Phytochemical constituents and antibacterial activity of some green leafy vegetables
Ramesa Shafi Bhat, Sooad Al-Daihan
Biochemistry Department, College of Science, King Saud University, P. O. Box 22452, Riyadh 11495, Saudi Arabia
Phytochemical constituents and antibacterial activity of some green leafy vegetables
Ramesa Shafi Bhat*, Sooad Al-Daihan
Biochemistry Department, College of Science, King Saud University, P. O. Box 22452, Riyadh 11495, Saudi Arabia
PEER REVIEW
Peer reviewer
Dr. Afaf K.El-Ansary, Professor, Biochemistry Department, Science College, King Saud University, Saudi Arabia.
Tel: 009661-4769137 ext. 1396
Fax: 009661-4769137
E-mail: elansary@ksu.edu.sa
Comments
This is a good and applicable study in which the author tested the efficacy of 5 different green leafy plants as antibacterial extracts. The obtained results are promising showing the possibility of using these plants to treat different bacterial infections through their antioxidant, detoxification and antimicrobial effects.
Details on Page 192
Objective:To investigate the antibacterial activity and photochemicals of five green leafy vegetables against a panel of five bacteria strains.
Green leafy vegetables, Antibacterial activity, Phytochemical screening.
1. Introduction
Green leafy vegetables have been used as medicine since ancient times and have been playing a very important role in our diet and nutrition. They are the most readily available sources of carbohydrates, fats, important proteins, vitamins, minerals, essential amino acids, and fibers[1]. Their bioactive substances have a wide range of biological functions, including antioxidant and antimicrobial activities[2-5] and can be helpful in management of oxidative stress and age related human aliments[6]. They are rich source of carotene, ascorbic acid, riboflavin, folic acids and minerals like calcium, iron and phosphorus[7]. Being a photosynthetic tissue, leafy vegetables have higher levels of vitamin K when compared with other fruits and vegetables due to direct involvement of vitamin K (phylloquinone) in photosynthesis process. Vegetables as medicinal plants contain none or less toxic effects[8,9], and have the ability to synthesize several secondary metabolites of relatively complex structures possessing antimicrobial activities[10-12]. Green leafy vegetables are also rich in compounds having antidiabetic[13], anti-histaminic[14], anti-carcinogenic[15]and hypolipidemic[16] properties and possess preventive or curative properties against cardiovascular disease, ageing, obesity, hypertension, insomnia and ageing[17-19]. Leafy vegetables are natural source of antioxidants and rich in phytochemicals[20,21]. The present work was therefore designed to investigate the antibacterial effects of five leafy vegetables namelyCoriandrum sativum(C. sativum), Lactuca sativa(L. sativa), Mentha piperita(M. piperita), Portulaca oleracea(P. oleracea) andRaphanus sativus(R. sativus) against some bacteria strains and their phytochemical screening.
2. Materials and methods
2.1. Collection of plant material
Fresh leaves ofC. sativum, L. sativa, M. piperita, P.oleraceaandR. sativusfree from disease were purchased from local farms in Al-Qassim. Samples were labeled and stored at 4 °C in polythene bags till they were processed. Collected materials were washed thoroughly in running tap water, rinsed in distilled water and shade dried for one week in open air, and then crushed using mortar and pestle, reduced to powder using Waring laboratory blender (MX-7011G) for 5 min at high speed and then stored in airtight closed bottles for two days before used for analysis. Fifty grams of all the fresh samples were stored for juice preparation.
2.2. Microorganisms
Bacteria cultures ofStaphylococcus aureus,Streptococcus pyogenes,Escherichia coli, Bacilllus subtillis, andPseudomonas aeruginosa(clinical isolates) were obtained from Botany Department of King Saud University. The strains were maintained on agar slant at 4 °C and activated at 37 °C for 24 h on nutrient agar (Sigma-Aldrich, Germany) before any susceptibility test.
2.3. Preparation of leaf extract
2.3.1. Juice preparation
Fifty grams of raw leave samples after washing with water were crushed by grinder without adding any solvent. The residue was removed by filtering through 8 layers of muslin cloth. The filtrate was collected in clean airtight bottle and stored at 4 °C until use for antibacterial activity test.
2.3.2. Aqueous extraction
Ten grams of dry powder of samples were dissolved in 30 mL of 0.01 mol/L HCl containing 0.15 mol/L NaCl. (sample:extract solution, 1:3 w/v). The residue was then removed by filtering through cheese cloth. The filtrate was then centrifuged at 8 100×g, for 5 min. These leaves and extract were subjected to antibacterial activity experiments and protein determination[22].
2.3.3. Methanol extraction
Ten grams of powdered sample was dissolved in 100 mL of methanol in a conical flask, plugged with cotton wool and then kept on a rotary shaker at 190-220 r/min for 24 h. The supernatant was collected slowly and evaporated in wide mouthed evaporating bowls at room temperature for 2-3 d till the final volume was reduced to one fourth of the original volume of the solvent used, giving the concentration of 400 mg/mL[23] and stored at 4 °C in airtight bottles.
2.4. Media preparation
Twenty three grams of nutrient agar (Sigma-Aldrich, Germany) were dissolved in 1 000 mL of distilled water and bring to boil. Agar was then autoclaved for 15 min at 121 °C and left to cool at room temperature. Once the LB medium was cooled (about 45 °C), it was poured into Petri dishes. Each Petri dish was left on the flat surface for 30-40 min until completely set.
2.5. Antibacterial activity
Antibacterial activity was assayed by disc diffusion method. For all bacteria strains, overnight culture grown in broth was adjusted to an inoculum’s density of 100 µL: 0.1A600 culture containing 3.2×108colony forming unit. Further, 20 µL was spread onto 20 mL of sterile agar plates by using a sterile cotton swab. The surface of the medium was allowed to dry for about 3 min. Sterile filter paper discs (5 mm in diameter) impregnated with different test extracts (100 µL disc) were then placed on the surface of inoculated agar plates. Kanamycin (30 µg/disc) was used as positive control. The plates were then incubated at 37 °C for 24 h after which microbial growth was determined by measuring the diameter of the inhibition zone (mm) using a transparent scale. Each extract was analyzed in triplicate, the mean values are presented. Kanamycin disc (30 µg/disc) was used for comparing the bioassay.
2.6. Phytochemical analysis
2.6.1. Molisch’s test for Carbohydrates
At first 0.5 g of each powder was dissolved separately in 5 mL of distilled water and filtered. Few drops of Molisch’sreagent were added to each solution, this was then followed by addition of 1 mL of concentrated H2SO4by the side of the test tube. The mixture was then allowed to stand for 2 min and then diluted with 5 mL of distilled water. Formation of a red or dull violet colour at the interphase of the two layers was taken as positive test[24].
2.6.2. Test for alkaloids
A given weight of 0.1 g of each powder was dissolved in 5 mL of methanol separately and then filtered. A volume of 2 mL of each filtrate from each sample were stirred with 5 mL of 1% aqueous HCl on water bath and then filtered. Of the filtrate, 1 mL was taken individually into 2 test tubes. To the first portion (1 mL), few drops of Dragendorff’s reagent were added. Occurrence of orange-red precipitate was taken as positive. To the second 1 mL, Mayer’s reagent was added and appearance of buff-colored precipitate was taken as positive test for the presence of alkaloids[24].
2.6.3. Liebermann-Burchard test for steroids
At the beginning 0.2 g of crude powder of each sample was dissolved in 2 mL of acetic acid separately. The solutions were cooled well in ice followed by the addition of concentrated H2SO4carefully. Color development from violet to blue or bluish-green indicated the presence of a steroidal ring[24].
2.6.4. Test for saponins
One gram of crude powder of each sample was boiled with 5 mL of distilled water separately and then filtered. To each filtrate, about 3 mL of distilled water was further added and shaken vigorously for about 5 min. Frothing which persisted on warming was taken as an evidence for the presence of saponins[24].
2.6.5. Shinoda’s test for flavonoids
About 0.5 g of each powder was dissolved in 5 mL of ethanol separately, warmed and then filtered. Three pieces of magnesium chips was then added to the filtrate followed by few drops of concentrated HCl. A pink, orange, or red to purple colouration indicates the presence of flavonoids[25].
2.6.6. Test for tannins
About 0.5 g of each portion of crude powder was stirred with about 10 mL of distilled water separately and then filtered. Few drops of 1% ferric chloride solution were added to 2 mL of each filtrate occurrence of a blue-black, green or blue-green precipitate indicates the presence of tannins[25].
2.7. Statistical analysis
The results were analyzed by using standard deviation (SD) statistical methods[26].
3. Results
3.1. Antibacterial activity of the vegetable extracts
The antibacterial activity of five green vegetables extract was assayedin vitroby agar disc diffusion against five bacterial species. The data in Table 1 show the antibacterial activities of the tested extracts on a panel of five Gram positive or Gram negative bacteria. All methanol extracts were found active against all the test bacterial strains. All the extracts ofC. sativumshowed maximum inhibitory activity against the entire test bacterial strains while all extracts fromM. piperitawere less active against test organism under study. The best activity was obtained with methanol extract fromC. sativumagainstE. coli.

Table 1 Antibacterial activity of various extracts of test samples against bacterial species tested by disc diffusion assay.
3.2. Chemical composition of the vegetable extracts
The results of the qualitative analysis showed that carbohydrates and proteins were found in all the leaves under study (Table 2). Flavonoids were present in all samples except inM. piperita. Only green leaves ofM. piperitaandR. sativuscontain steroids while alkaloids were found inL. sativa, P. oleraceaandR. sativus. Saponins were found inM. piperita, P. oleraceaandR. sativus,and tannin was found inC. sativum,M. piperitaandP. oleracea.
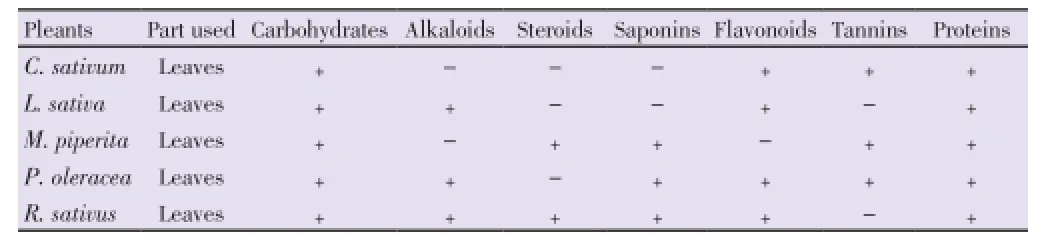
Table 2 Phytochemical composition of different salad leaves.
4. Discussion
The various leave extracts showed varied antimicrobial activity to the test organism which was species dependent. Gram-positive bacterial strains were more susceptible to the extracts when compared to Gram negative bacteria. Gram negative bacteria are surrounded by the cell wall which restricts diffusion of hydrophobic compounds through its lipopolysaccharide covering. The absence of this barrier in Gram positive bacteria allows the direct contact of the essential oil’s hydrophobic constituents with the phospholipids bilayer of the cell membrane, causing either an increase of ion permeability and leakage of vital intracellular constituents, or impairment of the bacterial enzyme systems[27]. Also two groups of bacteria differ in their structure of cell wall. Ability of tannin to disintegrate bacterial colonies is hinder with bacterial cell wall[28]. Medicinal plants which are rich in tannins are used to treat inflamed or ulcerated tissues[29].
Bioactive compounds are normally accumulated as secondary metabolites in all plant cells but their concentration varies according to the plant parts, seasons, climates and particular growth phases. Leaves are one of the highest sources of accumulation and are highly beneficial[30,31].
Most of the secondary metabolite identified in the test samples like flavonoids, saponins, tannin, steroids and alkaloids are phytoprotectants and are important for cell growth, replacement, and body building[32]. Their medicinal value is due to presence of some chemical substances that can produce a defined physiological action on human body with antioxidant, antibacterial, anti-inflammatory, antiviral, immune system stimulant and detoxification activities[33].
Green leafy vegetables contain various pharmacologically active compounds. On the whole the present investigation confirmed the traditional uses of the studied vegetables in the treatment of bacterial infections.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This research project was supported by a grant from the Research Center of the Center for Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University.
Comments
Background
The authors gave a strong and well written background on the medicinal uses of green leafy vegetables, their antioxidant and antimicrobial efficacy. The cited references covered a period from 1998 until 2013 which reflect the awareness of the author with the presented topic.
Research frontiers
Green leafy vegetables have phyto-protectants that are important for cell growth, replacement, and body building. Their detoxification and antimicrobial effects increase their nutritional values.
Related reports
The antimicrobial effects of the five green leafy plants were ascertained in this study.
Innovations and breakthroughs
The present study foucuses on the possibility of the traditional use of green leafy plant extracts in treating bacterial infections.
Applications
The research results indicate that the five plants could be used in developing antimicrobials.
Peer review
This is a good and applicable study in which the author tested the efficacy of 5 different green leafy plants as antibacterial extracts. The obtained results are promising, showing the possibility of using these plants to treat different bacterial infections through their antioxidant, detoxification and antimicrobial effects.
[1] Sharma HP, Kumar RA. Health security in ethnic communities through nutraceutical leafy vegetables. J Environ Res Develop 2013; 7(4): 1423-1429.
[2] Burt S. Essential oils: their antibacterial properties and potential applications in foods. A review. Int J Food Microbiol 2004; 94(3): 223-253.
[3] Chanda S, Baravalia Y, Kaneria M, Rakholiya K. Fruit and vegetable peels-strong natural source of antimicrobics. In: Mendez-Vilas A, editor. Current research, technology and education topics in apllied microbiology and microbial biotechnology. Spain: Formatex Research Center; 2010.
[4] Gutierrez J, Barry-Ryan C, Bourke P. The antimicrobial efficacyof plant essential oil combinations and interactions with food ingredients. Int J Food Microbiol 2008; 124(1): 91-97.
[5] Kim SJ, Cho AR, Han J. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their applications to meat product preservation. Food Control 2013; 29: 112-120.
[6] Gacch RN, Kabaliye VN, Dhole NA, Jadhav AD. Antioxidant potential of selected vegetables commonly used in diet in Asian subcontinent. Indian J Nat Prod Resour 2010; 1(3): 306-313.
[7] Fasuyi AO. Nutritional potentials of some tropical vegetable meals, chemical characterization and functional properties. Afr J Biotechnol 2006; 5(1): 49-53.
[8] Matasyoh JC, Maiyo ZC, Ngure RM, Chepkorir R. Chemical composition and antimicrobial activity of the essential oil of Coriandrum sativum. Food Chem 2009; 113: 526-529.
[9] Evarando LS, Oliveira LE, Freire LKR, Sousa PC. Inhibitory action of some essential oils and phytochemicals on growth of various moulds isolated from foods. Braz Arch Biol Technol 2005; 48: 234-241.
[10] Kubo I, Fujita K, Kubo A, Nihei K, Ogura T. Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J Agr Food Chem 2004; 52: 3329-3332.
[11] Hedges LJ, Lister CE. Nutritional attributes of some exotic and lesser known vegetables. Plant & food research confidential report No. 2325. New Zealand: New Zealand Institute for Plant & Food Research Limited; 2009.
[12] Dhiman K, Gupta A, Sharma DK, Gill NS, Goyal A. A review on the medicinal important plants of the family of Cucurbitaceae. Asian J Clin Nutr 2012; 4: 16-26.
[13] Kesari AN, Gupta RK, Watal G. Hypoglycemic effects of Murraya koengii on normal and alloxan-diabetic rabbits. J Ethnopharmacol 2005; 97: 247-251.
[14] Yamamura S, Ozawa K, Ohtani K, Kasai R, Yamasaki K. Antihistaminic flavones and aliphatic glycosides from Mentha spicata. Phytochem 1998; 48: 131-136.
[15] Rajeshkumar NV, Joy KL, Kuttan G, Ramsewak RS, Nair MG, Kuttan R. Antitumour and anticarcinogenic activity of Phyllanthus amarus extract. J Ethnopharmacol 2002; 81: 17-22.
[16] Khanna AK, Rizvi F, Chander R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J Ethnopharmacol 2002; 82: 19-22.
[17] Iyer SR, Sethi R, Abroham AA. Analysis of nitrogen and phosphate in enriched and non enriched vermicompost. J Environ Res Develop 2012; 7(2): 899-904.
[18] Vishwakarma KL, Dubey V. Nutritional analysis of indigenous wild edible herbs used in eastern Chhattisgarh, India. Emir J Food Agric 2011; 23(6): 554-560.
[19] Patro HK, Kumar A, Shukla DK, Mahapatra BS. Total productivity nutrient uptake and economics of rice wheat cropping system as influenced by Crotalaria junea green manuring. J Environ Res Develop 2011; 5(3): 532-541.
[20] Elias KM, Nelson KO, Simon M, Johnson K. Phytochemical and antioxidant analysis of methanolic extracts of four African indigenous leafy vegetables. Ann Food Sci Technol 2012; 13(1): 37-42.
[21] Raghavendra M, Reddy AM, Yadav PR, Raju AS, Kumar LS. Comparative studies on the in vitro antioxidant properties of methanolic leafy extracts from six edible leafy vegetables of india. Asian J Pharm Clin Res 2013; 6(3): 96-99.
[22] Franco, Murad OL, Leite AM, Mendes JR, Prates PA, Bloch MV, et al. Identification of a cowpea g-thionin with bacteria activity. FEBS J 2006; 273: 3489-3497.
[23] Harbone JB. Phytochemical methods. London: Chapman and Hill; 1973, p. 49-188.
[24] Sofowora A. Screening plants for bioactive agents. In: Medicinal plants and traditional medicinal in Africa. 2nd ed. Ibadan, Nigeria: Spectrum Books Ltd, Sunshine House; 1993, p. 134-156.
[25] Trease GE, Evans WC. Pharmacognosy. 15th ed. London: Saunders Publishers; 2002, p. 42-44, 221-229, 246-249, 304-306, 331-332, 391-393.
[26] Bhat RS, Al-daihan S. Antimicrobial activity of Litchi chinensis and Nephelium lappaceum aqueous seed extracts against some pathogenic bacterial strains. J King Saud Univ-Sci. Forthcoming 2013.
[27] Zhao WH, Hu ZO, Okubo S, Hara Y, Shimamura T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillinresistant Staphylococcus aureus. Antimicrob Agents Chemother 2001; 45: 1737-1742.
[28] Venkataswamy R, Doss A, Sukumar M, Mubarack HM. Preliminary phytochemical screening and antimicrobial studies of Lantana indica roxb. Indian J Pharmaceutical Sci 2010; 72(2): 229-231.
[29] Akinpelu DA, Onakoya TM. Antimicrobial activities of medicinal plants used in folklore remedies in south-western. Afr J Biotechnol 2006; 5(11): 1078-1081.
[30] Jain AK, Tiwari P. Nutritional value of some traditional edible plants used by tribal communities during emergency with reference to Central India. Indian J Trad Knowl 2012; 11(1): 51-57.
[31] Mensah JK, Okoli RI, Ohaju-Obodo JO, Eifediyi K. Phytochemical, nutritional and medical properties of some leafy vegetables consumed by Edo people of Nigeria. Afr J Biotech 2008; 7(14): 2304-2309.
[32] Kubmarawa D, Khan ME, Punah AM, Hassan. Phytochemical screening and antibacterial activity of extracts from Parkia clappertoniana Keay against human pathogenic bacteria. J Med Plant Res 2008; 2(12): 352-355.
[33] Johanna WL. Spicing up a vegetarian diet: chemopreventive effects of phytochemicals. Am J Clin Nutr 2003: 78: 579-583.
10.1016/S2221-1691(14)60230-6
*Corresponding author: Ramesa Shafi Bhat, Biochemistry Department, College of Science, King Saud University, P. O. Box 22452, Riyadh 11495, Saudi Arabia.
Tel: +96614785968 ext 1204
E-mail: ramesa.aftab@gmail.com, rbhat@ksu.edu.sa
Foundation Project: Supported by a grant from the Research Center of the Center for Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University.
Article history:
Received 25 Dec 2013
Received in revised form 3 Jan, 2nd revised form 11 Jan, 3rd revised form 20 Jan 2014
Accepted 29 Feb 2014
Available online 28 Mar 2014
Methods:Disc diffusion method was used to determine the antibacterial activity, while kanamycin was used as a reference antibiotic. The phytochemical screening of the extracts was performed using standard methods.
Results:All methanol extracts were found active against all the test bacterial strains. Overall maximum extracts shows antibacterial activity which range from 6 to 15 mm. Proteins and carbohydrates was found in all the green leaves, whereas alkaloid, steroids, saponins, flavonoids, tannins were found in most of the test samples.
Conclusions:The obtain result suggests that green leafy vegetables have moderate antibacterial activity and contain various pharmacologically active compounds and thus provide the scientific basis for the traditional uses of the studied vegetables in the treatment of bacterial infections.
 Asian Pacific Journal of Tropical Biomedicine2014年3期
Asian Pacific Journal of Tropical Biomedicine2014年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Isolation and structural elucidation of cytotoxic compounds from the root bark of Diospyros quercina (Baill.) endemic to Madagascar
- Comparative micromorphological study of wild and micropropagated Dioscorea bulbifera Linn.
- 15-Lipoxygenase inhibition of Commelina benghalensis, Tradescantia fluminensis, Tradescantia zebrina
- Acute and subacute oral toxicity study on the flavonoid rich fraction of Monodora tenuifolia seed in albino rats
- Chromatographic finger print analysis of anti-inflammatory active extract fractions of aerial parts of Tribulus terrestris by HPTLC technique
- Survey on cattle ticks in Nur, north of Iran
