudy, the authors demonstrated that hesperidin could be used for lowering breast cancer resistance to chemotherapy agents.
Applications
It is well-known that resistance to chemotherapy is one of the major problems to treat breast cancer. This study suggests that hesperidin can be used for reducing resistance to breast cancer chemotherapy, by single treatment or in combination with anti-cancer agents.
Peer review
This is an interesting and valuable research work. The authors demonstrated that hesperidin has cytotoxic effect on MCF-7/Dox cells with IC50of 11 µmol/L. Hesperidin did not increased the apoptotic induction combined with doxorubicin. Co-chemotherapy application of doxorubicin and hesperidin on MCF-7/Dox cells showed synergism effectthrough inhibition of Pgp expression.
[1] Wong HL, Bendayan R, Rauth AM, Xue HY, Babakhanian K, Wu XY. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J Pharmacol Exp Ther 2006; 317(3): 1372-1381.
[2] Emami A, Zeinali S, Motahari Z, Azizi E. Resistance to adriamycin alters the MDR1/P-gp, Topoisomerase II-alpha gene and protein expression levels in T47D human breast cancer cells. Conference Module of 2005 AAPS Annual Meeting and Exposition. 2005. [Online] Available from: http://abstracts.aaps. org/published/ContentInfo.aspx?conID=2788 [Accessed on 2 April, 2012].
[3] Li XQ, Lu Y, Liang K, Liu BL, Fan Z. Differential responses to doxorubicin-induced phosphorylation and activation of Akt in human breast cancer cells. Breast Cancer Res 2005; 7(5): R589-R597.
[4] Zhang S, Morris ME. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Ther 2003; 304(3): 1258-1267.
[5] Wang SW, Kotamraju S, Konorev E, Kalivendi SS, Joseph J, Kalyanaraman B. Activation of nuclear factor-kB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J 2002; 367: 729-740.
[6] El-Readi MZ, Hamdan D, Farrag N, El-Shazly A, Wink M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur J Pharmacol 2010; 626(2-3): 139-145.
[7] Lee CJ, Wilson L, Jordan MA, Nguyen V, Tang J, Smiyun G. Hesperidin suppressed proliferations of both human breast cancer and androgen-dependent prostate cancer cells. Phytother Res 2010; 24(Suppl 1): S15-19.
[8] Arafa HM, Aly HA, Abd-Ellah MF, El-Refaey HM. Hesperidin attenuates benzo[alpha] pyrene-induced testicular toxicity in rats via regulation of oxidant/antioxidant balance. Toxicol Ind Health 2009; 25(6): 417-427.
[9] Park HJ, Kim MJ, Ha E, Chung JH. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedecine 2008; 15(1-2): 147-151.
[10] Hermawan A, Meiyanto E, Susidarti RA. Hesperidin increase cytotoxic effect of doxorubicin in MCF-7 cells. Indonesian J Pharm 2010; 21(1): 8-17.
[11] Borska S, Sopel M, Chmielewska M, Zabel M, Dziegiel P. Quercetin as a potential modulator of P-glycoprotein expression and function in cells of human pancreatic carcinoma line resistant to daunorubicin. Molecules 2010; 15(2): 857-870.
[12] Chung SY, Sung MK, Kim NH, Jang JO, Go EJ, Lee HJ. Inhibition of P-glycoprotein by natural products in human breast cancer cells. Arch Pharm Res 2005; 28: 823-828.
[13] Fitriasari A, Wijayanti KN, Ismiyati N, Dewi D, Kundarto W, Sudarmanto BSA, et al. Selective estrogen receptor modulators (SERMs) potency of curcumin and its analogues: a docking on estrogen β receptors. Pharmacong 2008; 9(1): 27-32.
[14] Nugroho AE, Hermawan A, Putri DDP, Novika A, Meiyanto E. Combinational effects of hexane insoluble fraction of Ficus septica Burm. F. and doxorubicin chemotherapy on T47D breast cancer cells. Asian Pac J Trop Biomed 2013; 3(4): 297-302.
[15] Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 2004; 56: 185-229.
[16] Kim JY, Jung KJ, Choi JS, Chung HY. Hesperetin: a potent antioxidant against peroxynitrite. Free Radic Res 2004; 38: 761-769.
[17] Jung YS, Kang TS, Yoon JH, Joe BY, Lim HJ, Seong CM, et al. Synthesis and evaluation of 4-hydroxyphenylacetic acid amides and 4-hydroxycinnamamides as antioxidants. Bioorg Med Chem Lett 2002; 12(18): 2599-2602.
[18] Takahama U, Imamura H, Hirota S. Nitration of the salivary component 4-hydroxyphenylacetic acid in the human oral cavity: enhancement of nitration under acidic conditions. Eur J Oral Sci 2009; 117: 555-562.
[19] Sakata K, Hirose Y, Qiao Z, Tanaka T, Mori H. Inhibition of inducible isoforms of cyclooxygenase and nitric oxide synthase by flavonoid hesperidin in mouse macrophage cell line. Cancer Lett 2003; 199(2): 139-145.
[20] Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanism, intermediacy of H(2)O(2)-and p53-dependent pathways. J Biol Chem 2004; 279(24): 25535-25543.
[21] Kim JH, Chae M, Kim WK, Kim YJ, Kang HS, Kim HS. Salinomycin sensitizes cancer cells to the effects of doxorubicin and etoposide treatment by increasing DNA damage and reducing p21 protein. Br J Pharmacol 2011; 162(3): 773-784.
[22] Abdel-Raheem IT, Abdel-Ghany AA. Hesperidin alleviates doxorubicin-induced cardiotoxicity in rats. J Egypt Natl Canc Inst 2009; 21(2): 175-184.
[23] Kusharyanti I, Larasati, Susidarti RA, Meiyanto E. Hesperidin increases cytotoxic activity of doxorubicin on Hela cell line through cell cycle modulation and apoptotis induction. Indonesian J Cancer Chemoprev 2011; 2(2): 267-273.
10.1016/S2221-1691(14)60236-7
*Corresponding author: Prof. Agung Endro Nugroho, M.Sc., Ph.D. Cancer Chemoprevention Research Center, Faculty of Pharmacy, Universitas Gadjah Mada, Sekip Utara Yogyakarta, Indonesia 55281
Telp: (0274)543120
Fax : (0274)543120.
Email : agungendronugroho@yahoo.com; nugroho_ae@ugm.ac.id
Foundation Project: Supported by DP2M DIKTI (Directorate of higher Education) Ministry of Education Indonesia trough HKI research grant 2011.
Article history:
Received 1 Dec 2013
Received in revised form 12 Dec, 2nd revised form 21 Dec, 3rd revised form 29 Dec 2013
Accepted 13 Jan 2014
Available online 28 Mar 2014
Methods:The cytotoxic properties, 50% inhibition concentration (IC50) and its combination with doxorubicin in MCF-7 cell lines resistant to doxorubicin (MCF-7/Dox) cells were determined using MTT assay. Apoptosis induction was examined by double staining assay using ethidium bromide-acridine orange. Immunocytochemistry assay was performed to determine the level and localization of Pgp.
Results:Single treatment of hesperidin showed cytotoxic activity on MCF-7/Dox cells with IC50value of 11 µmol/L. Thus, combination treatment from hesperidin and doxorubicin showed addictive and antagonist effect (CI>1.0). Hesperidin did not increase the apoptotic induction, but decreased the Pgp expressions level when combined with doxorubicin in low concentration.
Conclusions:Hesperidin has cytotoxic effect on MCF-7/Dox cells with IC50of 11 µmol/ L. Hesperidin did not increased the apoptotic induction combined with doxorubicin. Cochemotherapy application of doxorubicin and hesperidin on MCF-7/Dox cells showed synergism effect through inhibition of Pgp expression.
 Asian Pacific Journal of Tropical Biomedicine2014年3期
Asian Pacific Journal of Tropical Biomedicine2014年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Isolation and structural elucidation of cytotoxic compounds from the root bark of Diospyros quercina (Baill.) endemic to Madagascar
- Comparative micromorphological study of wild and micropropagated Dioscorea bulbifera Linn.
- 15-Lipoxygenase inhibition of Commelina benghalensis, Tradescantia fluminensis, Tradescantia zebrina
- Phytochemical constituents and antibacterial activity of some green leafy vegetables
- Acute and subacute oral toxicity study on the flavonoid rich fraction of Monodora tenuifolia seed in albino rats
- Chromatographic finger print analysis of anti-inflammatory active extract fractions of aerial parts of Tribulus terrestris by HPTLC technique
