Acute and subacute oral toxicity study on the flavonoid rich fraction of Monodora tenuifolia seed in albino rats
Raphael Chukwuma Ekeanyanwu, Obioma Uzoma Njoku
1Department of Biochemistry, Faculty of Sciences, Imo State University Owerri, Imo State, Nigeria
2Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria Nsukka, Enugu State, Nigeria
Acute and subacute oral toxicity study on the flavonoid rich fraction of Monodora tenuifolia seed in albino rats
Raphael Chukwuma Ekeanyanwu1*, Obioma Uzoma Njoku2
1Department of Biochemistry, Faculty of Sciences, Imo State University Owerri, Imo State, Nigeria
2Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria Nsukka, Enugu State, Nigeria
PEER REVIEW
Peer reviewer
Prof. Dr. Ngbolua Koto-te-Nyiwa, PhD, Department of Biology, Faculty of Science, University of Kinshasa, Democratic Republic of the Congo
Tel: +243-8168-79527
E-mail: jpngbolua@unikin.ac.cd
Comments
This is an interesting study in which authors have demonstrated the safety of M. tenuifolia in rodent. Toxicological bioassays were assessed based on biochemical and hematological parameters, and histopathological observations. Flavonoid rich fraction of M. tenuifolia seeds were found to be no toxic and could be served as nutraceuticals.
Details on Page 201
Objective:To investigate the effects of the flavonoid rich fraction of Monodora tenuifolia seed on the haematology, histopathology and liver profile of Wistar albino rats.
Monodora tenuifolia, Toxicity, Haematological parameters, Biochemical parameters, Histology
1. Introduction
Monodorais a genus of plant in the family Annonaceae. It contains approximately 35 species, distributed throughout tropical Africa. Two of the species,MonodoramyristicaandMonodora tenuifolia(M. tenuifolia) are widely used as spices.M. tenuifoliais a plant with a rich ethnobotanical history. In traditional medicine practice, it is widely used to relieve toothache, dysentery, diarrhoea, dermatitis, headache, and as vermifuge[1].M. tenuifoliais a spice that has been shown to possess potent antioxidant activity[2]. Therefore, it can be hypothesized to be effective in the management and treatment of diseases caused by oxidative stress.
Spices have enjoyed widespread use for the treatment of several ailments, but still little is known about their toxicity and safety issue which are always a concern. Investigations on spices provide evidence for the presence of substances that offer potential human health benefits. However, it should be a vital requirement to determine the toxic effects of some of the substances contained in these plants[3]. Toxicity is an expression of being poisonous, indicating the state of adverse effects led by the interaction between toxicants and cells[4]. This interaction may varydepending on the chemical properties of the toxicants and the cell membrane, as it may occur on the cell surface, within the cell body, or in the tissues beneath as well as at the extra cellular matrix. The toxic effects may take place prior to the binding of the toxicants to the vital organs such as liver and kidneys. Hence, evaluation of toxic properties of a substance is crucial when considering for public health protection because exposure to chemicals can be hazardous and results to adverse effects on human being. In practice, the evaluation typically includes acute, subacute, chronic, carcinogenic and reproductive effects. The present study therefore aims at investigating the effects of the flavonoid rich fraction ofM. tenuifoliaseed on the haematology, histology and liver profile of Wistar albino rats as part of a wider study to evaluate the pharmacological potential of the seed, thereby evaluating its safety.
2. Materials and methods
2.1. Plant materials
Fresh seeds ofM. tenuifolia(Figure 1) were obtained between the month of May and June, 2013 from fruits harvested from the plant growing within the University of Nigeria Nsukka, Enugu State. The fruits and hence the seeds were authenticated by a botanist in the Department of Botany, University of Nigeria through comparison with a voucher specimen present in the herbarium.

Figure 1. M. tenuifolia seeds.
2.2. Experimental animals
All the experimental animals used were obtained from the animal house of the Zoological Garden, University of Nigeria Nsukka.
2.3. Preparation of M. tenuifolia seed flour
The fresh seeds ofM. tenuifoliawere shade dried for 48 h, then reduced in size to a coarse texture using a manual blender (Corona, Landers Colombia) and packed in airtight containers.
2.4. Extraction of flavonoid rich fractions
The aqueous-alcoholic extract and fractions of groundM. tenuifoliaseed was obtained by solvent-solvent extraction technique according to the method described by Harborne[5] with slight modifications. A quantity, 5.5 kg ground seeds ofM. tenuifoliawas macerated twice with 10 L of 70% ethanol for 72 h at room temperature. The combined maceration was passed through Whatman No.4 filter paper and mixed thoroughly with 1.8 L of chloroform to partition the aqueous-alcoholic extract. Two distinct layers were obtained, the upper aqueous layer and the lower chloroform layer. The two layers were drawn out separately and the chloroform fraction was dried in vacuo, weighed and called the chloroform fraction. A quantity, 2.75 L of the main (aqueous portion) extract was extracted three times with an equal volume of ethyl acetate for 48 h at room temperature. The ethyl acetate soluble extracts were pulled together and dried in vacuo, weighed and called the ethyl acetate fraction. The remaining aqueous extracts were stored away. Then 11.6 g of the dried ethyl acetate fraction was suspended in a relatively small volume (200 mL) of absolute methanol and allowed to settle. The dissolved part was separated from the rest by filtration through Whatman No.4 filter paper and then concentrated in vacuo weighed and called methanol fraction. The total flavonoid content of the different fraction was determined quantitatively by spectrophotometer method.
2.5. Total flavonoid concentration of fractions
The total flavonoid content of the fractional extracts and standard was determined according to the method of Changet al[6].
2.6. Toxicity study (LD50) of the flavonoid rich fraction
Acute toxicity study of the flavonoid rich fraction was carried out according to the method of Lorke[7] using 39 albino mice of both sexes of average weight between 13.2-19.2 g, that were dosed orally with different gradual doses (10-5 000 mg/kg body weight). In the first phase, mice were divided into three groups of nine mice each and were treated with the extract at doses of 10, 100 and 1 000 mg/kg body weight orally by means of a cannula. They were observed for 24 h for signs of toxicity, mortality and general behaviours. In the second phase, twelve mice were divided into four groups of three mice each and were also administered with the flavonoid rich fraction at doses of 1 000, 1 600, 2 900, and 5 000 mg/kg body weight orally. They were observed for 24 h for signs of toxicity, mortality and general behaviours. The LD50was calculated as the geometric mean of the highest non-lethal dose (with no death) and the lowest lethal dose (where death occurred).
LD50=√minimum toxic dose×maximum tolerated dose
2.7. 14 and 28 d oral toxicity study on the flavonoid fraction
2.7.1. Animal study
Adult male Wistar rats of age 8 weeks were used for the subacute toxicity profiling. They were fedad libitumwith standard feed, and had free access to water. They were also maintained under standard conditions of humidity, temperature, and 12 h light/dark cycle. The animals were acclimatised for a week before the commencement of the study. A standard protocol was drawn up in accordance with current guidelines for the care for laboratory animals and ethical guidelines for investigations of experiments in conscious animals[8].
2.7.2. Dosing
Twenty four albino rats of average weight between 95 to 140 g were selected by stratified randomisation and then divided into four groups of six rats. Group I, Group II, and Group III were given 100, 200 and 400 mg/kg body weight respectively of the flavonoid rich fraction orally for 14 d and 28 d respectively. One percent of DMSO served as the vehicle and was used to prepare the doses. Group IV served as the control group and received the diluted vehicle only. The first day of dosing was taken as Day 0 and blood was collected on Day 14 and 28 respectively and used for biochemical, haematological and histopathological analysis.
2.7.3. Mortality and clinical signs (General behaviour)
During the four-week dosing period, all the animals were observed daily for clinical signs and mortality patterns once before dosing, immediately after dosing and up to 2-4 h after dosing.
2.7.4. Weekly body weight measurement
The body weight of each rat was expressed using a sensitive balance during the acclimatisation period, once before commencement of dosing, once weekly during the period and once on the day of sacrifice.
2.7.5. Determination of haematological parameters
Blood samples were collected by the orbital technique. Blood sample for haematological determinations was collected from the retrobulbar plexus of the medial canthus of the eye to puncture the retrobulbar plexus and thus enable outflow of blood into a sample bottle containing EDTA. The sample bottle was shaken gently to mix up the blood with EDTA and prevent clothing. The values of the red blood cells (RBCs) count, total and differential white blood cells (WBCs) count, packed cell volume (PCV), erythrocyte sedimentation rate and haemoglobin (Hb) content were determined. The quantities of RBCs and WBCs were determined with the improved Neubauer Haemocytometre[9]. PCV was determined spectrophotometrically using the cyanomet haemoglobin method[10]. The erythrocyte sedimentation rate was determined by the method of Westergren as described by Mbakaet al[9]. All haematological parameters were determined at room temperature (27±0.5) °C.
2.7.6. Determination of biochemical parameters
Blood was collected by orbital technique. The blood sample was kept at room temperature for 30 min to clot. Afterwards, the test tube containing the clotted blood sample was centrifuged at 3 000 r/min for 10 min using a table centrifuge to enable a complete separation of the serum from the clotted blood. The clear serum supernatant was then carefully aspirated with syringe and needle and stored in a clean sample bottle for the biochemical tests. The values of blood glucose, total serum protein, serum albumin, serum cholesterol, serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), serum alkaline phosphatase (ALP), serum urea and serum creatinine were determined following standard laboratory procedures. All haematological parameters were determined at room temperature (27.0±0.5) °C.
2.7.7. Organ weight
The liver, kidney and heart of rats in the various groups were excised on the Day 14 and 28 immediately after blood collection. Following excision, the organs were trimmed of extraneous tissues, placed on a saline soaked gauze pad to retard desiccation and were immediately weighed (paired organs were weighed together) to one decimal place and calculated for organ weight ratio[11].
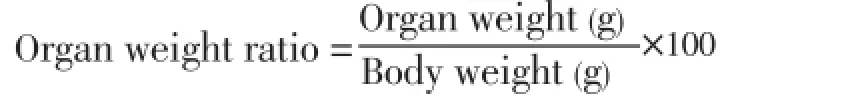
2.7.8. Histopathological examination
For postmortem, the rats in the various groups were dissected and careful examination of the liver was carried out. Tissue samples were fixed in 10% formalin and dehydrated overnight using upgraded ethanol series and embedded in paraffin blocks. Ultrathin sections were dewaxed by xylene, hydrated through a degraded ethanol series, and stained with haematoxylin and eosin. A pathologist, blinded to the treatments, performed the histopathologic examination with an optical microscope [Nikon Eclipse E600, USA (×400)]. Sections were assigned grades as reported by Djamiet al[12].
2.8. Statistical analysis
Values of treated groups were compared statistically with control by Independent Samplet-test, One-way ANOVA and Spearman correlation. Inferences were made from findings at 95% confidence level. Data obtained were presented as mean ±standard deviation and analysed by simple percentages.
3. Results
The effect of the flavonoid rich fraction of the aqueous alcoholic extract of the seed ofM. tenuifoliawas investigated on thehaematology, histopathology and liver profile of Wistar albino rats as part of a wider study to evaluate the pharmacological potential of the seed, thereby evaluating its safety.
3.1. Phytochemical study
3.1.1. Flavonoid determination
Table 1 shows the result of the total flavonoid content of the chloroform fraction, ethyl acetate fraction and methanol fraction of the aqueous-alcoholic extract ofM. tenuifoliaseed. The total flavonoid content of chloroform fraction, ethyl acetate fraction and methanol fraction was found to be (12.10 ±0.01) mg/g, (12.50±0.04) mg/g and (9.20±0.01) mg/g in terms of quercetin equivalent respectively.

Table 1 Total flavonoid content of the different fractions.
3.2. Toxicological testing
3.2.1. Acute toxicity assay
Table 2 shows the result of the acute toxicity study (LD50) of the flavonoid rich fraction ofM. tenuifoliaseed. There was no sign of toxicity or mortality up to the dose of 1 000 mg/kg. However, there was partial decrease in activity and increased respiratory rate in mice which lasted few minutes after administration of extract.
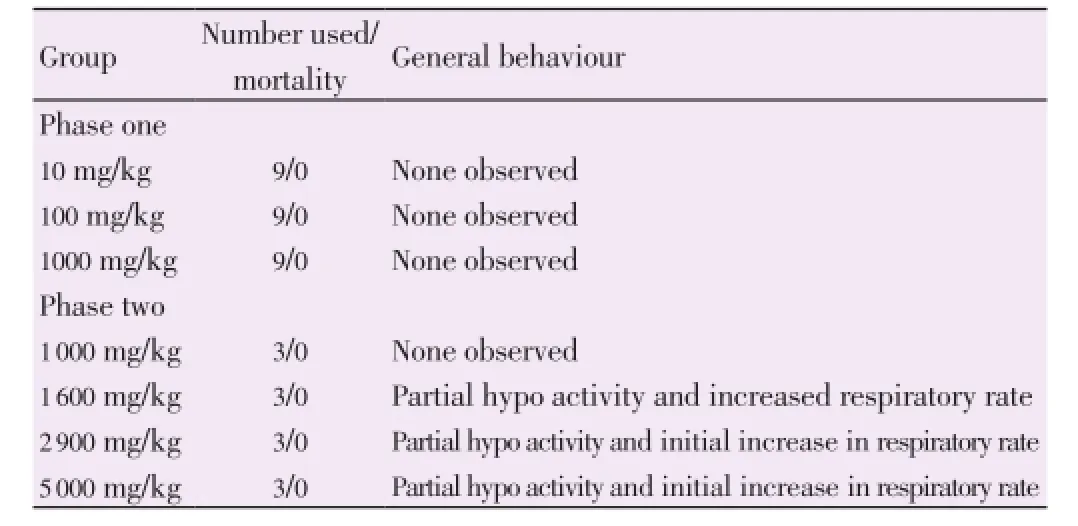
Table 2 Acute toxicity study on the flavonoid rich ethyl acetate fraction.
3.2.2. Mortality and clinical signs (general behaviour)
Table 3 shows the effect of oral administration of the flavonoid rich fraction ofM. tenuifoliaseed on the general behaviour of the rats. There was no noticeable deviation in the behaviour of the rats treated with 100, 200 and 400 mg/kg compared to that of the control.

Table 3 Effect of subacute administration of flavonoid rich fraction of M. tenuifolia seed on the general behaviour of the rats.
3.2.3. Body weight changes in rats
Table 4 illustrates the effect ofM. tenuifoliaon the changes in bodyweight of rats over the dosing. Overall, the rats grew at rates of (2.13±0.04), (2.20±0.56), (2.11±0.24) and (2.22±0.23) g/d respectively for the control, 100, 200, and 400 mg/kg dose groups respectively. After 28 d of exposure toM. tenuifolia, an increase in growth in all the groups was observed. The result suggested that the 28 d of subacute oral ingestion ofM. tenuifoliadid not affect the weight of rats.

Table 4 The effect of M. tenuifolia on the changes in body weight of rats over the dosing period.
3.2.4. Weight of organs
Table 5 illustrates the organ weight and relative organ weight of rats. There was significant (P<0.05) increase in liver of rats in group IV treated with flavonoid rich fraction of the seed extract ofM. tenuifoliaafter the Day 14 but no significant (P>0.05) difference was observed in liver of rats after the Day 28. No significant (P>0.05) difference was observed in kidney and heart for the groups treated with flavonoid rich fraction ofM. tenuifoliaafter the Day 14 and 28.
3.2.5. Biochemical markers
Table 6 shows the effect of the flavonoid rich fraction ofM. tenuifoliaseed on kidney function and liver function parameters. After the Day 14, AST was significantly (P<0.05) reduced in Group IV. There was also significant (P<0.05) decrease in ALT in Group II and III and a significant decrease (P<0.01) in Group IV. Alkaline phosphatase was markedly increased in the entire treated group but not significantly (P>0.05) different from the control. There was dose dependent decrease in total serum protein for all treated groups but not significantly (P>0.05) different from the control group. There was no statistically significant (P>0.05) difference in urea, creatinine, albumin and globulin. The ratio of albumin to globulin was elevated in all the treated groups but was not significantly (P<0.05) different from the control. After the Day 28, there was no significant (P>0.05) difference in AST, ALP, urea, creatinine, total protein, albumin and globulin in all the groups compared to the control. The ratio of albumin to globulin was not significantly (P>0.05) different in all treated groups compared to the control.

Table 5 Organ weight and relative organ weight of rats in parenthesis.

Table 6 Levels of biochemical markers of kidney profile and liver profile in rats administered flavonoid rich fraction of M. tenuifolia.
3.2.6. Haematological parameters
Table 7 shows the effect of flavonoid rich fraction of the seed extract ofM. tenuifoliaon haematological parameters in rats. After the Day 14, there was no significant (P>0.05) effect on total WBC in treated group compared to the control group. There was no significant (P>0.05) effect on the basophil, eosinophil, lymphocyte, monocyte and neutrophil count in the treated groups compared to the control group. RBC, Hb, erythrocyte sedimentation rats, and packed cell volume were not significantly different (P>0.05) in the test groups compared to the control group. There was also no significant (P>0.05) effect on mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) in the treated groups compared with the control group. After the Day 28, packed cell volume and haemoglobin concentration were elevated significantly (P<0.05) in Group IV compared to the control group. Erythrocyte sedimentation rate was decreased significantly (P<0.05) in Group IV compared to the control group. There was an increase in RBC concentration but not significantly. However total WBC, basophil, eosinophil, lymphocyte, monocyte, neutrophil, MCV, MCH, MCHC were not significantly (P>0.05) affected in the treated groups compared to the control group.
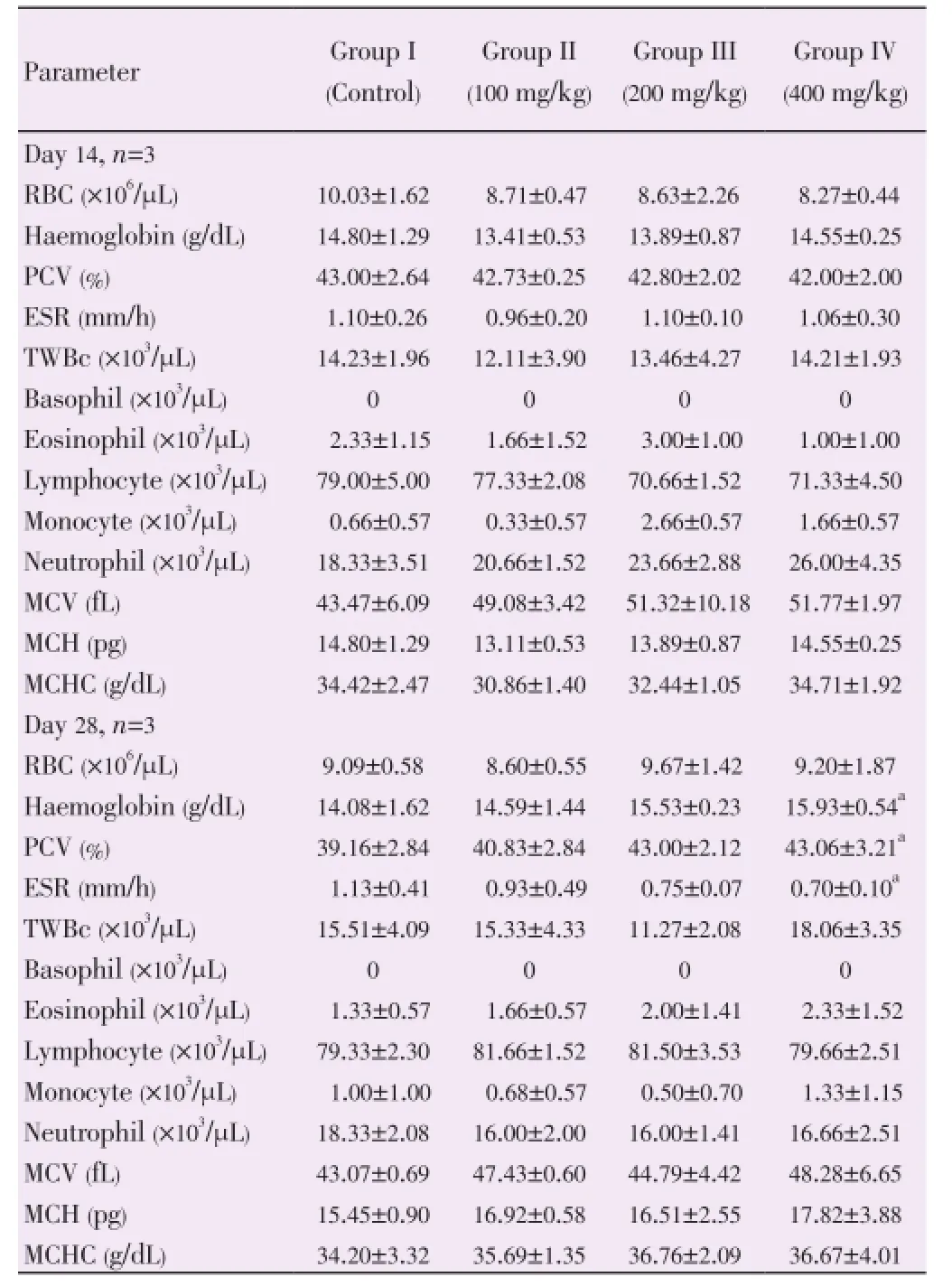
Table 7 Levels of some haematological markers in rats on ethyl acetate seed extract of M. tenuifolia.
3.2.7. Results of histopathological assessment of organs
Figure 2 illustrates the photomicrograph of liver section from Group I (Control) rats showing normal portal area. There was no abnormal activity seen around the bile duct area and blood vessel after the Day 14 showing normal portal activity. Figure 3 and 4 illustrates the photomicrograph of liver section from rats given 100 mg/kg and 200 mg/kg of the flavonoid rich fraction ofM. tenuifoliaseed respectively showing peribiliary hepatitis. There was mobilisation of inflammatory cells around the bile duct area after the Day 14 showing peribilliary hepatitis. Figure 5 illustrates the photomicrograph of liver section from rats given 400 mg/kg of the flavonoid rich fraction ofM. tenuifoliaseed showing portal hepatitis. There was mobilisation of inflammatory cells around the bile duct area and blood vessels after the Day 14 showing complete portal hepatitis.

Figure 2. Photomicrograph of liver section from Group I (Control) rats showing normal portal area.BV: blood vessel; Arrow: bile ducts. H and E×40.

Figure 3. Photomicrograph of liver section from rats given 100 mg/kg of the flavonoid rich fraction of M. tenuifolia seed showing peribilliary hepatitis.Arrow: inflammatory cells; BD: bile duct. H and E ×40.

Figure 4. Photomicrograph of liver section from rats given 200 mg/kg of the flavonoid rich fraction of M. tenuifolia seed showing peribilliary hepatitis.Arrows: inflammatory cells; BD: bile duct showing cholestasis. H and E ×40.

Figure 5. Photomicrograph of liver section from rats given 400 mg/kg of the flavonoid rich fraction of M. tenuifolia seed showing portal hepatitis.Arrow: inflammatory cells; BD: bile duct. H and E ×40.
4. Discussion
To determine the safety of drugs and plants products for human use, toxicological evaluation is carried out in various experimental animals to predict toxicity and to provide guidelines for selecting a “safe” dose in humans. The highest overall concordances of toxicity in animals with humans are with haematological, gastrointestinal and cardiovascular adverse effects[13], with certain adverse effects in humans, especially hypersentivity and idiosyncratic reactions, and are poorly correlated with toxicity observed in animals. Furthermore, it is quite difficult to ascertain adverse effects in animals such as headache, abdominal pain, dizziness, and visual disturbances. In addition, interspecies differences in the pharmacokinetic parameters make it difficult to translate some adverse effects from animals to humans.
Chloroform was the highest extracting solvent amongstthe other solvents because it generally extracted the highest quantity of plant material from the aqueous alcoholic extract compared to ethyl acetate. It shows that the seeds ofM. tenuifoliacontain more nonpolar material than polar material since chloroform extracted the highest quantity of plant material. The total flavonoid content of chloroform fraction, ethyl acetate fraction and methanol fraction was found to be (12.10±0.01) mg/g, (12.50±0.04) mg/g and (9.20± 0.01) mg/g in terms of quercetin equivalent respectively. The ethyl acetate fraction had the highest content of total flavonoid and was used as the flavonoid fraction in subsequent animal studies.
Acute toxicity tests are generally the first test conducted in any toxicity study. They provide data on the relative toxicity likely to arise from a single brief exposure to any substance. Different plants extracts have been known to possess different levels of bioactive compounds inherent in the plants[14]. In the acute toxicity study, flavonoid rich fraction ofM. tenuifoliaseed extract did not show any mortality up to the dose of 5 000 mg/kg during the observational period of 48 h. However, minor changes in behaviour, breathing, and activity were observed in mice administered 1 600, 2 900 and 5 000 mg/kg of the fraction few minutes after administration. These results showed that in single dose, there was no adverse effect of flavonoid rich fraction ofM. tenuifoliaseed extract, indicating that the LD50is higher than 5 000 mg/kg for both male and female mice. Accordingly, about one-twelfth of the maximum tolerated dose, that is, 400 mg/kg was considered as the high dose of the flavonoid rich fraction ofM. tenuifoliaseed extract and used for the subsequent animal studies.
A summary of the results of the mortality and gross symptoms of toxicity seen in rats administered orally with the flavonoid rich fraction ofM. tenuifoliaseed extract over 28 d. Firstly, there was no noticeable deviation in the behaviour of the rats administered orally with 100, 200, and 400 mg/kg compared to that of the control (Group I, no dose) group and essentially all the dosed rats remained healthy during the 28 d period of oral administration of the flavonoid fraction ofM. tenuifoliaseed. Moreover, no deaths occurred with any of the doses up to 400 mg/kg given over 28 d confirming that the LD50for subacute dosing with flavonoid rich fraction ofM. tenuifoliaseed was higher than 400 mg/kg.
Changes in the body weight have been used as an indicator of adverse effects of drugs and chemicals[15]. There was slight increase in weight at the onset of the 28 d study which peaked subsequently suggesting that the extract does not exert any deteriorative effect on the weight and growth of the animals. The increase in weight of the animals suggests that they increasingly accumulated calories from the normal rat diet. Although the animals used in this study were fed with normal rat diet, theM. tenuifoliaseed extract might have allowed proper absorption and utilisation of the nutrients. Low level of active/toxic principles may have stimulated appetite and increased feed utilization resulting in increased weight gain. The seed ofM. tenuifoliais used as a spice[1]. There have not been any reported cases of toxicity in humans.
Organ weight is also an important index of physiological and pathological status in animals. The relative organ weight is fundamental to establish whether the organ was exposed to the injury or not. The heart, liver, kidney, spleen, and lungs are the primary organs affected by metabolic reaction caused by toxicant[16]. The liver, being a key organ in the metabolism and detoxification of xenobiotics, is vulnerable to damage induced by a huge variety of chemicals[16]. Thus, the observed significant (P<0.05) increase in liver weight of the group administered orally with 400 mg/kg body weight of the flavonoid rich fraction after the Day 14 could be attributed to high rate of metabolism of the liver resulting from the brief exposure to extract (containing several constituents).
Analysis of blood parameters is relevant to risk evaluation and the changes in the haematological system have a higher predictive value for human toxicity, when the data are translated from animal studies[17]. The assessment of haematological parameters could be used to reveal the deleterious effect of foreign compounds including plant extract on the blood constituents of animals. They can also be used to determine possible alterations in the levels of bio molecules, metabolic products, haematology, normal functioning and histomorphology of the organs[16]. The increase in erythrocytes (RBC), PCV, MCH, MCHC, MCV and Hb after the 28 day study may be due to over production of haematopoietic regulatory elements such as colonystimulating factors, erythropoietin and thrombopoietin by the stroma cells and macrophages in the bone marrow[18] thus providing the local environment for haematopoiesis[18]. Since MCHC, MCH and MCV relate to individual RBCs, while Hb, RBC and PCV relate to the total population of RBCs in the blood[19], it could encourage haemoglobin incorporation into RBCs and a consequent increase in oxygen exchange. MCV reflects the size of RBCs. The MCH and MCHC reflect the haemoglobin content of RBCs.
Urea and creatinine are considered as a suitable prognostic indicator of renal dysfunction and kidney failure for any toxic compounds[20]. In this study, the absence of significant differences in these parameters after the Day 14 and the Day 28 means thatM. tenuifoliaseed extract has no harmful effect on the kidney.
The evaluation of adverse effects of subacute oral dosing based on biochemical and histological analysis in experimental animal may be more relevant in determining the overall toxicity of the flavonoid rich fraction ofM. tenuifoliaseed extract. When liver cell plasma membrane is damaged, a variety of enzymes normally located in the cytosol are released into the blood stream. Measurements of the activities of serum marker enzymes like ALT, AST and ALP have provided a powerful tool for the assessment of liverfunction[21]. The reduction in the levels of AST and ALT by the flavonoid rich fraction ofM. tenuifoliaafter the Day 14 is an indication of stabilisation of the integrity of the cell membrane of the hepatocytes, keeping the membrane intact and the enzyme enclosed. ALT is a cytosolic enzyme found in very high concentration in the liver[21], and an increase of this specific enzyme indicates hepatocellular damage, while AST is less specific than ALT as an indicator of liver function.
Alkaline phosphatase is membrane bound and its alteration is likely to affect the membrane permeability and produce derangement in the transport of metabolites. There was increase but not significantly in the level of alkaline phosphate after the Day 14. A rise in serum ALP level is usually a characteristic finding in cholestatic liver disease[22,23]. No significant effect was noticed after the Day 28. An elevated serum ALP level is often associated with various disorders such as extrahepatic bile obstruction, intrahepatic cholestasis, infiltrative liver disease, as well as hepatitis and bone disease[16].
Histopathology of the liver sections of the control rats showed normal hepatic architecture and normal liver lobular structure with well-preserved cytoplasm, prominent nucleus and nucleolus. The effect of the flavonoid rich fraction ofM. tenuifoliaon the liver was confirmed from histopathological sectioning which indicated various degrees of peribiliary hepatitis in Group II (100 mg/kg), Group III (200 mg/kg) and Group IV (400 mg/kg) rats due to obstruction of bile flow in the liver after the Day 14. These changes also confirms that the significant increases in ALP and liver weight observed within the groups administered 100, 200 and 400 mg/kg of the flavonoid rich fraction after the Day 14 due to hepatobilliary obstruction leading to cholestasis.
Cholestasis, extrahepatic or intrahepatic, is a common pathophysiological process in many human diseases leading to the accumulation of toxic bile salts within the liver[24]. It is known that increased concentration of bile acids induce lipid peroxides, probably related to the stimulation of phagocytic activity in the polymorphonuclear leucocytes and inflammatory cells, which are present after biliary tract obstruction and enhance the tissue injury[24,25].
Cholestasis (lack of bile flow) results from the blockage of bile ducts or from a disease that impairs bile formation in the liver itself. Alkaline phosphatase level typically rises to several times the normal level after several days of bile duct obstruction or intra-hepatic cholestasis[26]. The results obtained in this study clearly show that the medicinal plant examined exerted an initial effect on bile production and/or flow, the fact that the serum ALP, very low density lipoprotein and triacylglycerol levels eventually normalised after the Day 28 implies that the bile obstruction and/or impairment of liver bile synthesis, was a temporary event that fizzled out. From the results obtained in this study, flavonoid rich fraction ofM. tenuifoliaseed appears to be practically safe when administered acutely to mice through the oral route. When administered orally to rats for Day 14 and 28 toxicity study, the flavonoid rich fraction was found to have a membrane stabilizing effect on the hepatocytes, the flavonoid rich fraction did not have any deleterious effect on the haematopoietic system, serum ALP significantly increased after the Day 14. However, it normalised after the Day 28. The histopathological examination of the liver section showed various degrees of peribiliary obstruction after the Day 14 which normalised after the Day 28. The result therefore suggests that the seed extract was potentially safe for consumption orally. The result therefore suggests that the seed extract was potentially safe for consumption orally.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors thank the lecturers and post graduate students of the Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria Nsukka, Enugu State, Nigeria for their constructive inputs. This research was funded by Imo State University Owerri, Nigeria with grant No. 2012.
Comments
Background
The World Health Organization encourages the use of plants for the treatment of health problems particularly in developing countries. Recent findings have shown that many of these medicinal plants are potentially toxic, mutagenic and carcinogenic. The present study evaluated some of such information and confirms the safety of traditional use ofM. tenuifoliaseed.
Research frontiers
The present research work describesin vivoacute and subacute oral toxicity study on the flavonoid rich fraction ofM. tenuifoliaseed in rodent assessed by estimating mortality rate, body and vital organs weights change and evaluating kidney and liver biomarkers and hematological markers and the effect of extract on liver at graded concentrations.
Related reports
Several works were reported in the literature on this plant species as biopesticide, antidiarrheal. This is the first time report on the pharmacological potential of the flavonoid rich fraction ofM. tenuifoliaseed on the haematology, histology and liver profile of Wistar albino rats.
Innovations and breakthroughs
M. tenuifoliaseeds are widely used in Western Africa to treat various ailments including toothache, dysentery, diarrhoea, dermatitis, headache,etc. In the present study, authors provide consistent evidence of the safety of this plant species in rodent model.
Applications
The pharmaceutical relevance of findings from this study derives from the possibility of formulating the flavonoid rich fraction ofM. tenuifoliaas phytomecines to be used in Africa (where plants have constituted the basis of traditional medicine systems for thousands of years) as well as in other parts of the world.
Peer review
This is an interesting study in which authors have demonstrated the safety ofM. tenuifoliain rodent. Toxicological bioassays were assessed based on biochemical and hematological parameters, and histopathological observations. Flavonoid rich fraction ofM. tenuifoliaseeds were found to be no toxic and could be served as nutraceuticals.
[1] Ezenwali MO, Njoku OU, Okoli CO. Studies on the antidiarrhoeal properties of seed extract of Monodora tenuifolia. Int J Appl Res Nat Prod 2009; 2(4): 20-26.
[2] Njoku UO, Akah PA, Okonkwo CC. Antioxidant activity of seed extracts Monodora tenuifolia (Annonoaceae). Int J Basic Appl Sci 2012; 12(2): 80-87.
[3] Bellini MF, Cabrioti LN, Terezan AP, Jordao BQ, Ribeiro LR, Mantavani M. Cytotoxicity and genotoxicity of Agricus blazei methanolic extract fractions assessed using gene and chromosomal mutations assays. Genet Mol Biol 2008; 31(1): 122-127.
[4] Sujatha R. The effect of vegetarian diet, plant foods, and phytochemicals on hemostasis and thrombosis. Am J Clin Nutr 2003; 78(Suppl 3): 552-558.
[5] Chukwuma ER, Uzoma NO. Biochemical studies on Nigerian Monodora tenuifolia seed. Am J Agric Biol Sci 2013; 8(4): 257-267.
[6] Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 2002; 10(3): 178-182.
[7] Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol 1983; 54(4): 275-287.
[8] Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16(2): 109-110.
[9] Mbaka GO, Adeyemi OO, Oremosu AA. Acute and subchronic toxicity studies of the ethanol extract of the leaves of Sphenocentrum jollyanum (Menispermaceae). Agric Biol J N Am 2010; 1(3): 265-272.
[10] Mbaka GO, Adeyemi OO. Toxicity study of ethanol root extract of Sphenocentrum jollyanum (Menispermaceae) Pierre. Asian J Exp Biol Sci 2010; 1(4): 869-874.
[11] Akhtar N, Srivastara MK, Raizada RB. Assessment of chlorpyrifos toxicity on certain organs in rat, Rattus norvegicus. J Environ Biol 2009; 30(6): 1047-1053.
[12] Thierry TA, Acha AE, Paulin N, Aphrodite C, Pierre K, Tazoacha A. Subacute toxicity study of the aqueous extract from Acanthus montanus. Electronic J Biol 2011; 7(1): 11-15.
[13] Ogbonnia SO, Mbaka GO, Igbokwe NH, Anyika EN, Nwakakwu N. Antimicrobial evaluation, acute and subchronic toxicity studies of Leone Bitters, a Nigerian polyherbal formulation in rodents. Agric Biol J N Am 2010; 1(3): 366-376.
[14] Saha P, Mazumber UK, Halder PK, Islam A, Kumar SRB. Evaluation of acute and subchronic toxicity of Legenaria siceraria aerial part. Int J Pharm Sci Res 2011; 2(6): 1507-1512.
[15] Nandy S, Datta R. Acute and subacute toxicity studies of methanolic leaves extract of Pterospermum acerifolium L wild in rodents. Int J Pharm Life Sci 2012; 3(3): 1519-1529.
[16] Jothy SL, Zakaria Z, Chen Y, Lau YL, Latha LY, Sasidharan S. Acute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules 2001; 16: 5268-5282.
[17] Ibrahim MY, Abdul ABH, Ibrahim TAT, Abdelwahab SI, Elhassan MM, Syam MM. Evaluation of acute toxicity and the effect of single injected doses of zerumbone on the kidney and liver function of Sprague Dawley rats. Afr J Biotechnol 2010; 9(28): 4442-4450.
[18] Kafaie S, Loh SP, Mohtarrudin N. Acute and sub-chronic toxicological assessment of Nannochloropsis oculata in rats. Afr J Agric Res 2012; 7(7): 1220-1225.
[19] Gautam MK, Singh A, Rao CV, Goel RK. Toxicological evaluation of Murraya paniculata L. leaves extract on rodents. Am J Pharmacol Toxicol 2012; 7(2): 62-67.
[20] Gnanamani A, Sudha M, Deepa G, Sudha M, Deivanai K, Sadulla S. Haematological and biochemical effects of polyphenolics in animal models. Chemosphere 2008; 72: 1321-1326.
[21] Aliyu R, Adebayo AH, Gatsing D, Garba IH. The effects of ethanolic leaf extract of Commiphora africana (Burseraceae) on rat liver and kidney function. J Pharmacol Toxicol 2006; 2: 373-379.
[22] Illodigwe EE, Akah PA, Nwosu CS. Evaluation of the acute and subchronic toxicities of ethanol leaf extract of Spathodea campanulata P. Beauv. Int J Appl Res Nat Prod 2010; 3(2): 17-21.
[23] Angelico F, Del Ben M. Towards predicting the therapeutic response in patients with hepatitis C: author’s reply. Aliment Pharmacol Ther 2010; 31: 339-340.
[24] Hussein MA, Abdel-Gawas SM. Protective effect of Jasonia montana against ethinylesteradiol-induced cholestasis in rats. Saudi Pharm J 2010; 18(1): 27-33.
[25] Forchielli ML, Bersani G, Tala’ S, Grossi G, Munarini A, Martini L, et al. Phytosterols and lack of occurrence of cholestasis in rats nourished parenterally or orally. In: Tripodi V, Lucagioli S, editors. Cholestasis. Croatia: InTech; 2012.
[26] Omonkhua AA, Onoagbe IO. Effects of long-term oral administration of aqueous extracts of Irvingia gabonensis bark on blood glucose and liver profile of normal rabbits. J Med Plant Res 2010; 6(13): 2581-2589.
10.1016/S2221-1691(14)60231-8
*Corresponding author: Raphael Chukwuma Ekeanyanwu, Department of Biochemistry, Faculty of Sciences, Imo State University Owerri, P.M.B 2000, Imo State, Nigeria.
E-mail: ekeanyanwuraphael@yahoo.com
Tel: +234-8032-744572
Foundation Project: Supported by Imo State University Owerri, Nigeria with the grant No. 2012.
Article history:
Received 28 Nov 2013
Received in revised form 6 Dec, 2nd revised form 20 Dec, 3rd revised form 29 Dec 2013
Accepted 18 Feb 2014
Available online 28 Mar 2014
Methods:Toxicity study was investigated on the flavonoid rich fraction of Monodora tenuifolia in rats administered different concentrations orally for 28 d using standard laboratory procedures.Results:The LD50of the flavonoid rich fraction was found to be above 5 000 mg/kg body weight in mice observed for 48 h. After the Day 14, biochemical markers of liver injury such as serum alanine aminotransferase, and aspartate aminotransferase decreased significantly (P<0.05 at doses of 100 and 200 mg/kg body weight and P<0.01 at 400 mg/kg) while serum alkaline phosphatase increased non-significantly (P>0.05). There was non-significant (P>0.05) effect observed across the groups in the levels of serum total protein, albumin, globulin, urea and creatinine. The result of histological examination showed various degrees of peribiliary hepatitis after the Day 14 which fizzled out after the Day 28.
Conclusions:The result therefore suggests that the seed extract is potentially safe.
 Asian Pacific Journal of Tropical Biomedicine2014年3期
Asian Pacific Journal of Tropical Biomedicine2014年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Isolation and structural elucidation of cytotoxic compounds from the root bark of Diospyros quercina (Baill.) endemic to Madagascar
- Comparative micromorphological study of wild and micropropagated Dioscorea bulbifera Linn.
- 15-Lipoxygenase inhibition of Commelina benghalensis, Tradescantia fluminensis, Tradescantia zebrina
- Phytochemical constituents and antibacterial activity of some green leafy vegetables
- Chromatographic finger print analysis of anti-inflammatory active extract fractions of aerial parts of Tribulus terrestris by HPTLC technique
- Survey on cattle ticks in Nur, north of Iran
