Comparative micromorphological study of wild and micropropagated Dioscorea bulbifera Linn.
Mubo A. Sonibare, Adedapo A. Adeniran
Department of Pharmacognosy, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria
Comparative micromorphological study of wild and micropropagated Dioscorea bulbifera Linn.
Mubo A. Sonibare*, Adedapo A. Adeniran
Department of Pharmacognosy, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria
PEER REVIEW
Peer reviewer
Professor Abiodun E. Ayodele, Department of Botany, University of Ibadan, Ibadan, Nigeria.
Tel: +23408023255482
E-mail: bayodele@yahoo.com
Comments
This is a good paper elucidating the importance of micro characters in the confirmation of the identity of both micro propagated and wild samples of D. bulbifera. The paper also provided information on the best conditions for obtaining a high level of genetic materials for the conservation of the plant.
Details on Page 182
Objective:To study the leaf epidermis of wild and micropropagated Dioscorea bulbifera Linn. (D. bulbifera) in order to document useful diagnostic features that may be employed for correct crude drug identification and to clear any taxonomic uncertainties in the micropropagated medicinal plant.
Dioscorea bulbifera, Micropropagation, Microscopy, Stomata, Trichome, Standardization, Medicinal plant
1. Introduction
Dioscorea bulbiferaLinn. (D. bulbifera) belongs to the family Dioscoreaceae assigned to the order Dioscorales. It is commonly called air potato, air yam or bulbil-bearing yam. It is a vigorous climber plant native of West Africa predominantly found in forest gaps and forest edges[1]. Clockwise twinning direction was reported inD. bulbiferaand measures up to 12 m[2]. The leaves are shiny green, alternate with a long petiole. Just like every yam plant,D. bulbiferapossesses an annual vegetative cycle. The aerial bulbil serves as the main storage organ rather than underground tubers or bulbils[3]. At the beginning of the growing season, tubers and new bulbils produce thick sphagetti-like root from the rhizomatous head. Vegetatively,D. bulbiferais cultivated by planting the whole bulbils,but it is not grown much commercially because of the unpalatable bitter taste[4].
In Southwest Nigeria,D. bulbiferagrows under the shade that contains a substrate composed of high levels of organic material and often over the tops of low-lying vegetation and into tree canopies. Most farmers regard the plant as weed competing with their farm products and clear them from the farm. Consequently, the plants are destroyed and are not eaten. In spite of the great medicinal application ofD. bulbifera, preference is largely given by local people to the flavor of other yam species. This has led to the near extinction status of this plant in the southeastern part of Nigeria[5]. It is therefore imperative to consider conserving this medicinally useful plant genetic resource. The use of micropropagation techniques for endangered plant genetic resources conservation was recognized late in the 70s[6]. At the moment,in vitrotechnique has found increasing significance as a complementary means toin situplant conservation, especially for vegetatively propagated and recalcitrant seed-producing species[7]. Although the development of successfulin vitropropagation and storage protocols are reliable methods that make possible the establishment of extensive germplasm collections, plants cultivatedin vitropresent certain anatomical and physiological characteristics intrinsic to culture environment[8]. Maintaining the genetic identity of donor plant and a completely viable acclimatized plant yield afterin vitrostorage are the essential prerequisites for usingin vitroculture techniques forex-situconservation purpose. Morpho-anatomical, biochemical and molecular parameters have also been used to monitor integrity and stability ofin vitrogrown plants[9].
Leaf micro-characters can be used for distinguishing crude drugs even when they are fragmented[10]. A combination of different diagnostic features (quantitative and microscopic parameters such as stomata and trichome types with sizes, shapes and sizes of epidermal cell, thickness of cell and cell inclusions) has been used for taxonomic distinction and recognition in the angiosperm family[11-15]. Species mis-identification of herbal drugs due to numerous local names given to the same species or several species of crude drug with the same local name is common in Africa[16]. Therefore, foliar micromorphological description of crude drug may complement macroscopic description of medicinal plants which could be useful in preparing a monograph for its identification, thus providing a useful tool for collection and preservation of the species.
D. bulbifera, besides being important as an edible yam, is used in traditional medicine[17]. Among the many documented medicinal folk uses, the plant is used to paste produced from the tubers to treat swelling and as a cure for snakebite and scorpion stings in Africa and Asia, and the bulbils are used to cure thyroid diseases and cancer in traditional Chinese medicine[18-20]. The plant had been reported to possess analgesic, anti-inflammatory and antimicrobial properties as well as used for diseases such as leprosy, tumours, piles, dysentery, syphilis and ulcers[21-23].
In view of the biological and medicinal importance ofD. bulbifera, and the report of its near extinction in southeast region of Nigeria, the present study was designed to compare qualitative and quantitative micro-morphological characteristics of the wild and micropropagated plants which may be employed for correcting crude drug identification.
2. Materials and methods
2.1. Plant material
Bulbils and leaves ofD. bulbiferawere collected in November, 2011 from the field at Alabata village in Moniya, Ibadan, Southwest Nigeria. The plant was identified by Mr. O. A. Osiyemi at the Forest Herbarium Ibadan where the voucher specimen with number “FHI 109529” was deposited.
2.2. Source of explant
Following dormancy break of the bulbils (after 16 weeks), they were dipped into black polythene bags with holes, filled with top soil and kept in the green house to minimize microbial contamination of plants. Healthy vines with active buds were collected from 12 weeks old plants ofD. bulbiferaraised and maintained in the green house. The vines were excised from the mother plant with single node intact (used as explants). Similarly, explants with single node were excised from underground bulbil (12 weeks old) sprouting after dormancy break but kept indoor away from light source (the sun).
2.3. Micropropagation
Healthy nodal segments (12 weeks old) obtained from the green house and underground bulbil grown indoor (Figure 1A and 1B) were disinfected using a drop of Tween 20 in distilled water in a sterilized bottle with constant shaking for 3 min.

Figure 1. Healthy nodal segments from the green house and underground bulbil grown indoor.A: Purple nodal segments obtained from underground bulbil following dormancy break; B: Greenish nodal segments from green house.
They were rinsed in distilled water until explants wereclean and free from Tween 20. Thereafter, they were soaked in 70% alcohol for 10 min and finally treated with 3% sodium hypochloride for 15 min and then rinsed thoroughly by intermittent shaking with sterilized distilled water (3× ). The surface-decontaminated explants were trimmed on the edges and cut to the required length (1.0-1.5 cm) before vertically in Murashige Skoog (MS) medium-filled culture tubes (one explant per tube)[24].
2.4. Culture medium and conditions
MS medium was used as the basal medium for shoot and root proliferation. The medium was supplemented with 30 g/L sucrose and gelled with 5 g/L agar. The pH of the medium was adjusted to 5.8 prior to autoclaving. The basal medium was fortified with cysteine (20 mg/L), combination of α-naphthaleneacetic acid (NAA) (0.0-1.5 mg/L) and 6-benzylamino purine (BAP) (0.0-1.0 mg/L), NAA (2.0 mg/ L) and NAA [2.0 mg/L+BAP (0.5 mg/L)]. Routinely, 10-15 mL each of molten medium was dispensed into sterile (20×150) mm culture tubes. For each treatment, 10 replicates were used and all the experiments were repeated three times. The cultures were incubated at (27±1) °C with a photoperiod of 16 h at an intensity of 10-20 µmol m-2s-1. After 3-6 weeks of culturing, cultures were subcultured in fresh basal medium depending on the experiments.
2.5. Estimation of growth of D. bulbifera in cultures
Growth of plant was assessed 4-6 weeks. Shoot and root proliferation were scored and measured after 6 weeks of culturing. The growth factors used were number of leaves, callus formation, shoot number, number of nodal segments (buds) on shoots and shoot length.
2.6. Epidermal surface tissue preparation
Fresh leaves of wild andin vitrocultivated plant ofD. bulbiferacut in the standard median position and then soaked in concentrated nitric acid for 3-5 h in a covered Petri dish were used for epidermal study. The Petri dish was placed on a hot water bath until bubbles were noticed on leaves which signified readiness of epidermal peel removal. The epidermal peel was rinsed (5×) to remove concentrated nitric acid and thereafter cleared with soft Carmel hair brush to dissociate tissue remains from the surfaces. The abaxial and adaxial surfaces were carefully mounted on a clean glass slides and dehydrated by graded series 50%, 70%, 80% and 100% ethyl alcohol and later stained with safaranin O for 2 min; excess stain was removed by adding few drops of 70% ethyl alcohol. Glycerin was used as mountant, and epidermises were covered with coverslips and the edges of the slide sealed with nail varnish to prevent dehydration. Slides were examined for assessment of the qualitative diagnostic features such as stomata type, cuticular striation on walls, mucilage and shape of epidermal cell with quantitative parameters such as epidermal cell size, stomata and epidermal wall dimension were recorded.
The mean±SE, minimum and maximum values were calculated for all variables. Photomicrograph (Ziess standard 25) of the epidermises was obtained using Motic camera mounted on a compound light microscope and attached to a Pentium IV computer. The techniques utilized in this study were in accordance with methodology previously reported in literature[25-27].
3. Results
3.1. Growth responses of micropropagated plant
In the present study, MS medium+BAP (0.05 mg/L)+NAA (0.01 mg/L) produced the highest mean root length of (27.00±1.25) mm with an elongated single shoot of mean length (38.00± 11.09) mm (Figure 2A, Table 1) representing 70% explants response after 8 weeks of culturing.

Figure 2. Multiple root formation with single elongated shoot and no shoot and root formation.A: Multiple root formation with single elongated shoot; B: Bud break with single leaf (no shoot and root formation).

Table 1 Callus, shoot, leaf and root formation from nodal segments of D. bulbifera (parameters of growth scored after 6 weeks).
Responses of nodal segments ofD. bulbiferain MS media supplemented with BAP (1.5 mg/L) and NAA (0.3 mg/L) were poor as 20% of growth was recorded with no shoot elongation and root formation after culturing for more than 6 weeks (Figure 3B). MS medium supplemented with BAP (1.0 mg/ L)+NAA (0.2 mg/mL)+cysteine (20 mg/L) cysteine showed significant response of nodal segments ofD. bulbiferawith more than 80% growth response in which bud break was achieved within 2 weeks (Figure 3A and 3B). The quality of shoots and overall growth response in terms of average shoot length and number of leaves was best in this combination in which an average number of leaves (3.00±0.31), average number of shoots (1.33±0.17), average shoot length (53.33±3.50) mm and average root length (25.00±10.61) mm were recorded.

Figure 3. Nodal segments of D. bulbifera with elongated shoots.A: Nodal segments from green house with elongated shoot and leaves; B: Nodal segments from underground bulbil with elongated shoots and root proliferation.
Subculturing of nodal segments ofD. bulbiferaobtained from Figure 3A and 3B after 6 weeks of growth in the same media resulted in formation of multiple shoots, 3.60±0.87 [mean length (49.20±5.71) mm] within 6 weeks of culturing, which was higher compared to multiple shoots, 2.40±0.40 [mean length of (33.60±7.48) mm] (Table 2) obtained from excised nodal segments from green house (Figure 4A and 4B).

Figure 4. Multiple shoots from subcultured nodal segments.A: Multiple shoots obtained from green house; B: Multiple shoots obtained from underground bulbil.
Generally, parameters of growth, such as number of leaves, mean multiple shoots and mean length showed optimal and better growth response in nodal segments obtained from underground bulbils (100% free of contamination) compared with nodal segments obtained from the green house (70% free of contamination) (Table 2). Introduction of excised nodal segments from 6 weeks old plant in fresh MS media+BAP (1.0 mg/L)+NAA (0.2 mg/L)+cysteine (2.0 mg/L) resulted in formation of a single bulbil with aerial root after 4 weeks of culturing (Figure 5A). Similarly, introduction of 6 weeks old cultured plant from MS media+BAP (1.0 mg/L)+NAA (0.2 mg/ L)+cysteine (20 mg/L) in fresh MS media supplemented with NAA (2.0 mg/L)+cysteine (20 mg/L) resulted in 50% production of single nodal bulbil with aerial root attached to the bulbil and profuse whitish callusing at the base of the nodal vine after 8 weeks of culturing (Figure 5B).

Figure 5. Single bulbil with aerial root and single bulbil at the nodal base.A: Formation of single bulbil with aerial root; B: Single bulbil at the nodal base.
In all media treatments, 10%-30% callus was generated (Figure 6A). Also, subculturing of 6 weeks oldin vitroplants from MS media+BAP (1.0 mg/L)+NAA (0.2 mg/L)+cysteine (20 mg/L) in MS media+NAA (1.0 mg/L)+BAP (0.2 mg/L) resulted in profuse white callus at the base after 8 weeks of subculturing with suppressed shoot growth (Figure 6B).

Figure 6. Eight weeks old callus and profuse whitish callus.A: Eight weeks old callus from nodal explants of D. bulbifera; B: Profuse whitish callus with shoot suppression.

Table 2 Subcultured nodal segments from N1 and N2 in same media composition (parameters of growth scored after 6 weeks).
3.2. Macroscopy characteristics
Morphologically, the leaf description of the wild and micropropagated plants were similar in terms of leaf venation which was parallel and vein convergence at the base, leaf shape in both was cordate with long petiole and alternate arrangement, leaf margin was entire with aerial bulbils produced from the node in both wild and micropropagated plants.
3.3. Microscopy of wild and micropropagated plants
Comparative qualitative and quantitative features of foliar epidermis of wild and micropropagatedD. bulbiferaare presented in Table 3. The useful diagnostic features included amphistomatic leaves, anomocytic and anisocytic stomata and smooth epidermal wall as shown in Figures 7 and 8.

Figure 7. Foliar microscopic features of abaxial epidermis of D. bulbifera. A & B: Wild plant; C & D: In vitro plant (400×).
Slight variations included closed stomata pore in the wild and open in thein vitrocultivated plants, cuticularization of epidermal walls was observed in the adaxial and abaxial surfaces of the wild whereas it was noticed only on the abaxial surfaces of thein vitroplant. Wild plant had thicker lignified walls than the micropropagated plant and mass of mucilages were evenly spread in the epidermal cell of the wild but condensed and scattered in thein vitropropagated plant (Figures 7 and 8).

Figure 8. Foliar microscopic features of adaxial epidermis of D. bulbifera. A & B: Wild plant; C & D: In vitro plant (400×).
4. Discussion
Production of plantlets with profuse rootingin vitrois important for successful establishment of regeneratedplants in soil[28]. The combination and interaction of BAP and NAA had been reported to play an important role inin vitropropagation of nodal explants for multiple shoot induction[29]. NAA, an auxin, can effectively induce shoot emergence when combined with an adenine derivative (BAP). It is possible that the combination of BAP (1.0 mg/L)+NAA (0.20 mg/L)1+cysteine (20 mg/L) in this study was responsible for shoot regeneration as control without these plant growth regulators failed to grow after observation in culture medium for more than 6 weeks. Earlier workers reported shoot regeneration of 42%-75% inDioscorea rotundatawhen cultured in MS medium supplemented with NAA (0.1 µmol/ L)+BAP (0.20 µmol/L) and shoot plantlet regeneration of 60%-82% obtained in media made of NAA (0.05 µmol/L)+BAP (0.20 µmol/L) or BAP (0.46 µmol/L)+kinetin (0.50 µmol/L) inDioscorea alata[29]. Similarly, MS medium supplemented with NAA (1.0 mg/L) and BAP (0.5-1.0 mg/L) was reported as the best concentration for multiple shoot bud induction inDioscorea opposita. InDioscorea hispida, BAP (2.0 mg/L)+NAA (0.50 mg/L) with ascorbic acid 100 mg/L, elicited optimal response in shoot in which an average of (6.00±0.18) shootlets with a mean shoot length of (5.00±0.28) cm per explant was recorded[30].
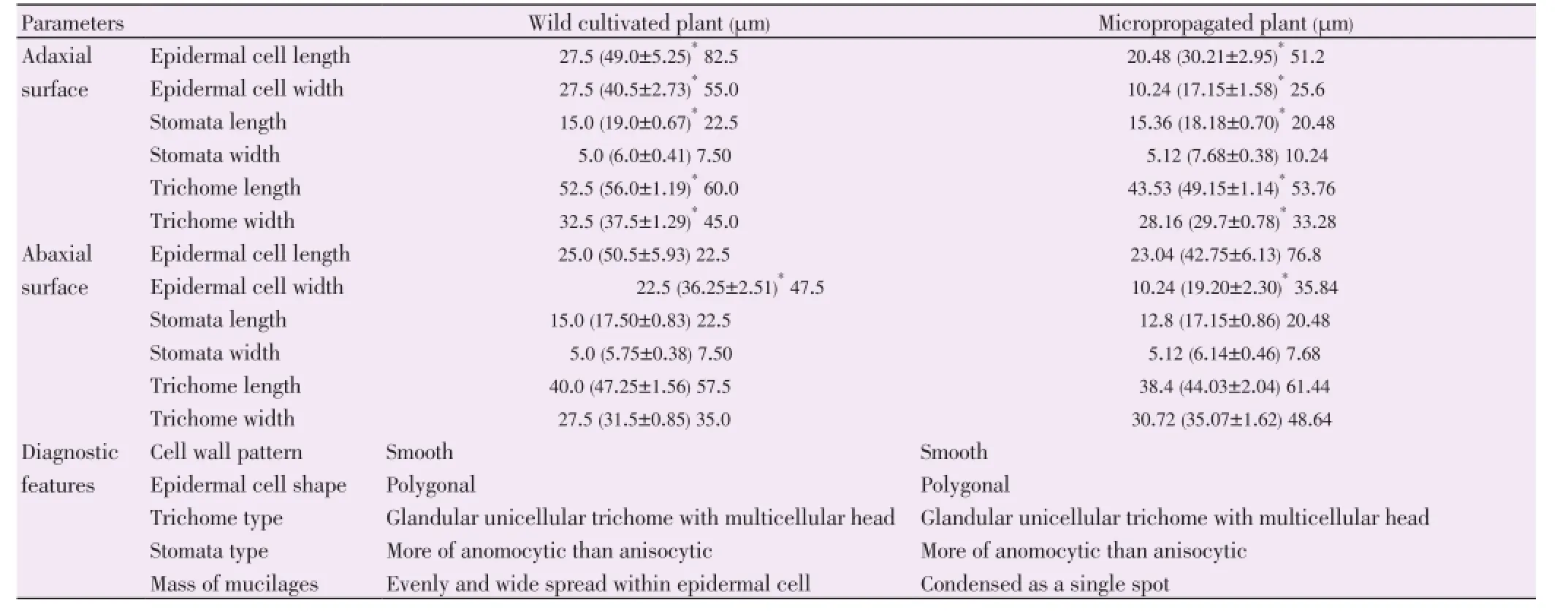
Table 3 Comparative qualitative and quantitative features of foliar epidermis of wild and micropropagated D. bulbifera.
In vitrobulbil induction in this study agrees with a previous study in which incorporation of BAP (2.0 mg/L) in MS medium resulted in the formation of a single oval bulbil from the lowest node ofD. bulbifera[31]. In the same report, NAA (0.50 mg/L) along with BAP (2.0 mg/L) in MS medium induced formation of a maximum of eight spherical bulbils. However, in the present study, induction of bulbil with aerial root (whitish) in MS media+NAA (2.0 mg/L)+cysteine (20 mg/L) is suggested rather than the use of BAP (2.0 mg/L) or NAA (0.50 mg/L)+BAP (2.0 mg/L) in MS medium.
The observation of whitish callus at the nodal base ofD. bulbiferawith resultant effect in suppression of shoot growth may be attributed to the presence of NAA at 1.0 mg/L in the MS media. Naphthalene acetic acid had been reported to inhibit root elongation in a wide range of concentrations from 0.01 µmol/L to 1 µmol/L[32]. The presence of mass of mucilage within the epidermal cell and the presence of glandular unicellular trichomes with multicellular head are indicative of the medicinal lodgments of bioactive principles in the plant and in a way may justify its use in traditional medicine[33]. A combination of these features had been found useful for taxonomic distinction and recognition in angiosperm families.
Mucilages are generally normal products of metabolism formed within the cell (intracellular formation) and or produced without injury to the plant[34]. Mucilages are evenly and widely spread within the epidermal cell of the wild plant than the micropropagated plant. Previous workers have reported that phytochemicals including saponins, polyphenols and mucilage are the likely active components ofDioscoreaspecies[35-37]. Also, mucilages have been reported in the epidermis of senna (Cassia acutifoliaDelile), Alexandrian senna (Cassia augustifoliaVahl),Cassia auriculataLinn. andThespia populneaLinn.[38,39].
Anomocytic stomata type reported in this work agrees with a previous report of anomocytic stomata in the order Dioscorales as in the case ofDioscorea hispida[40]. Similarly, the foliar epidermal anatomy ofDioscorea rotundata,Dioscorea cayenensis,Dioscorea dumetorum, andDioscorea alatawas reported to consist of anomocytic stomata only for the four species ofDioscoreastudied[41]. However, the present anatomical studies of the foliar epidermises ofD. bulbiferarevealed not only anomocytic stomata type but anisocytic. The presence of anisocytic stomata inD. bulbiferatogether with anomocytic stomata may serve as distinguishing character for its identification from otherDioscoreaspecies.
Cuticular striated wall has been reported in the epidermal imprint of the lower surface of the leaf ofD. bulbiferausing non-toxic mucilage[42]. Our observation of cuticular striations on the adaxial and abaxial epidermises of both wild and micropropagatedD. bulbiferais in line with this report and can therefore be used as a diagnostic feature for standardization of the crude drug.
It is well known that most histological traits of plant are under the control of environmental conditions. Organs of an individual plant living under stress undergo adaptive changes which determine their more effective function[43].In vitroculture explants are exposed to specific artificial conditions which differ from those in the natural environment. These specific conditions are responsible for the structural changes occurring in micropropagated plants. The main determinant factors are high relative air humidity, air composition and culture media content. The air humidity in culture vessels is very high ranging from 95%-100% and plant response is to enhance water diffusion into the cells, with subsequent increase in both parenchymatic cell sizes and intercellular spaces. Futhermore, the continuing cell enlargement retards the development of secondary walls (lignification and cutinization) resulting in thinner cell wall, reduced deposition of epicuticular waxes and poor mechanical tissue formation[44]. This supports the thin cell wall observed in the micropropagated plant in this study and may explain the slight variation of anatomical features in the foliar epidermis when compared with the wild. Thin cuticle seems to be a common feature for allin vitrocultivated plant species. Johanssonet al.demonstrated the developmental differences in the cuticle of rose plants which occur in various micropropagation stages[45]. Therefore, in the case of shifting fromin vitrotoex vitrocultures, the cutine is gradually synthesized, with the decrease relative humidity of the medium in which the plant lives.
In water saturated flask atmosphere such as the one presented in tissue culture experiments, the accumulation of specific gases (ethylene) in the confined atmosphere causes a dramatic increase in the transpiration rate leading to the failure of stomata to close[46]. This indeed may explain the opening of stomata pore and possibly the reason for having larger average width of stomata sizes in the micropropagated plant [adaxial surface (7.68±0.38) µm and abaxial surface (6.14±0.46 µm)] compared to the wild. Generally, mean lengthof stomata size, epidermal cell size and trichomes of the abaxial and adaxial surfaces in wild plant were larger than micropropagatedD. bulbiferawhile the average epidermal trichome in abaxial surface was larger compared with the wild.
Morphological, anatomical and phytochemical investigations are the integral part of herbal science. Anatomical characters can help in the identification of plant when morphological features are not distinct. Microscopic evaluation is an indispensable tool for identification of medicinal herbs and is one of the essential parameter in modern monograph. In this regard, the important microscopic features of the leaf ofD. bulbifera(an important medicinal plant) such as anomocytic and anisocytic stomata, glandular trichomes with unicellular stalk and multicellular head, cuticular striations, presence of mucilage within epidermal cell and polygonal epidermal wall with smooth surfaces may serve as useful diagnostic features.
In conclusion, the diagnostic features reported in the wild and micropropagated plants could serve as a basis for proper identification and authentication ofD. bulbifera.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are grateful to the management of National Centre for Genetic Resources and Biotechnology for providing technical support and an enabling environment during the study. Mr. O. A. Osiyemi is gratefully acknowledged for plant identification. This work was financially supported by University of Ibadan Senate Research Grant (Grant No. SRG/FP/2010/4A).
Comments
Background
Micro morphological characters are indispensable in the identification and authentication of medicinal plants samples. These have been employed in this paper to confirm the identity of the wild and micro propagated samples ofD. bulbifera.
Research frontiers
The near endangered status ofD. bulbiferamakes it imperative to find appropriate means of its conservation. However, we can only conserve what we know hence the need to be sure that what is propagatedin vitrois the same plant in the wild. This study combined taxonomic skill with tissue culture.
Related reports
The use of micro-propagation techniques for endangered plant genetic resources conservation was recognized late in the seventies (Ivanovaet al., 2011). The techniques utilized in this study were in accordance with methodology previously reported in literature (Saheed and Illoh, 2010; Smillie and Khan, 2010; Santoset al., 2008).
Innovations and breakthroughs
Though many papers have reported the importance of micro characters, this report focused on the comparison between wild and micro propagated relatives using such characters and thus introducing another research dimension in biodiversity conservation.
Applications
The study further confirms the taxonomic importance of micro morphological characters in plant taxa as well as the identification of crude drug samples even when fragmentary.
Peer review
This is a good paper elucidating the importance of micro characters in the confirmation of the identity of both micro propagated and wild samples ofD. bulbifera. The paper also provided information on the best conditions for obtaining a high level of genetic materials for the conservation of the plant.
[1] Wilkin P. Dioscoreaceae of South-Central Africa. Kew Bull 2001; 56(2): 361-404.
[2] Islam MT, Chowdhury RU, Afroz R, Rahman S, Haque MM. Characterization and maintenance of yam (Dioscorea spp.) germplasm. Bangladesh J Agric Res 2011; 36(4): 605-621.
[3] Coursey DG. Yams: An account on the nature, origin, cultivation and utilization of the useful members of Dioscoreaceae. London: Longmans; 1967, p. 230.
[4] Kay DE. Root crops. London: Tropical Development and Research Institute; 1987, p. 343.
[5] Okeke EC, Eneobong HN, Uzuegbunam AO, Ozioko AO, Kuhnlein H. Igbo tradional food system: documentation, uses and research needs. Pak J Nutr 2008; 7(2): 365-376.
[6] Ivanova T, Gussev C, Bosseva Y, Stoeva T. In vitro conservation of micro-propagated Ruscus aculeatus L. (Liliaceae) plants. Botanica Serbica 2011; 35(1): 61-66.
[7] Pence VC. Evaluating costs for the in vitro propagation and preservation of endangered plants. In vitro Cell Dev Biol-Plant 2011; 47(1): 176-187.
[8] Soares JD, Pasqual M, De Araujo AG, De Castro EM, Pereira FJ, Braga FT. Leaf anatomy of orchids micrpropagated with different silicon concentrations. Acta Sci Agron 2012; 34(4): 413-421.
[9] Paunescu A. In vitro and in vivo variability of histological traits of Dianthus callizonus (Caryophyllaceae) aerial vegetative organs.Phytologia Balcanica 2008; 14(3): 417-423.
[10] Essiett UA, Bala DN, Agbakahi JA. Pharmacognostic studies of the leaves and stem of Diodia scandens Sw in Nigeria. Arch Appl Sci Res 2010; 2(5): 184-198.
[11] Yasmiri G, Khan MA, Shaheen N, Hayat MQ. Micromorphological investigation of foliar anatomy of genera Aconogonon and Bistorta of family Polygonaceae. Int J Agric Biol 2009; 11(3): 285-289.
[12] Abere TA, Onwukaeme DN, Eboka CJ. Pharmacognostic evaluation of the leaves of Dissotis rotundifolia Triana (Melastomataceae). Afr J Biotechnol 2009; 8(1): 113-115.
[13] El-Sayed ZI, Ateya AM, Fekry M. Macro and micromorphological study of the leaf, stem, flower and root of Hibiscus rosa-sinensis L. J Appl Sci Res 2012; 8(1): 34-56.
[14] Sonibare MA, Jayeola AA, Egunyomi A, Murata J. A survey of epidermal morphology of the genus Ficus Linn. (Moraceae). Bot Bull Acad Sin 2005; 46: 231-238.
[15] Adedeji O, Jewoola OA. Importance of leaf epidermal characters in the Asteraceae family. Not Bot Horti Agrobo 2008; 36(2): 7-16.
[16] Sofowora A. Medicinal plants and traditional medicine in Africa. Hoboken: John Wiley and Sons Ltd.; 2008, p. 200-204.
[17] Ahmed Z, Chishti MZ, Johri RK, Bhagat A. Antihyperglycemic and antidyslipidemic activity of aqueous extract of Dioscorea bulbifera tubers. Diabetologia Croatica 2009; 38(3): 63-72.
[18] Wang J, Ji L, Liu H, Wang Z. Study of the hepatotoxicity induced by Dioscorea bulbifera L. rhizome in mice. Biosci Trends 2010; 4(2): 79-85.
[19] Gao H, Wu L, Kuroyanagi M. Seven compounds from Dioscorea bulbifera L. Nat Med 2001; 55(5): 277.
[20] Wang JM, Ji LL, Branford-White CJ, Wang ZY, Shen KK, Liu H, et al. Antitumour activity of Dioscorea bulbifera L. rhizome in vivo. Fitoterapia 2012; 83(2): 388-394.
[21] Mbiantcha M, Kamanyi AR, Teponno AL, Tapondjou B, Watcho P, Nguelefack T. Analgesic and anti-inflammatory properties of extracts from the bulbils of Dioscorea bulbifera L. var sativa (Dioscoreaceae) in mice and rats. Evid Based Compl Med 2011; doi: 10.1155/2011/912935.
[22] Kuete V, Betrandteponno R, Mbaveng AT, Tapondoju LA, Meyer JJ, Barboni L, et al. Antibacterial activities of the extract, fractions and compounds from Dioscorea bulbifera. BMC Complement Altern Med 2012; 12: 228.
[23] Shriram V, Jahagirdar S, Latha C, Kumar V, Puranik V, Rojatkar S, et al. A potential plasmid-curing agent, 8-epidiosbulbin E acetate, from D. bulbifera L. against multidrug-resistant bacteria. Int J Antimicrob Agents 2008; 32(5): 405-410.
[24] Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 1962; 15: 473-497.
[25] Saheed SA, Illoh HC. A taxonomic study of some species in Cassiinae (Leguminosae) using leaf epidermal characters. Not Bot Horti Agrobo 2010; 38(1): 21-27.
[26] Smillie TJ, Khan IA. A comprehensive approach to identifying and authenticating botanical products. Clin Pharmacol Ther 2010; 87(2): 175-186.
[27] Santos L, Thadeo LD, Larema L, Alves RM, Ferreira FA. Foliar anatomy and histochemistry in seven species of Eucalyptus. Rev Árvore 2008; 32(1): 769-779.
[28] Bejoy M, Dan M, Anish NP, Anjana RG, Nair BJ, Radhika K, et al. Micropropagation of an Indian ginger (Curcuma vamana Sabu and Mangaly): a wild relative of turmeric. Biotechnol 2012; 11(6): 333-338.
[29] Diaz MS, PalacioL, Figueroa AC, Goleniwski ME. In vitro propagation of Muna-Muna (Clinopodium odorum (Griseb.) Harley). Biotechnol Res Int 2012; doi: 10.1155/2012/196583.
[30] Behera KK, Sahoo S, Prusti AB. Effect of plant growth regulator on in vitro micropropagation of bitter yam (Dioscorea hispida Dennst.). Int J Integr Biol 2008; 4(1): 50-54.
[31] Asha KI, Nair GM. In vitro bulbil induction in Dioscorea species. J Root Crops 2007; 33(2): 81-87.
[32] Alarcón MV, Lloret PG, Iglesias DJ, Talón M, Salguero J. Comparison of growth responses to auxin 1-naphthalene acetic acid and the ethylene precussor 1-aminocyclopropane-1-carboxylic acid in maize seedling root. Acta Biol Cracov Ser Bot 2012; 54: 16-23.
[33] Ajayi GO, Kadiri AB, Egbedi ME, Oyeyemi OO. Pharmacognistic study of two medicinal species of Rytigynia (Rubiaceae) from Nigeria. Phytologia Balcanica 2011; 17(3): 355-359.
[34] Umeshkumar MD, Vilas ND, Diniesh MS. Natural gums and mucilage’s in NDDS: applications and recent approaches. Int J PharmaTech Res 2012; 4(2): 799-814.
[35] Sautour M, Mitaine-Offer A, Lacaille-Dubois M. The Dioscorea genus: a review of bioactive steroid saponins. J Nat Med 2012; 61(2): 91-101.
[36] Murugan M, Mohan VR. In vitro antioxidant studies of Dioscorea esculenta (Lour.) Burkill. Asian Pac J Trop Biomed 2012; 2(Suppl 3): S1620-S1624.
[37] Prakash G, Hosetti BB. Bio-efficacy of Dioscorea pentaphylla from Midmid-western Ghats, India. Toxicol Int 2012; 19(2): 100-105.
[38] Wadekar J, Sawant R, Honde B. Anthelmintic and antibacterial potential of Cassia auriculata roots. Int J Pharm Res 2011; 1(2): 93-98.
[39] Venkata AS, Sai KD. Pharmacognostic and pharmaceutical studies of Thespesia populnea Linn. J Chem Pharm Res 2011; 3(4): 237-244.
[40] Salmah T, Nashriyah M, Abdul-Ghani Y, Shamsul BA. Anatomical study, petiole, leaf, tuber, root and flower of Dioscorea hispida Dennst. (Dioscoreaceae) by using optical microscope, SEM and TEM. J Agrobiotechnol 2013; 4: 32-41.
[41] Aina OD, Atumeyi S. Foliar epidermal anatomy of four species of Dioscorea. Adv Appl Sci Res 2011; 2(4): 21-24.
[42] Shah GL, Gopal BV. Preparation of epidermal imprints of living plant organs by the use of non toxic mucilage and latex. Stan Technol 1969; 44(3): 123-126.
[43] Yang F, Miao LF. Adaptive responses to progressive drought stress in two poplar species originating from different attitude. Silva Fennica 2010; 44(1): 23-37.
[44] Singh IP, Parthasarathy VA, Handique PJ. Comparative growth of micropropagated plantlets and seedlings of citrus varieties. Agrotrópica 2003; 15(1): 9-16.
[45] Johansson M, Kronestedt-Robards EC, Robards AW. Rose leaf structure in relation to different stages of micropropagation. Protoplasma 1992; 166(3-4): 165-176.
[46] Kevers C, Franck T, Strasser RJ, Dommes J, Gaspar T. Hyperhydricity of micropropagated shoots; a typically stressinduced change of physiological state. Plant Cell Tiss Organ Cult 2004; 77(2): 181-191.
10.1016/S2221-1691(14)60228-8
*Corresponding author: Dr. Mubo A. Sonibare, Senior Lecturer, PhD, Department of Pharmacognosy, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria.
Tel: 00234-8134901273
E-mail: sonibaredeola@yahoo.com
Foundation Project: Supported by University of Ibadan Senate Research Grant (Grant No. SRG/FP/2010/4A).
Article history:
Received 12 Dec 2013
Received in revised form 19 Dec, 2nd revised form 26 Dec, 3rd revised form 29 Dec 2013
Accepted 27 Jan 2013
Available online 28 Mar 2014
Methods:Growth responses of micropropagated D. bulbifera were observed on Murashige Skoog medium supplemented with 6-benzylamino purine (1.0 mg/L)+α-naphthaleneacetic acid (0.2 mg/ L)+cysteine (20 mg/L) using nodal segments as explants. Leaves of the wild and micropropagated plants were studied microscopically.
Results:More than 80% shoot regeneration and formation of 10%-30% whitish-brown callus were observed within 3 weeks. The highest root proliferation was obtained from Murashige Skoog medium of 6-benzylamino purine (0.05 mg/L) and α-naphthaleneacetic acid (0.01 mg/L) with mean root length of (27.00±1.25) mm and elongated single shoot of mean length (38.00±11.09) mm. Leaf epidermal features that revealed similarities between the wild and micropropagated plants included amphistomatic condition, presence of mucilage, glandular unicellular trichome with multicellular head, polygonal cells with smooth walls, stomata type and shape. Slight variations included thick cuticular wall with closed stomata in wild plant compared to thin walled opened stomata in the in vitro plant. Opening of stomata accounted for larger average stomata sizes of (7.68±0.38) µm and (6.14±0.46) µm on the adaxial and abaxial surfaces, respectively of the micropropagated plant compared to the wild.
Conclusions:The diagnostic features obtained in the study could serve as a basis for proper identification for quality control for standardization of the medicinal plant.
 Asian Pacific Journal of Tropical Biomedicine2014年3期
Asian Pacific Journal of Tropical Biomedicine2014年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Isolation and structural elucidation of cytotoxic compounds from the root bark of Diospyros quercina (Baill.) endemic to Madagascar
- 15-Lipoxygenase inhibition of Commelina benghalensis, Tradescantia fluminensis, Tradescantia zebrina
- Phytochemical constituents and antibacterial activity of some green leafy vegetables
- Acute and subacute oral toxicity study on the flavonoid rich fraction of Monodora tenuifolia seed in albino rats
- Chromatographic finger print analysis of anti-inflammatory active extract fractions of aerial parts of Tribulus terrestris by HPTLC technique
- Survey on cattle ticks in Nur, north of Iran
