Isolation and structural elucidation of cytotoxic compounds from the root bark of Diospyros quercina (Baill.) endemic to Madagascar
Fatiany Pierre Ruphin, Robijaona Baholy, Randrianarivo Emmanuel, Raharisololalao Amelie, Marie-Therese Martin, Ngbolua Koto-te-Nyiwa
1Department of Organic Chemistry, Faculty of Sciences, P.O. Box 187, University of Toliara, 601 Toliara, Madagascar
2Malagasy Institute of Applied Research, Avarabohitra Itaosy lot AVB 77, P. O. BOX 3833, 102 Antananarivo, Madagascar
3Antananarivo Poly-technique High School, University of Antananarivo, 101Antananarivo, Madagascar
4Department of Organic Chemistry, Faculty of Sciences, P.O. Box 906 University of Antananarivo, 101Antananarivo, Madagascar
5Institute of Natural Products Chemistry, National Centre for Scientific Research CNRS 91198, Gif Sur Yvette-Paris, France
6Department of Biology, Faculty of Science, University of Kinshasa, P.O. BOX 190 Kinshasa XI, Democratic Republic of the Congo
Isolation and structural elucidation of cytotoxic compounds from the root bark of Diospyros quercina (Baill.) endemic to Madagascar
Fatiany Pierre Ruphin1, Robijaona Baholy2, Randrianarivo Emmanuel3, Raharisololalao Amelie4, Marie-Therese Martin5, Ngbolua Koto-te-Nyiwa6*
1Department of Organic Chemistry, Faculty of Sciences, P.O. Box 187, University of Toliara, 601 Toliara, Madagascar
2Malagasy Institute of Applied Research, Avarabohitra Itaosy lot AVB 77, P. O. BOX 3833, 102 Antananarivo, Madagascar
3Antananarivo Poly-technique High School, University of Antananarivo, 101Antananarivo, Madagascar
4Department of Organic Chemistry, Faculty of Sciences, P.O. Box 906 University of Antananarivo, 101Antananarivo, Madagascar
5Institute of Natural Products Chemistry, National Centre for Scientific Research CNRS 91198, Gif Sur Yvette-Paris, France
6Department of Biology, Faculty of Science, University of Kinshasa, P.O. BOX 190 Kinshasa XI, Democratic Republic of the Congo
PEER REVIEW
Peer reviewer
Dr. Iteku Bekomo Jeff, Northeast Normal University (P. R. China)/ Kinshasa University (D. R. Congo).
Tel: +86-131-9436-6880
E-mail: jeffitekubekomo@gmail.com
Comments
This is a valuable research work in which the authors have demonstrated the cytotoxicity of three compounds of Diospyros quercina in vitro. The structural elucidation was determined based on chemical and spectroscopic studies. The bioactivity was assessed based on cell proliferation assay. Diospyros quercina is then a promising anticancer agent.
Details on Page 174
Objective:To isolate and characterize the cytotoxic compounds from Diospyros quercina (Baill.) G.E. Schatz & Lowry (Ebenaceae).
Diospyros quercinia, Cytotoxic activities, Isodiospyrin, 6’-Ethoxy-1’, 3’-dihydroxy-4, 6-dimethyl-1,2’-binaphthyl-2,5’,8,8’tetraones, (E)-5,6-Dimethyl-2-(2-methyl-3-(prop-1-enyl) phenyl)-2H-Chromene, Madagascar
1. Introduction
Madagascar is reputed for the extraordinary richness of its flora (biodiversity) and boasts a wide variety of indigenous species[1]. These plants species represent an enormous reservoir of new molecules with potential therapeutic activity waiting to be discovered.
In Madagascar as well as in the others parts of Africancontinent, the majority of people rely on traditional medicine for their health care needs because the costs of conventional drugs are unaffordable[2-4]. In addition, this big island is unique because of the endemicity of its flora which makes 85% of Malagasy biodiversity[5]. During an ethno-botanic survey conducted in the south-west part of Madagascar, it was reported that the aerial part of the plant known under the vernacular name Maintifototse (Malagasy name), and scientifically namedDiospyros quercina(Baill.) G.E. Schatz & Lowry (Ebenaceae) is used by the local communauties to treat malaria and incurable wounds. The genusDiospyrosis represented in Madagascar by 21 endemic species and the majority of species are mostly distributed in the south and southwest parts of Madagascar[6,7]. This plant is a well-known and very important recipe in these regions because of its therapeutic values in the Malagasy traditional medicine.
The aim of the present study was to isolate and characterize the cytotoxic compounds fromDiospyros quercina(Baill.) G.E. Schatz & Lowry (Ebenaceae). Thus, if admitted thatDiospyros quercinacontains such cytotoxic compounds, the use of this plant as antimalarial or antidiabetic by traditional healers should be carefully adjusted or discouraged because recent findings have shown that many plants used in traditional medicine are potentially toxic, mutagenic and carcinogenic[8]. Such information would be useful in preventing the wide use of the plant by local communities and will guide laboratory research in identifying new anticancer lead compounds.
In this present study, the crude extract of the root bark ofDiospyros quercina(Baill.) G.E. Schatz & Lowry (Ebenaceae) exhibited a good activity with the IC50value of 0.85 µg/ mL at 5 µg/mL concentration. This interesting preliminary result has conducted us to undertake further analyses in order to purify and to elucidate the chemical structure(s) of the bioactive compounds using a combination and spectroscopic techniques (1D and 2D NMR and mass spectrometry in positive mode IES.SM-TOF 6.97Ev).
2. Materials and methods
2.1. Selection and collection of plant material
Ethnobotanical information about plant species selected for this study was obtained by interviewing traditional healers during field work which was conducted in the south of Madagascar from July to August 2010.Diospyros quercina(Baill.) G.E. Schatz & Lowry (Ebenaceae) was collected in theIzombitse SakarahaNational Park at nearly 165 km from Toliara town, in the south of Madagascar. The plant was identified by comparison with reference specimen available at the Department of Botany; “Parc Botanique et Zoologique de Tsimbazaza” in Antananarivo, Madagascar. Voucher specimen assigned sample number MQ-01 was deposited at the herbarium of the Applied Chemistry Laboratory of the University of Toliara, Madagascar.
2.2. Extraction and bioguided isolation
The air-dried and powdered root barks ofDiospyros quercina(Baill.) G.E. Schatz & Lowry (2 kg) were repeatedly extracted by cold percolation with ethanol 90° (3×3 h, 15 L) at room temperature on a shake. Pooled organic solvents were dried over Na2SO4and evaporated until dryness at 40 °C, under reduced pressure to yield 30 g of crude extract. Twenty-five grams of the ethanol crude extract was suspended in water and partitioned successively withn-hexane, dichloromethane, ethyl acetate andn-butanol to yield the corresponding soluble extract fractions. Bioassay-guided extraction revealed interesting activity only with the dichloromethane extract fraction, which exhibited an inhibition rate of leukemia P388 cell growth of 94.67% at 2 µg/mL. Ten grams of the dichloromethane crude extract was first subjected to fractionation, using a silica gel column chromatography eluted withn-hexane and gradient of ethyl acetate (9:1 to 6:4) resulting into eight fractions (F1-F8). Two fractions, F5and F6, showed strong cytotoxic activities with an inhibition rate of 96.08% and 92.43% respectively at 0.5 µg/mL. These fractions were checked for purity by analytical TLC, and the zone was detected with a UV lamp at 254 and 365 nm and by spraying with sulfuric vanillin acid, followed by heating at 120 °C for 1-5 min. F5and F6were combined on the basis of TLC profile similarity and resubmitted to seperation by silica gel column chromatography eluting with a mixture of diethyl ether/chloroform/methanol (2/7.5/0.5); the column was in isocratic regime and at the end, it resulted into seven fractions.
The fraction F63showed cytotoxic activity with an inhibition rate of 99.012% at 0.25 µg/mL. Then 100 mg of F63was subjected to further purification, using a silica gel column chromatography, eluting withn-hexane and gradient of ethyl acetate furnished a pure compound (6 mg) and a mixture of two compounds (40 mg) which were further separated by preparative TLC usingn-hexane/ethyl acetate/ acetone (2/6/2) as the solvent system affording compounds 2 (4 mg) and 3 (5 mg). The three pure compounds showedstrong cytotoxic activities against murine P388 leukemia cell lines after biological test. The purity of compounds was proved by HPLC analysis. The mobile phase consisted of a CHCl3/MeOH (1/1) mixture of chloroform and methanol; the chromatography was performed with isocratic regime during 25 min. Eluted compounds were detected on the basis of their UV absorption in the wavelength range from 190 nm to 315 nm. The purity of each product was respectively 99.92% at λ=202 nm, 98.99% at λ=198 nm, and 99.42% at λ=245 nm.
2.3. Cytotoxicity assay
The crude extract, the fractions (n-hexane, dichloromethane, ethyl acetate,n-butanol and aqueous extracts) and the pure compounds were systematically submitted to cytotoxicity test. In that way, murine P388 leukemia cells were grown in RPMI 1640 medium containing 0.01 nmol/L of β-mercapto-ethanol, 10 mmol/ L of L-glutamine, 100 IU/mL of G-penicillin, 100 µg/mL of streptomycin, 50 µg/mL of gentamycin, and 50 µg/mL of nystatine, supplemented with 10% of fetal calf serum. Cells were maintained at 37 °C in a moisturized atmosphere with 5% CO2. The inoculums seeded at 104cells/mL at an optimal volume of 0.1 mL per well, were introduced into flat-bottomed 95-well plates containing a serie of concentrations of compounds (0.001, 0.01, 0.1 and 1 µg/ mL for compound/extract having IC50<1 µg/mL or ranging from 0.976 to 50 µg/mL for extract/compound having IC50<50 µg/mL), alongside untreated controls. Culture was then incubated at 37 °C for 71 h in the required atmosphere. Thereafter, cells were incubated at 37 °C with 0.02% neutral red dissolved in methanol/water (1/9), 0.1 mL per well for 1 h and then washed with 1 mol/L PBS and finally lyzed with 1% SDS. After a brief agitation on a micro-culture plate shaker, the plates were transferred to a Titertek Twin reader equipped of a 540 nm filter to measure absorbance of the extracted dye. Cell viability was expressed as the percentage of cells incorporating dye relative to the untreated controls, and IC50values were determined by linear regression method.
2.4. Statistical analysis
All statistical calculations were carried out with GraphPadPrism4 software. The results are expressed as the mean±standard error of mean (SEM) of n independent experiments with individual values. Unpaired Student’st-test was used for statistical comparison,Pvalues less than 0.01 were considered as significantly different against the control.
3. Results
3.1. Phytochemical studies
The structures of three isolated compounds named TR-21, TR-22, and TR-23 are summarized in Figure 1.
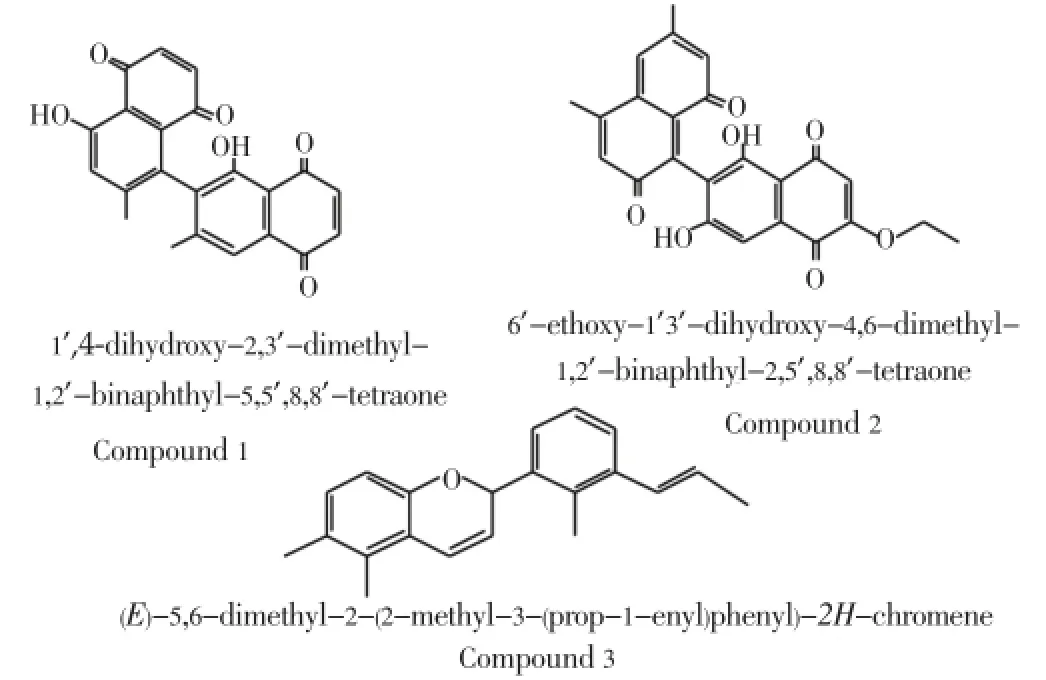
Figure 1. Structures of cytotoxic compounds 1, 2 and 3.
The bioassay-guided fractionation of the crude extract of the root bark ofDiospyros quercinausing repeated silica gel column chromatography resulted in the isolation of three pure compounds as evidenced by analytical TLC and HPLC analysis.
The isolated compound 1 (TR-21), showed a quasimolecular ion atm/z= 375.1942 [M+H]+in the ESI.TOF.MS spectrum which corresponds to the molecular formula C22H14O6. The1H-NMR spectrum showed characteristic singlet attributed to two methyl groups δ1.99, δ2.00 and six protons δ6.75(d), δ6.89(d), δ6.92(d), δ6.93(d), δ7.27(s) and δ7.60 typical for benzenes skeleton, and at the end two protons acid attributed to hydroxyl protons of phenol at δ12.00 and δ12.43. Examination of the13C NMR (broad brandand DEPT), and the HSQC spectra data of the pure compound revealed the presence of a four carbonyls carbons at δC184.4 (C-1), δC184.6(C-4), δC190.1(C-4’), δC190.3(C-1’) and sixteen alcens carbons (C=C) double bond typical for benzenes skeleton at δC113.1(C-1a); δC114.2(C-1b’); δC121.3(C-8); δC125.7(C-6’); δC128.6(C-6’b); δC128.7(C-6a); δC130.2(C-8’), δC135.0(C-6), δC137.7(C-3’); δC138.8(C-3); δC139.5(C-2); δC140.1(C-2’); δC145.5(C-7); δC148.3(C-7’); δC158.5(C-5); δC161.5(C-5’) and two of carbons methyl groups (Table 1). The1H and13C chemical shift values of individual spin systems were determined by correlation in the 2D HSQC spectrum. The individual1H and13C chemical shift assigned by the1H-1H COSY spectrum and 2D HSQC andHMBC correlation spectra, respectively (Table 1).
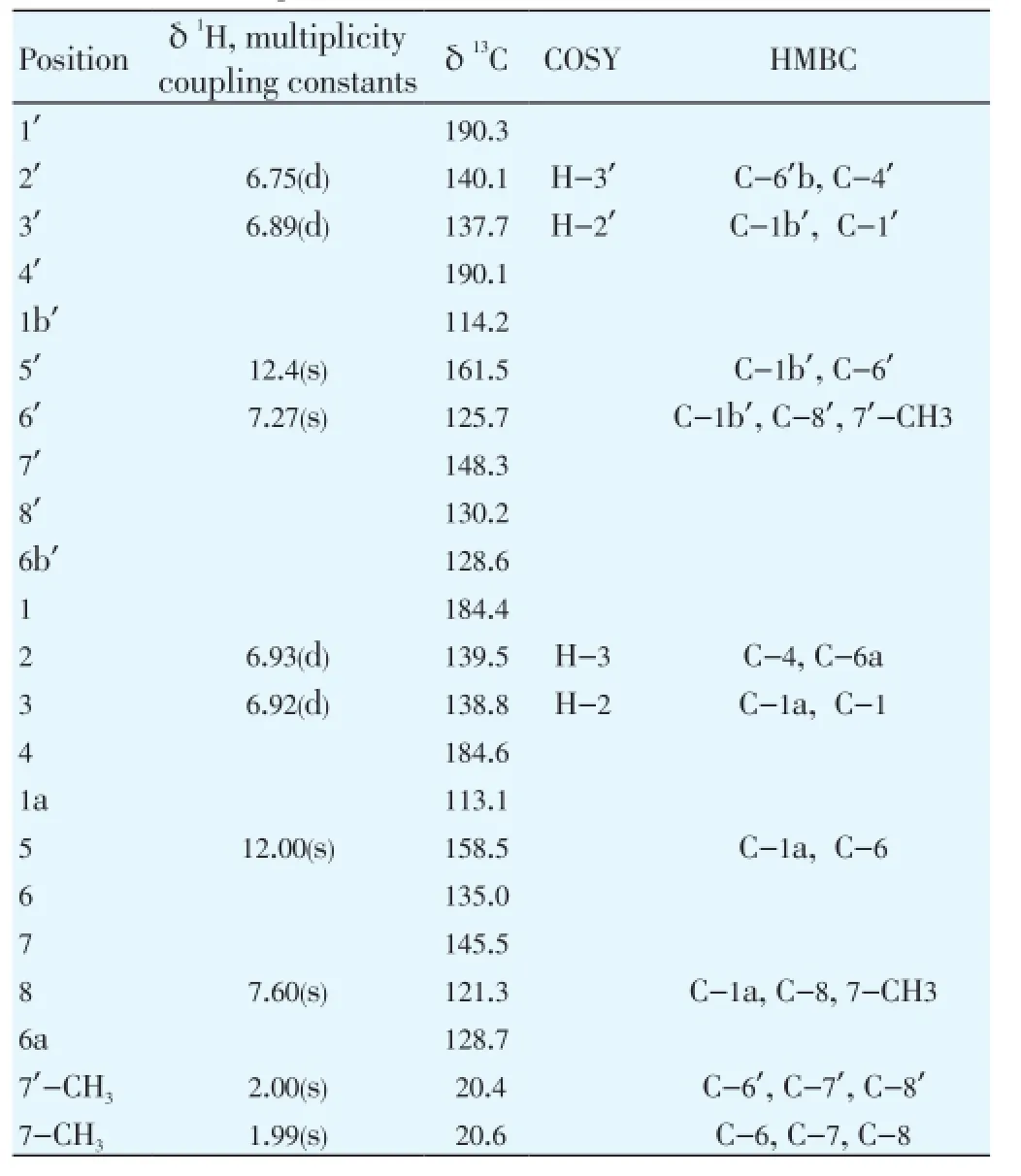
Table 11H and13C chemical shift, the1H-1H COSY, and important HMBC correlations of compound 1 (TR-21).
Compound 2 (TR-22) had as molecular formula C24H18O7from ESI.TOF.MS (m/z=419.0814 [M+H]+calculated). Its1HNMR spectrum exhibited two singlets at δ2.21, δ2.35 and a doublet at δ1.22 characteristic of the three methyl groups, one triplet at δ3.85 was attributed by the protons in the OCH2group and thereafter others singlets at δ6.92, δ6.98, δ7.16, δ7.52 and δ7.59 characteristic attributed to the benzenic protons and at the end two hydroxyls protons between δ11.85, δ12.52 typical for a phenol. The 1D13C (Broad Band) NMR spectrum contained twenty-four (24) signals including the carbonyls between δC184.20(C-5’), δC184.40(C-8), δC188.13(C-2), δC190.00(C-8’). Examination of the 1D13C (DEPT), and the 2D HSQC spectra data of the compound 2 revealed the presence of a fourteen alcens carbons (C=C) double bond typical for benzenes skeleton at δC117.58(C-1); δC121.21(C-3); δC148.70(C-4); δC142.76(C-4b’); δC120.72(C-5); δC146.60(C-6); δC124.21(C-7), δC113.60(C-8b), δC159.95(C-1’); δC112.51(C-2’); δC162.04(C-3’); δC139.95(C-4’); δC132.45(C-4’a); δC117.89(C-6’); δC138.2(C-7’); δC1124.41(C-8’a) and two of carbons methyl groups, and at the end, one ethoxy group (OCH2CH3) (Table 2). The1H and13C chemical shift values of individual spin systems were determined by correlation in the 2D HSQC spectrum. The individual1H and13C chemical shift assigned by the1H-1H COSY spectrum and 2D HSQC and HMBC correlation spectra, respectively (Table 2).
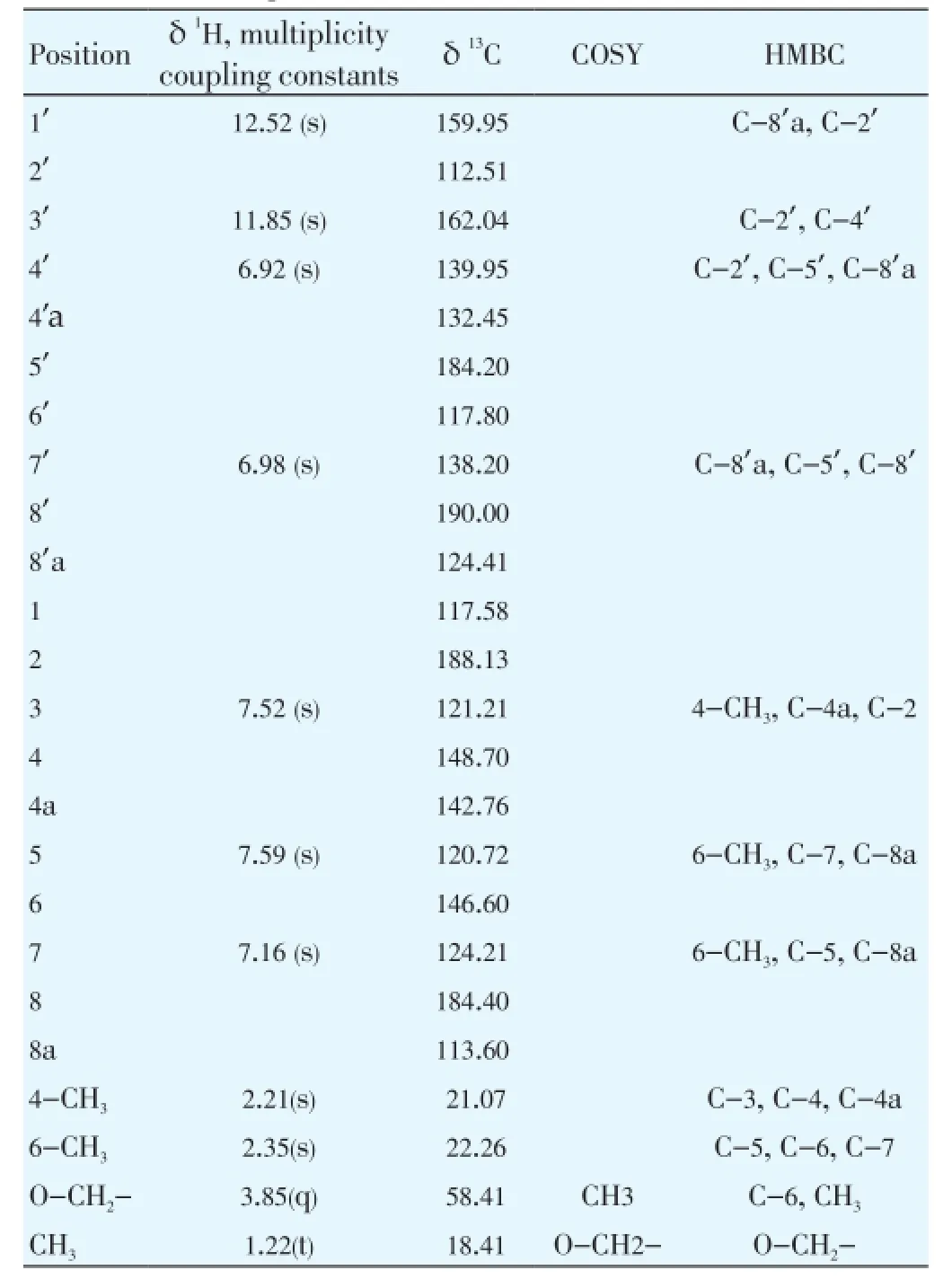
Table 21H and13C chemical shift, the1H-1H COSY, and important HMBC correlations of compound 2 (TR-22).
The molecular formula of compound 3 (TR-23) was determined to be C21H22O by ESI.TOF.MS and NMR experiments. The1H-NMR spectrum showed singlet characteristic attributed to four methyl groups δ1.62, δ2.03, δ2.21, δ2.35 and eleven signals between δ8.19(d), δ8.16(d), δ8.13(t), δ8.09(t), δ7.73(dd), δ7.53(t), δ7.45(t), δ6.40(d), δ6.14(d), δ5.57(d), δ5.43(dd), attributed to characteristic of signals alcens proton typical for benzenes skeleton. The 1D13C (Broad Band) NMR spectrum contained twenty one (21) signals of the carbons. Examination of the 1D13C (DEPT), and the 2D HSQC spectra data of the compound 2 revealed the presence of about ten (10) alcens carbons (C=C) double bond typical for benzenes skeleton at δC
125.94; δC133.51; δC126.71; δC125.21; δC123.93; δC126.39; δC122.29, δC117.50, δC128.05; δC123.10, six quaternary C at δC135.50; δC112.8; δC143.00; δC148.00; δC110.04; δC1142.01 and four of carbons methyl groups (Table 3). The1H and13C chemical shift values of individual spin systems were determined by correlation with the 2D HSQC spectrum. The individual1H and13C chemical shift assigned by the1H-1H COSY spectrum and 2D HSQC and HMBC correlation spectra,respectively (Table 3).

Table 31H and13C chemical shift, the1H-1H COSY, and important HMBC correlations of of compound 3 (TR-23).
3.2. Biological screening
Crude extract, five fractions (n-hexane, dichloromethane, ethyl acetate,n-butanol and aqueous extracts), and the three pure compounds, from the root bark ofDiospyros quercinawere screened for cytotoxicity assay against P388 cell lines using a serie of concentrations. The results for the cytotoxic bioassay are dose-response curves for each fractions/ compounds. These curves are plots of P388 cells survival percentage against logarithm of concentration. The IC50values from these graphs are summarized in Table 4.

Table 4 In vitro cytotoxic activities of crude extract, different fractions and the pure compounds from the root bark of Diospyros quercina (Baill.) G.E. Schatz & Lowry (n=6).
The results indicate that crude extract, dichloromethane soluble fraction, F5, F6, F63, Compounds 1, 2 and 3 can be accepted as potent cytotoxic agents because their IC50values are below 20 µg/mL[9].
It can also be seen from the Table 4 that compound 1 displayed a very good activity similar to that of known anticancer actinomycin (0.018 µg/mL) and camptothecin (0.016 µg/mL) than taxol (0.032 µg/mL) and vincristin (0.023 µg/mL). Compounds 2 and 3 are less active than all positive control
cited[10].
4. Discussion
The goal of ethnopharmacology is to preserve the cultural heritage by documenting information on medicinal plants and their isolates compounds. This is due to the fact that the first line of treatment for poor people in the developing countries is the use of medicinal plants at home. In the present study, three cytotoxic compounds were isolated and characterized from the medicinal plantDiospyros quercinaendemic to Madagascar.
The lower activity of compound 3 compared to that of compound 1 or 2 may be linked to the difference in their chemical structures and the difference in biological activity of naphtylquinone derivatives compounds could be linked to the ethoxy side chain which could reduce the toxicity of these leads. The quinone reductive functions are able to react with amine function of nucleotides to form imines’derivatives responsible for their probable cytotoxic properties as intercalating agents[11].
There are several cancerous cell lines used in different academic and pharmaceutical laboratories of the world for cytotoxicity evaluation. Among them P388 lymphocytic leukemia cell lines which have been used in this study. The nuclear proto-oncogenes c-myc in such cells is frequently overexpressed and constitutes an indication of early response of cell proliferation[12]. We postulated in this study that cytotoxic compounds could induce apoptosis in P388 cells by down-regulating/suppressing c-myc expression. This study revealed that herbal and plants based traditional medicine are good source of chemical structures that might be effective in attacking invading cancer cells. Indeed, the plant kingdom offers a way of hope because of its enormous chemical diversity[13]. Several known anticancer drugs have been derived from medicinal plants and some of these include vincristine, vinblastine and taxol[10].
The chemical structures of the active compounds isolated from the root bark ofDiospyros quercina(Baill.) G.E. Schatz & Lowry revealed that these leads are closely linked toDiospyrosgenus. Indeed, recent findings showed thatphenolic complex compounds such as naphtoquinone could play a key role in chemotaxonomic classification of plants species (chemical systematic) or in ecological biochemistry research[14]. The compound 1 was obtained as pure major component and had a red color. According to spectroscopic data, its molecular formula C22H14O4was elucidated as 1’,4-dihydroxy-2’, 3-dimethyl-2’binaphtyl-5, 5’, 8, 8’ tetraone or isodiospyrin. This compound exhibited a very good cytotoxic activity with the IC50<0.1 µg/mL. Isodiospyrin, a naphtoquinone derivative, was previously reported in the literature to have a broad spectrum of biological activities includingin vitro12(S)-HETE inhibitory effects[15], antitermitic[16], analgesic, antipyretic and anti-inflammatory properties[17] and cytotoxic activities[18].Diospyrosgenus elaborated a large number of 1, 4-naphtoquinone secondary metabolites belonging to the juglone class[19]. About 75% ofDiospyrosphytochemicals reported in the literature are 1,4-naphtoquinones which include several monomers, dimmers and derivatives. In fact, these secondary metabolites can serve as specific markers ofDiospyrosgenus.
The molecular formula C24H18O7for compound 2 was established by positive ESI.TOF.MS (m/z=419.0814 for [M+H]+calculated). The NMR spectral data resembled closely to those of compound 1, except for the presence of ethoxy group. From our knowledge, this molecule was not previously reported in the literature.
Compounds 1 and 2 were identified as 1,4’-dihydroxy-2,3’ dimethyl-1,2’binaphthyl-5,5’,8,8’tetraone and 6’ethoxy-1’,3’dihydroxy-4,6 dimethyl-1,2’binaphthyl-2,5’,8,8’tetraones respectively. They had the same binaphtylquinone ring and differed by their substituent on the hydroxyl group at position 3’ and by the carbonyls carbons at position 2, at the end presence of ethoxy group at position 6 in the compound 2.
Compound 2 also exhibited a very good cytotoxic activity with an IC50<0.1 µg/mL.
The compound 3 has got as molecular formula C21H22O with molecular massm/z=291.386 [M+H]+according to spectroscopic data, this compound was elucidated as a derivative of cyanidin: (E)-5, 6-dimethyl-2-(2-methyl-3-(prop-1-enyl) Phenyl)-2H-Chromene. From our knowledge, this molecule is also reported for the first time in theDiospyrosgenus. The compound 3 exhibited good to moderate cytotoxic activity with an IC50value of 1.27 µg/mL. Others chemical investigations of the plant genusDiospyrosalso yielded many compounds with interesting and exciting biological activities[15-19].
This study focused on the isolation and characterization of the cytotoxic compounds fromDiospyros quercinawhich have been selected through ethno-botanical survey. At the end of this study, we have demonstrated that ethnobotanical survey and ethnopharmacology are powerful tools for selecting and validating the biological activity of an appropriate medicinal plant species traditionally used by indigenous community to treat commonly diseases. The combination of biological experimentsin vitro, chromatographic and spectroscopic modern techniques (CC/HPLC/MS/NMR) has led to the isolation and structural elucidation of three cytotoxic compounds fromDiospyros quercina: Isodiospyrin (compound 1), 6’ethoxy-1’, 3’-dihydroxy-4, 6-dimethyl-1,2’-binaphthyl-2,5’,8,8’tetraones (compound 2), and (E)-5,6-dimethyl-2-(2-methyl-3-(prop-1-enyl) phenyl)-2H-Chromene (compound 3). Compounds 2 and 3 are reported for the first time in theDiospyrosgenus. All lead compounds displayed very interesting cytotoxic properties thus justifying the wide use ofDiospyros quercina(Baill.) G.E. Schatz & Lowry (Ebenaceae) in Malagasy folk medicine to treat cancer. Further studies on these cytotoxic compounds involvingin vivoanti-neoplastic activity are necessary for promoting these molecules as anticancer new lead compounds. It can also be concluded that the use of this plant as antimalarial or antidiabetic by traditional healers should be carefully adjusted or discouraged because of the presence of potentially toxic compounds for healthy human.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This research was founded by the Third World Academy of Science (TWAS Fellowship for Research and Advanced Training FR number: Grant No. 3240224121 and the International Foundation for Science (IFS, Stockholm, Sweden) and the Organization for the Prohibition of Chemical Weapons (OPCW) (IFS Research Grant N0 F/4921-2) offered to Dr. Ngbolua K.N.
Comments
Background
In recent years, cancer has threatened human living and life and thrown human into a panic. However, modern medicine does not work effectively for cancers and they exhibit side effects. Thus, there is need of new and potent anticancer agents without side effects.
Research frontiers
The present research study describes the anti-proliferativeeffect and the structure of three compounds isolated from the alcohol extract ofDiospyros quercina(Baill).
Related reports
Recent findings showed that phenolic compounds such as naphtoquinone could play a key role in chemotaxonomic classification of plants species or biochemistry research. Isodiospyrin, a naphtoquinone derivative, is reported to have a broad spectrum of bioactivities includingin vitro12(S)-HETE inhibitory effects, antitermitic, analgesic, antipyretic and anti-inflammatory and cytotoxicity.
Innovations and breakthroughs
An ethnobotanic survey conducted in Madagascar reported that the aerial part ofDiospyros quercina(Baill.) is used as medicinal plant to treat malaria and incurable wounds. In the present study, the authors have demonstrated the antiproliferative effect of three purified compounds isolated fromDiospyros quercina(Baill.).
Applications
From the ethnobotanical survey it has been found thatDiospyros quercinais safe to humans. This scientific research support and suggest the use of this plant as an anticancer agent.
Peer review
This is a valuable research work in which the authors have demonstrated the cytotoxicity of three compounds ofDiospyros quercinain vitro. The structural elucidation was determined based on chemical and spectroscopic studies. The bioactivity was assessed based on cell proliferation assay.Diospyros quercinais then a promising anticancer agent.
[1] Pearson RG, Raxworthy CJ. The evolution of local endemism in Madagascar: water shed vesus climatic gradient hypotheses evaluated by null biogeographic models. Evolution 2009; 63(4): 959-967.
[2] Marimuthu S, Padmaja B, Nair S. Phytochemical screening studies on Melia orientalis by GC-MS analysis. Pharmacognosy Res 2013; 5(3): 216-218.
[3] Kumar VS, Navaratnam V. Neen (Azadirachta indica): prehistory to contempory medicinal uses to human kind. Asian Pac J Trop Biomed 2013; 3(7): 505-514.
[4] Maroyi A. Traditional use of medicinal plants in South-Central Zimbabwe: review and perspective. J Ethnobiol Ethnomed 2013; 9: 31.
[5] Ngbolua KN, Rafatro H, Rakotoarimanana H, Ratsimamanga US, Mudogo V, Mpiana PT, et al. Pharmacological screening of some traditionally-used anti-malarial plants from the Democratic Republic of Congo compared to its ecological taxonomic equivalence in Madagascar. Int J Biol Chem Sci 2011; 5(5): 1797-1804.
[6] The plant list. Version 1. [Online] Available from: http://www. theplantlist.org/. [Accessed on 30 June, 2013].
[7] Schatz GE, Lowry II PP. Nomenclatural notes on Malagasy Diospyros L. (Ebenaceae). Adansonia 2011; 33(2): 271-281.
[8] Maestro EL, Mota SF, Lima EB, Bernardes BM, Goulart FC. Genotoxicity and mutagenicity of Rosmarinus officinalis (Labiatae) essential oil in mammalian cells in vivo. Genet Mol Res 2010; 9(4): 2113-2122.
[9] Ramkumar KM, Manjula C, Elango B, Krishnamurthi K, Saravana DS, Rajaguru P. In vitro cytotoxicity of Gymnema montanum in huma leukaemia HL-60 cells; induction of apoptosis by mitochondrial membrane potential collapse. Cell Prolif 2013; 46(3): 263-271.
[10] Rasoanaivo P, Ratsimamanga Urverg S. Biological evaluation of plants with reference to the Malagasy flora. Monograph prepared for the IFS-NAPRECA workshop on bioassay held in Antananarivo, Madagascar. Antananarivo: NAPRECA; 1993, p. 65-71.
[11] Ruphin FP, Baholy R, Emmanue A, Amelie R, Martin MT, Koto-Te-Nyiwa N. Antiplasmodial, cytotoxic activities and characterization of a new naturally occurring quinone methide pentacyclic triterpenoid derivative isolated from Salacia leptoclada Tul. (Celastraceae) originated from Madagascar. Asian Pac J Trop Biomed 2013; 3(10): 780-784.
[12] Yamada Y, Hidaka H, Seki N, Yoshino H, Yamasaki T, Itesako T, et al. Tumor-suppressive microRNA-135a inhibit cancer proliferation by targeting the c-MYC oncogene in renal cell carcinoma. Cancer Sci 2013; 104(3): 304-312.
[13] Radulovic NS, Blagojevic PD, Stojanovic-Raclic ZZ, Stojanovic NM. Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr Med Chem 2013; 20(7): 932-952.
[14] Nematollahi A, Aminimoghadam Farouj N, Wiart C. Reviews on 1,4 naphtoquinones from Diospyros L. J Asian Nat Prod Res 2012; 14(1): 80-88.
[15] Wube AA, Streit B, Gibbons S, Asres K, Bucar F. In vitro 12(S)-HETE inhibitory activities of naphtoquinones isolated from the root bark of Euclea racemosa ssp. schimperi. J Ethnopharmacol 2005; 102(2): 191-196.
[16] Ganapaty S, Thomas PS, Fotso S, Laatsch H. Antitermitic quinones from Diospyros sylvatica. Phytochemistry 2004; 65(9): 1265-1271.
[17] Trongsakul S, Panthong A, Msonthi JD, Taesotikul T. The analgesic, antipyretic and anti-inflamatory activity of Diospyros variegata Kruz. J Ethnopharmacol 2003; 85(2-3): 221-225.
[18] Baikar S, Malpathak N. Secondary metabolites as DNA topoisomerase inhibitors: a new era towards designing of anticancer drugs. Pharmcogn Rev 2010; 4: 12-26.
[19] Mallavadhani UV, Panda AK, Rao YR. Pharmacology and chemotaxonomy of Diospyros. Phytochemisty 1998; 49(4): 901-951.
10.1016/S2221-1691(14)60227-6
*Corresponding author: Ngbolua Koto-te-Nyiwa, PhD, Associate Professor, Department of Biology, Faculty of Science, University of Kinshasa, P.O. BOX 190 Kinshasa XI, Democratic Republic of the Congo.
Tel: +243-8168-79527
E-mail: jpngbolua@unikin.ac.cd
Foundation Project: Supported by the Third World Academy of Science, (TWAS Fellowship for Research and Advanced Training FR number: Grant No. 3240224121 and the International Foundation for Science (IFS, Stockholm, Sweden) and the Organization for the Prohibition of Chemical Weapons (OPCW) (IFS Research Grant N0 F/4921-2).
Article history:
Received 20 Dec 2013
Received in revised form 1 Jan, 2nd revised form 8 Jan, 3rd revised form 15 Jan 2014
Accepted 18 Feb 2014
Available online 28 Mar 2014
Methods:An ethno-botanical survey was conducted in the south of Madagascar from July to August 2010. Bio-guided fractionation assay was carried out on the root bark of Diospyros quercina, using cytotoxicity bioassay on murine P388 leukemia cell lines as model. The structures of the cytotoxic compounds were elucidated by 1D and 2D NMR spectroscopy and mass spectrometry.
Results:Biological experiments resulted in the isolation of three bioactive pure compounds (named TR-21, TR-22, and TR-23) which exhibited very good in vitro cytotoxic activities with the IC50values of (0.017 5±0.0060) µg/mL, (0.089±0.005) µg/mL and (1.027±0.070) µg/mL respectively. Thus, they support the claims of traditional healers and suggest the possible correlation between the chemical composition of this plant and its wide use in Malagasy folk medicine to treat cancer.
Conclusions:The ability of isolated compounds in this study to inhibit cell growth may represent a rational explanation for the use of Diospyros quercina root bark in treating cancer by Malagasy traditional healers. Further studies are, therefore, necessary to evaluate the in vivo antineoplastic activity of these cytotoxic compounds as effective anticancer drugs.
 Asian Pacific Journal of Tropical Biomedicine2014年3期
Asian Pacific Journal of Tropical Biomedicine2014年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Comparative micromorphological study of wild and micropropagated Dioscorea bulbifera Linn.
- 15-Lipoxygenase inhibition of Commelina benghalensis, Tradescantia fluminensis, Tradescantia zebrina
- Phytochemical constituents and antibacterial activity of some green leafy vegetables
- Acute and subacute oral toxicity study on the flavonoid rich fraction of Monodora tenuifolia seed in albino rats
- Chromatographic finger print analysis of anti-inflammatory active extract fractions of aerial parts of Tribulus terrestris by HPTLC technique
- Survey on cattle ticks in Nur, north of Iran
