Enhancement of vitamin A combined vitamin D supplementation on immune response to Bacille Calmette-Guérin vaccine revaccinated in Chinese infants
Ying Zheng, Xue-Gang Li, Qiu-Zhen Wang, Ai-Guo Ma*, Ib Christian Bygbjerg, Yong-Ye Sun, Yong Li, Ming-Ci Zheng, Xi Wang
1Institute of Human Nutrition, Medical College of Qingdao University, Qingdao 266021, PR China
2Department of International Health, University of Copenhagen, DK-1014 Copenhagen, Denmark
3Department of Pediatrics, Guilin Medical College Affiliated Hospital, Guilin 541001, PR China
4Department of Orthopaedic Surgery, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou 510655, PR China
Enhancement of vitamin A combined vitamin D supplementation on immune response to Bacille Calmette-Guérin vaccine revaccinated in Chinese infants
Ying Zheng1#, Xue-Gang Li1#, Qiu-Zhen Wang1, Ai-Guo Ma1*, Ib Christian Bygbjerg2, Yong-Ye Sun1, Yong Li1, Ming-Ci Zheng3, Xi Wang4
1Institute of Human Nutrition, Medical College of Qingdao University, Qingdao 266021, PR China
2Department of International Health, University of Copenhagen, DK-1014 Copenhagen, Denmark
3Department of Pediatrics, Guilin Medical College Affiliated Hospital, Guilin 541001, PR China
4Department of Orthopaedic Surgery, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou 510655, PR China
Objective: To investigate whether there is an association between diameter of bacille Calmette-Guérin (BCG) scars and effect of purified protein derivative (PPD) reaction and to determine whether vitamin A (VA) combined vitamin D (VD) supplementation influences the immune response to BCG revaccinated in Chinese infants. Methods: A cross-section and 3-month community-randomised trial was conducted. A total of 5 629 infants at 3, 6 and 12 months of age in Junan County of China were examined for BCG scar formation. Then, 597 revaccinated infants were randomly assigned to supplementation (n=307) and control (n=290) groups. The supplementation group were daily assigned to 1 500 IU VA and 500 IU VD for 3 months. Then all infants were subjected to skin test with PPD. Results: The diameter of BCG scars was positively correlated with diameter of skin indurations of PPD (r=0.17, P<0.05) in the 5 629 infants. The rate of positive response to PPD was higher in the supplementation group than in the control group (96.1% versus 89.7%, P<0.05, prevalence ratio 1.07, 95% CI 1.02-1.12). The prevalence ratio of PPD response for the supplementation group compared with that for the control group was 1.07 (95% CI 1.01-1.13) for the males and 1.08 (95% CI 1.00-1.17) for the females. For the supplementation group, the males got larger tuberculin induration than the females [(0.73±0.21) cm versus (0.67±0.20) cm, P<0.05) after intervention. Conclusions: The diameter of BCG scars was effectively correlated with PPD response, which indicates BCG scar formation may be an useful tool to evaluate the effect of tuberculosis prevention.VA combined VD supplementation may play an immunoregulatory role in BCG revaccination. This may contribute to the prevention of childhood tuberculosis.
ARTICLE INFO
Article history:
Received 10 October 2013
Received in revised form 15 December 2013
Accepted 15 January 2014
Available online 20 February 2014
Tuberculosis
Bacille Calmette-Guérin
Purified protein derivative
Vitamin A
Vitamin D
Revaccination
1. Introduction
Bacille Calmette-Guérin (BCG) vaccination is administered in infancy in China with the aim to protect against mycobacterial infections such as tuberculosis (TB) and leprosy[1]. Failure of BCG vaccination and little effect on preventing TB are contributed to the influence of environmental mycobacteria, differences between BCG strains, inefficient immune response after BCG stimulation, genetic factors and malnutrition of populations[2,3].
Micronutrient deficiencies like vitamin A (VA) and vitamin D (VD) are more common in TB patients[4,5]. In recent years, there has been renewed interest in the biological effects of VA and VD on TB due to the growing evidence of the immunomodulatory properties of VA andVD. VA supplementation has been shown to modulate T helper 2 lymphocyte responses in childhood[6]. The active VD metabolite, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], has been shown to be an important regulator of innate and adaptive immune function and modulates the host response toMycobacterium tuberculosisinfection. Toll like receptor mediated activation of macrophages up-regulates expression of the VD receptor, leading to the induction of the antimicrobial peptide cathelicidin and growth restriction ofMycobacterium tuberculosis[7].
The correlation to vaccine efficacy may be affected by sex. For instance, boys develop scars and purified protein derivative (PPD) responses more frequently than girls[8], but there is no evidence boys are better protected against TB after BCG vaccination. Some previous studies have suggested sex-differential effect of VA and on response to vaccines, when the analyses were conducted separately for each sex[9-12].
As the induced immunity is mainly cellular, the immune response to BCG vaccination is difficult to be quantified. Instead, scar development and response to an immunogenic component of BCG, PPD ofMycobacterium tuberculosis, are used to measure vaccine response in epidemiologic studies. Both scar development and PPD response were reported correlated positively with a history of BCG vaccination in Asia population[13].
We aimed to investigate whether there is an association between diameter of BCG scars and effect of PPD reaction and to examine whether VA combined VD can influence the immune response to BCG revaccination evaluated as scar formation and response to PPDin vivoin infants.
2. Material and methods
2.1. Subject selection
Ethical permission for all aspects of this research was obtained from the Ethic Review Committees of Center for Disease Control of Junan County, and studies were conducted according to the principles outlined in the Declaration of Helsinki. Parents of study subjects were informed, and each final participant submitted informed consent before the trials. Healthy infants, who did not respond to PPD test at 3, 6, 12 months of ages respectively, were enrolled in the intervention study. Exclusion criteria included severe or chronic illness, moderate to severe injury, surgery during the previous month, chronic renal failure, liver disease, heart failure, irritability of BCG, and history of other preventive inoculation less than 2 weeks.
2.2. Enrollment
Follow the policy of government, all infants in Junan County, Shandong Province were vaccinated intradermally in the upper left deltoid region with 0.1 mL BCG vaccine (contains 5 tuberculin units ) at birth. A total of 5 629 infants at 3, 6 and 12 months of age in 20 towns of Junan County from 2011 to 2012 were enrolled in our study.
2.3. Anthropometrics
Measurements were made by trained assistants when the infants were taken to local centers for disease control. The length of the infants was measured supinely using a wooden measuring board. The weight of the undressed infant was measured by weighing machine. The head circumference was measured by using a tape rule.
2.4. Scar formation and in vivo PPD response
Local assistants documented health status, health care contacts, household characteristics and vaccination status and also measured the size of the BCG scar. The BCG scars of all infants were measured. Subsequently, 0.1 mL PPD was injected intradermally on the ventral side of the left forearm. Three days later, the infant was taken to the center again, and the induration was measured by trained assistants. The infants with a PPD response (≥0.5 cm) were categorized as“PPD-responders”. Diameter of skin indurations of PPD ≥1.5 cm accompanied with blister or necrosis was judged as a strong response, and X-ray and sputum examination were used to determine whether the infants had been infected byMycobacterium tuberculosis.
2.5. Intervention assay
A community-randomised trial was performed. Of the 5 629 infants, 608 infants did not respond to PPD test (<0.5 cm) and needed to be revaccinated. Because of the difficulty to find a legal drug manufactory to make placebo in rural area of China, the double-blind trials were not used. Randomization was applied to the entire 20 towns of Junan County (using a random number generator). The infants in 10 towns were divided into supplementation group, while those in other 10 towns into control group. One infant had moved to another place and parents of 10 infants refused to participate into intervention. Finally, 307 infants were enrolled in the supplementation group and 290 in the control group. All subjects were given BCG revaccination. The subjects in the supplementation group received VA (1 500 IU) combined VD (500 IU) drops (Qingdao Double Whale Pharmaceutical), 1capsule/d for 3 months. The drops were distributed by staffs from local centers for disease control. The infants were taken to give PPD re-test after 3 months.
2.6. Statistical analysis
All the statistical analysis was performed using SPSS, version 11.5. Data were tested for normal distribution using the Kolmogorov-Smirnov test. Length, weight, head circumference and other summarized data are expressed as mean ± standard deviation (SD). Diameter of tuberculin induration of different age groups was analyzed by one-way ANOVA. Size of scar and PPD was analyzed using Student’sttests between and within the intervention groups. Prevalence of PPD response between the intervention groups was usedChi-square test. Estimates were reported as prevalence ratio with 95% confidence intervals (CI) after intervention for the supplementation group compared with the control group. Correlations between BCG scar formation and tuberculin induration were estimated using Pearson’s correlation coefficient. Differences and associations were considered significant atP<0.05.
3. Results
3.1. Effect of age on scar formation and PPD response
Details of scar formation and tuberculin testing at each age group are shown in Table 1. Real scar formation (>0.2 cm) were developed in 5 119 (90.9%) of the vaccinated infants, a tiny scar (≤0.2 cm) in 400 (7.1%), and no scar in 110 (2.0%). About 89.2% (5 021/5 629) infants responded to PPD (≥0.5 cm). Totally, 4 994 (88.7%) subjects gave a positive tuberculin reaction, 27 (0.5%) gave a strong positive induration, and 608 (10.8%) gave a PPD-negative response. X-ray and sputum examination revealed no evidence of active TB in 65 infants with a tuberculin induration greater than 1.5 cm or displaying blister or necrosis.
The median scar size was larger in the males than in the females [(0.40±0.15) cm versus (0.38±0.13) cm,P<0.05] in the 6 months group. The rate of response to PPD was higher in the males than in the females in the 3 months group (92.0% versus 88.7%,P<0.05) but was higher in females than in males in the 6 months group (88.1% versus 91.5%,P<0.05). The relation between sex and diameter of tuberculin induration showed no differences in all age groups.
3.2. Correlation between tuberculin induration and scar formation
There was a positive correlation (r=0.17,P<0.05) between scar formation and tuberculin induration (Figure 1). About 90.3% of the infants with scar formation responded to tuberculin skin test, compared to only 33.6% of those whose scar formation were not visible (P<0.05). Instead, 99.3% of the infants with positive tuberculin skin testing exhibited scar formation compared to 88.0% of those who were negative for tuberculin reactivity (P<0.05). The infants with no scars more likely did not respond to tuberculin skin testing compared those with scars (P<0.05). The PPD negative subjects were more likely to develop a tiny scar or no scar than the subjects with a positive PPD test (P<0.05).
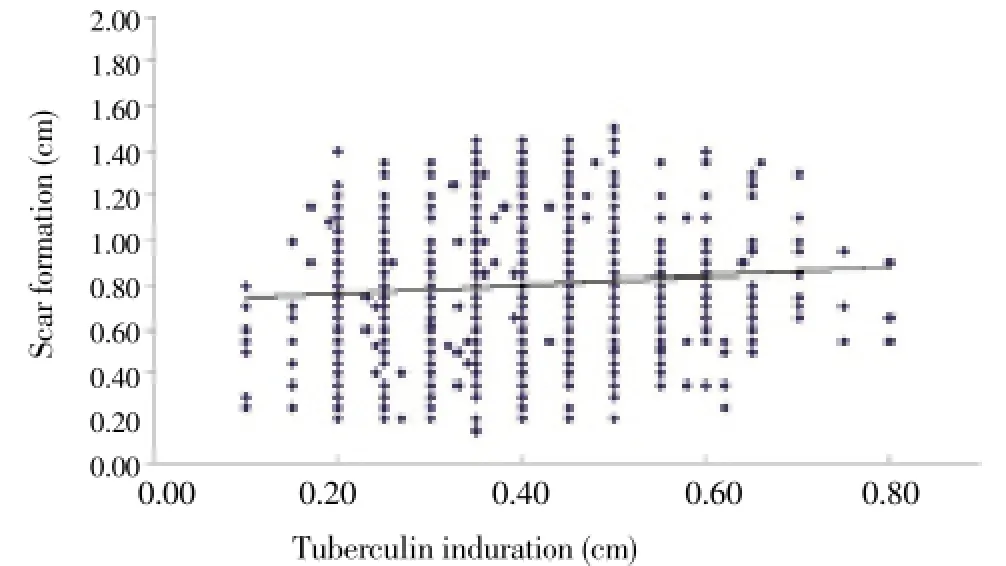
Figure 1. Correlation between tuberculin induration and scar formation.Correlations between BCG scar formation and tuberculin induration were estimated using Pearson’s correlation coefficient, due to the normal distribution. There was a positive correlation (r= 0.17, P<0.05) between scar formation and tuberculin induration according to all infants (n=5 629).
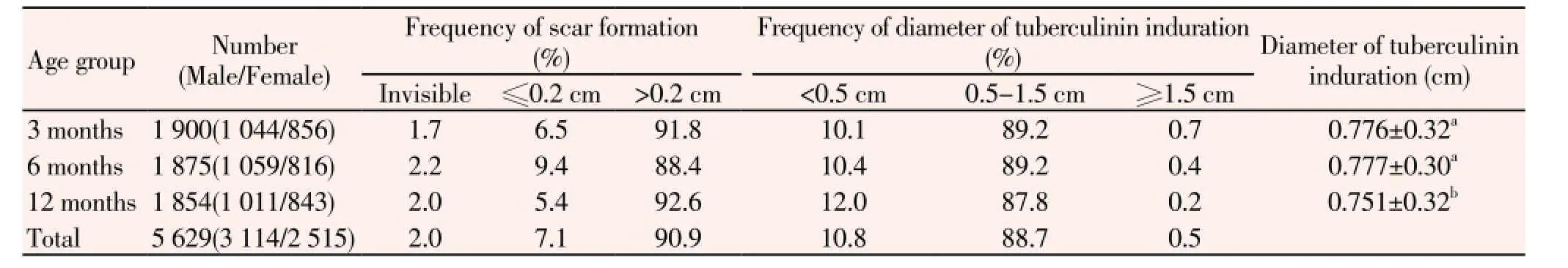
Table 1 Status of scar formation and tuberculin testing in different age groups.
3.3. Effect of VA combined VD on in vivo PPD response
Background characteristics were compared between the two intervention groups (Table 2). Sex, mean age, and other background factors of the infants did not differ between the supplementation group and control group. After 3 months, all 597 intervention subjects were returned to give PPD test and check the response. Compararison between the intervention groups is shown in Table 3. The rate of response to PPD was higher in the supplementation group than in the control group (P<0.05), and the prevalence ratio of PPD response was 1.07 (95% CI: 1.02-1.12). At 15 months of age, 97.4% responded to PPD in the supplementation group, compared to 89.7% in the control group (P<0.05), and the prevalence ratio of PPD response was 1.09 (95% CI: 1.01-1.17). The males and females in the supplementation group showed higher ratio of PPD response than those in the control group (P<0.05) respectively, and the prevalence ratios of PPD response for the supplementation group compared with that for the control group was 1.07 (95% CI: 1.01-1.13) for the males and 1.08 (95% CI: 1.00-1.17) for the females.
The rate of response to PPD was not different between sexes within the supplementation group and control group respectively (Table 4). For the supplementation group, the mean diameter of tuberculin induration was larger in the females than in the males (P<0.05) before the intervention. However, after 3 months of intervention, the males got larger tuberculin induration than the females (P<0.05). For the control group, the mean diameter of tuberculin induration was larger in the females than in the males (P<0.05) before the intervention, while no significant difference was found between the males and females after 3 months. The interaction analysis between two groups showed no differences in sexes.
4. Discussion
The BCG protective efficacy has been widely reported in Asia[14]. Despite continued efforts have been directed to improve TB control programs at the national level, China remains as a major region with the greatest burden of TB. As TB prevalence rate was high in children[15], BCG vaccination is still the important strategy in China before the appearanceof more efficient vaccines. In our study, we found there was a positive correlation between tuberculin induration and scar formation among infants. This finding is similar to a study which confirmed a highly positive correlation between scar formation and vaccination tuberculin sensitivity among preschool children[16]. The size of BCG scar was associated with considerable enhancement in sensitization to tuberculin[17,18]. Studies showed that 98% out of 361 Asian neonates[19] and 95% of 193 Asian vaccinees[20] revealed a positive post-vaccination tuberculin. Conflicting results from the United Kingdom showed that only 45%-46% of vaccinated children were positive in Mantoux when being tested between the ages of 3 months and 2 years[21]. Although the tuberculin state after vaccination was not generally thought to influence the protection offered by BCG, the higher increase of TB was in the mainly unimmunized cohorts born in a European country after 1975, compared with the mainly BCG immunized cohorts born there between 1969 and 1974[22]. It is too difficult to collect blood samples to evaluate immunological activity of infants in large population, because blood collecting is more harmful for infants and low income countries may not afford the cost. We think BCG scar and tuberculin induration are more harmless and visible for evaluation. According to previous study, our findings confirm the trends that the prevalence of PPD positivity was also consistently higher among individuals compared to those without a BCG scar.

Table 2 Background factors of intervention groups.

Table 3 Prevalence of PPD response in intervention groups after 3-month intervention (%).
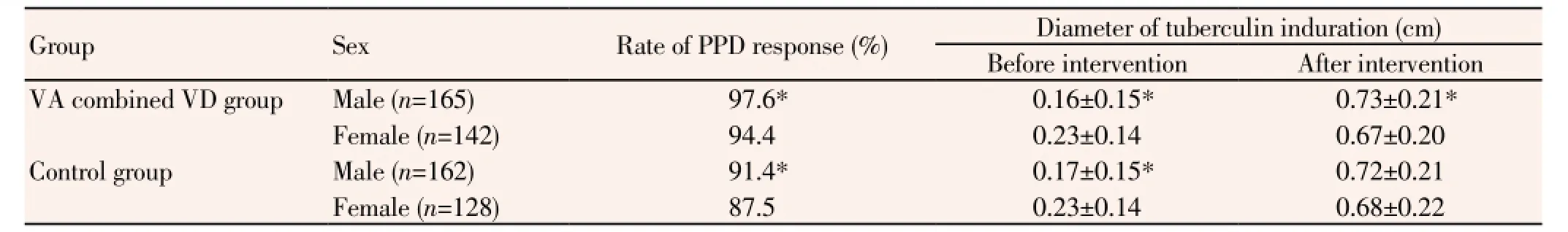
Table 4 Comparison on PPD response between different sexes of intervention groups before and after intervention.
In Malawian studies, boys develop scars and PPD responses more frequently than girls[8]. Our investigation showed larger scar formation in the males than in the females in the 6 months group, higher rate of response to PPD test in the males than in the females in the 3 months group, but higher rate of response to PPD test in the females than in the males in the 6 months group. Totally, the rate of all infants was not different between sexes. The relationship between sexes and PPD response is not clear in our study and needs further investigation.
Studies in north and south China showed a high prevalence of VA and VD deficiency among children and infants[23,24]. The high-risk groups were young, dwelled in rural areas with low socioeconomic status, poorly trained doctors and limited medical equipment, had parents with poor education and did not take a regular VA and VD containing supplement. It is associated with high prevalence of TB in rural of China[25]. Epidemiological relationships and largely clinical observation showed a protective role of VD in TB[26]. A study demonstrated that a single dose of 2.5 mg VD for adults significantly enhanced immunity against mycobacteria[27]. Studies from Asia suggested that it might be beneficial to provide VA supplementation at birth to decrease mortality[9,28]. A series of studies in Africa offered high-dose VA (200 000 IU/4 months or 50 000 IU/month) plus BCG vaccination and showed VA supplementation with BCG vaccination does not appear to interfere with the long-term immune response to BCG. VA supplementation at birth can not reduce the mortality and seems to increase the mortality of girls, and diphtheria-tetanus-pertussis vaccinations appeared to affect the rise in retinol binding protein concentrations negatively in girls[11,29]. In our study, we chose 1 500 IU VA and 500 IU VD for 3 months, lower than the tolerable upper intake levels (6 667 IU and 800 IU respectively) and far more lower than the VA provided by studies held in Africa[11,29], so the safety can be insured. According to the above study, many researches depended on VA or VD supplementation and there are both VA and VD deficiency in China, so we chose VA combined VD supplementation in our study. In our findings, the rate of response to PPD was higher in the VA combined VD supplementation group than in the control group. It proved that proper dose of VA and VD supplementation may be beneficial to enhance BCG effects on TB prevention. Two trials in Guinea-Bissau showed that VA supplementation at birth showed no effect and estimated a negative effect on girls and a potentially beneficial effect on boys. There are indications that boys may have a more pronounced Th1 profile than girls since they have a stronger delayed hypersensitivity response[10]. For our supplementation group, the mean diameter of tuberculin induration was larger in the females than in the males before the intervention, but the males got larger tuberculin induration than the females after 3 months of intervention. It indicated that the males may get better effects of TB protection after VA combined VD supplementation.
In conclusion, the correlation between tuberculin induration and scar formation indicated the BCG scar formation may be an useful tool to evaluate the effect of TB prevention. VA combined VD supplementation could raise the rate of PPD positive response and may be beneficial for countries with high prevalence of TB and related VA and VD deficiency. Further study should continue to confirm the effect of VA combined VD in TB prevention by follow-up, longitudinal investigation.
Conflicts of interest statement
All authors declare that they have no conflict of interest.
Acknowledgments
The study was funded by the National Natural Science Foundation of China (81172662) and Specialized Research Fund for the Doctoral Program of Higher Education(20123706110004). We thank all staffs and participants of the study, infants and their parents, staffs of Junan County Center for Disease Control and Prevention and Institute of Human Nutrition, Medical College of Qingdao University.
[1] Chowdhury AR, Dey RK. Penile tuberculosis following intravesical Bacille Calmette-Guérin immunotherapy. Indian J Urol 2013; 29(1): 64-66.
[2] Abubakar I, Matthews T, Harmer D, Okereke E, Crawford K, Hall T, et al. Assessing the effect of foreign travel and protection by BCG vaccination on the spread of tuberculosis in a low incidence country, United Kingdom, October 2008 to December 2009. Euro Surveill 2011; 16: 1-7.
[3] Elliott AM, Mawa PA, Webb EL, Nampijja M, Lyadda N, Bukusubaa W, et al. Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunization. Vaccine 2011; 29: 247-255.
[4] Visser ME, Grewal HM, Swart EC, Dhansay MA, Walzl G, Swanevelder S, et al. The effect of vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: A randomized controlled trial. Am J Clin Nutr 2011; 93(1): 93-100.
[5] Mastala Y, Nyangulu P, Banda RV, Mhemedi B, White SA, Allain TJ. Vitamin D deficiency in medical patients at a central hospital in Malawi: A comparison with TB patients from a previous study. PLoS One 2013; 8(3): 59017.
[6] Benn CS. Combining vitamin A and vaccines: convenience or conflict? Dan Med J 2012; 59(1): 4378
[7] Ní Cheallaigh C, Keane J, Lavelle EC, Hope JC, Harris J. Autophagy in the immune response to tuberculosis: clinical perspectives. Clin Exp Immunol 2011; 164(3): 291-300.
[8] Floyd S, Ponnighaus JM, Bliss L, Warndorff DK, Kasunga A, Mogha P, et al. BCG scars in northern Malawi: sensitivity and repeatability of scar reading, and factors affecting scar size. Int J Tuberc Lung Dis 2000; 4: 1133-1142.
[9] Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, et al. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr 1996; 128: 489-496.
[10] Benn CS, Aaby P, Bale C, Jensen H, Lisse I M, Aaby P. Randomised trial of effect of vitamin A supplementation on antibody response to measles vaccine in Guinea-Bissau, west Africa. Lancet 1997; 350: 101-105.
[11] Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N. Adverse event reports following yellow fever vaccination. Vaccine 2008; 26: 6077-6082.
[12] Diness BR, Fisker AB, Roth A, Yazdanbakhsh M, Sartono E, Whittle H, et al. Effect of high-dose vitamin A supplementation on the immune response to Bacille Calmette-Guérin vaccine. Am J Clin Nutr 2007; 86: 1152-1159.
[13] Setiawati L, Endaryanto A, Kusumadewi A, Lestari P. Effect of BCG vaccination and non-tuberculous Mycobacterium infection on interferon gamma specific assay and a tuberculin skin test among children with a tuberculosis contact in Surabaya, Indonesia. Southeast Asian J Trop Med Public Health 2011; 42(6): 1460-1468.
[14] Chan PC, Yang CH, Chang LY, Wang KF, Kuo YC, Lin CJ, et al. Lower prevalence of tuberculosis infection in BCG vaccinees: A cross-sectional study in adult prison inmates. Thorax 2013; 68(3): 263-268.
[15] Yang XY, Zhang NM, Diao X, Mao X, Li YP. Epidemiological analysis of pulmonary tuberculosis in Sichuan Province, China, 2000-2006. Int J Infect Dis 2008; 12: 534-541.
[16] Djuardi Y, Sartono E, Wibowo H, Supali T, Yazdanbakhsh M. A longitudinal study of BCG vaccination in early childhood: The development of innate and adaptive immune responses. PLoS One 2010; 5(11): 14066.
[17] Burl S, Adetifa UJ, Cox M, Touray E, Whittle H, McShane H, et al. The tuberculin skin test (TST) is affected by recent BCG vaccination but not by exposure to non-tuberculosis mycobacteria (NTM) during early life. PLoS One 2010; 5(8): 12287.
[18] Chang CM, Lee NY, Lee HC, Wu CJ, Chen PL, Lee CC, et al. Positive tuberculin skin tests in nursing home residents in Southern Taiwan. Arch Gerontol Geriatr 2010; 51(3): 129-132.
[19] Handfield JW, Allan J, Windebank WJ. Sensitivity of neonates to tuberculin after BCG vaccination. BMJ 1986; 292: 989-991.
[20] Packe GE, Innes JA. Protective effect of BCG vaccination in infant Asians: A case-control study. Arch Dis Child 1988; 63: 277-281.
[21] Lumb KM, Bandaranayake R, Beran PJ. BCG vaccination in infancy. Public Health 1986; 100: 54-55.
[22] Romanus V. Tuberculosis in Bacillus Calmette-Guerin-immunized and unimmunized children in Sweden: A ten-year evaluation following the cessation of general Bacillus Calmette-Guerin immunization of the newborn in 1975. Pediatr Infect Dis J 1987; 6: 272-280.
[23] Jiang JX, Lin LM, Lian GL, Greiner T. Vitamin A deficiency and child feeding in Beijing and Guizhou, China. World J Pediatr 2008; 4: 20-25.
[24] Strand MA, Perry J, Zhao J, Fischer PR, Yang J, Li S. Severe vitamin D-deficiency and the health of North China children. Matern Child Health J 2009; 13: 144-150.
[25] Li W, Zhang R, Zhang K, Song L, Hong PH, Zhang Y, et al. Reduced vitamin D levels are associated with autoimmune response in tuberculosis patients. Ann Rheum Dis 2012; 71: 790.
[26] Grange JM, Davies PD, Brown RC, Woodhead JS, Kardjito T. A study of vitamin D levels in Indonesian patients with untreated pulmonary tuberculosis. Tubercle 1985; 66: 187-191.
[27] Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 2007; 176: 208-213.
[28] Rahmathullah L, Tielsch JM, Thulasiraj RD, Katz J, Coles C, Devi S, et al. Impact of supplementing newborn infants with vitamin A on early infant mortality: Community based randomised trial in southern India. BMJ 2003; 327: 254-259.
[29] Benn CS, Fisker AB, Napirna BM, Roth A, Diness BR, Lausch KR, et al. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: Two by two factorial randomized controlled trial. BMJ 2010; 340: 1-10.
*Corresponding author: Ai-Guo Ma, Institute of Human Nutrition, Medical College of Qingdao University, NO. 38, Dengzhou Road, Qingdao 266021, PR China.
Tel: +86-532-82991503
Fax: +86-532-83812434
E-mail: jasminemaki@126.com
#These authors contributed equally to this work.
Foundation project: The study was funded by National Natural Science Foundation of China (81172662) and Specialized Research Fund for the Doctoral Program of Higher Education (20123706110004).
 Asian Pacific Journal of Tropical Medicine2014年2期
Asian Pacific Journal of Tropical Medicine2014年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Diagnosis and multi-modality treatment of adult pulmonary plastoma: Analysis of 18 cases and review of literature
- Mathematical modeling for selecting center locations for medical and health supplies reserve in Hainan Province
- Bond strength analysis of the bone cement- stem interface of hip arthroplasties
- Comparison on serum biomarkers for anovulatory and ovulatory dysfunctional uterine bleeding in Lizu females
- Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
- Hepatic effect of NAC on sevear acute pancteatise of rats
