Recovery of Cyclospora cayetanensis among asymptomatic rural Thai schoolchildren
K Thima, H Mori, R Praevanit, S Mongkhonmu, J Waikagul, D Watthanakulpanich*
1Department of Protozoology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
2Bangkok School of Tropical Medicine, Mahidol University, Bangkok, Thailand
3Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Recovery of Cyclospora cayetanensis among asymptomatic rural Thai schoolchildren
K Thima1, H Mori1, R Praevanit2, S Mongkhonmu2, J Waikagul3, D Watthanakulpanich3*
1Department of Protozoology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
2Bangkok School of Tropical Medicine, Mahidol University, Bangkok, Thailand
3Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Objective: To obtain the prevalence with clinical symptoms of Cyclospora cayetanensis (C. cayetanensis), a coccidian protozoan parasite, in Thailand which is the cause of an intestinal infection characterized by sporadic-to-frequent explosive diarrhea. Methods: In a field survey conducted by the Faculty of Tropical Medicine, Mahidol University, as part of the existing parasite-control program, a total of 2 540 faecal samples from villagers in Nan Province, Thailand, were collected and examined to determine the prevalence and clinical characteristics of parasitic infections. Results: Twelve cases of C. cayetanensis infection were found during faecal examination of schoolchildren aged 5-12 years. None exhibited obvious clinical symptoms, especially evidence of diarrhea; 5 of 12 had loose faeces, one reported frequent symptoms of abdominal discomfort, and another had pale conjunctiva with low hematocrit. The children were generally asymptomatic. Conclusions: This finding confirms a public-health issue with potentially serious consequences whereby children can be exposed to an environment contaminated with food-and water-borne transmitted oocysts, and can hence become infected with C. cayetanensis.
ARTICLE INFO
Article history:
Received 10 October 2013
Received in revised form 15 December 2013
Accepted 15 January 2014
Available online 20 February 2014
Cyclospora cayetanensis
1. Introduction
Cyclospora cayetanensis(C. cayetanensis) is an emerging coccidian parasite, first described by Ashford from three Papua New Guinea patients[1]. It has recently been recognized as a new cause of prolonged diarrheal illness worldwide, but with greater prevalence in tropical and subtropical area[2,3]. Its mode of person-to-person transmission is via oral-faecal route by oocysts in contaminated water or food[3,4].C. cayetanensisappeared to be associated mainly with prolonged self-limiting diarrhea (typically 7-9 weeks) in both immunocompetent and immunocompromised patients[5-11] and also as a cause of travelers’ diarrhea confined to adult foreigners infected while visiting endemic regions[3,12,13]. Patients normally report symptoms of nausea, vomiting, anorexia leading to weight loss, abdominal cramping and pain with increased gas, flatulence, fatigue and watery diarrhea[14,15]. In developing countries, especially among populations where sanitation is poor and insufficient, this protozoa is also associated with diarrhea among children. However, asymptomatic excreters have also been reported[4].
FewCyclosporainfected Thai cases have been reported recently in the literature[16-21]. Most reports dealt with immunocompromised hosts. The limited number of reported cyclosporiasis cases in Thailand is probably due to a lack of experience and awareness among laboratory workers. It is hoped that our findings will stimulate microscopists to be alert of potentialCyclosporainfection in Thailand evenwhen faecal samples are not collected from HIV/AIDS-infected patients or patients with prolonged diarrhea. The normal or immunocompetent hosts can probably serve as carriers of the disease without any obvious clinical symptoms or symptoms that might probably occur later. More research is required to monitorCyclosporaorganisms in immunocompetent hosts and the environment. This should yield further data to help clarify the real incidence and prevalence of cyclosporiasis in Thailand.
2. Materials and methods
2.1. Study area and study group
This study was approved by the Ethics Committees of the Faculty of Tropical Medicine, Mahidol University, Thailand. The hill-tribe people of Thailand mostly inhabit the North provinces such as Mae Hong Son, Chiang Rai, Chiang Mai and Nan Provinces. They principally earn their livelihoods through agriculture. Mountainous areas in tropical zones, such as Nan Province, are favourable for the development of the parasite and transmission of the disease, due to the traditional lifestyle and poor hygiene of hill-tribe people, who may harbor several different kinds of parasites. Epidemiological survey results for intestinal helminthiases have been reported in details elsewhere[22]. Among the total 2 540 faecal samples, 497 were identified to have protozoan infections by direct smear technique. Most of the protozoa found wereBlastocystis hominis (B. hominis)(22.2%) whilst the others wereGiardia intestinalis (G. intestinalis)(1.9%),Sarcocystis hominis (S. hominis)(0.7%),Entamoeba histolytica (E. histolytica)(0.7%) andamoeba trophozoite(0.7%). Some non-pathogenic protozoa were also found, such asEntamoeba coli (E. coli)(5.5%),Endolimax nana (E. nana)(5.5%) andTrichomonas hominis (T. hominis)(0.1%). In addition, human migration is common in the area, therefore disease transmission from place to place is possible. In view of this, the hill-tribe people in Nan Province were selected as the target group to investigate the prevalence of hiddenC. cayetanensisinfection. The prevalence ofC. cayetanensisin faecal specimens was evaluated among the hill-tribe people in all ages in both sexes, male and female, who were willing to join the project, in Thung Chang and Chalerm Phrakiat Districts, Nan Province, northern Thailand, on the Thai-Lao borders.
2.2. Faecal examination
Faecal samples were collected from residents of Thung Chang and Chalerm Prakiet Districts, Nan Province, northern Thailand. A plastic container was provided to each participant for faecal specimen collection. Faecal examination was examined for both protozoan and helminthic infections. For protozoan infections, samples were examined by direct-smear technique, in both normal saline solution and 1% iodine solution[23]. The modified cellophane thick-smear method was used for helminthic infections[24]. Subjects whose faecal samples were found by direct wet mount to contain Cryptosporidium-liked organisms were selected for further laboratory investigation with rapid DMSO modified acid-fast stain to identify forC. cayetanensisinfection[25].
2.3. Physical examination and clinical laboratory investigation
The students/villagers were approached at the selected schools/communities to participate in the study for those who were positive forC. cayetanensisand treated. The participants were asked to have an interview according to the questionnaires was taken in a private area. Physical examination was performed by medical doctors for all participants corresponding with the positive results ofC. cayetanensis-positive results and the signs and symptoms of each patient were recorded. About 1 mL of blood was drawn for evaluation of CBC only in the Thung Chang Community Hospital where was nearby the study area. The follow up of the treatment was performed by faecal examination after a month to evaluate the effectiveness of medicine.
2.4. Molecular study
DNA was extracted and analyzed by PCR, using a PSP Spin Stool DNA kit (Stratek, Germany), according to the manufacturer’s instructions. Nested PCR, targeting small subunit rRNA, was performed. Primary primers CYCF1 (5´-ATT ACC CAA TGA AAA CAG TTT-3´) and CYCR2 (5´-TGC AGG AGA AGC CAA GGT AGG-3´) were used for the first PCR. Secondary primers CYCF3 (5´-GCC TTC CGC GCT TCG CTG CGT-3´) and CYCR4 (5´- TCG TCT TCA AAC CCC CTA CTG-3´) were used for the second PCR. Amplification with these primers generated a 294-bp fragment from theC. cayetanensissmall subunit rRNA coding region[26]. Each 25 μL PCR mixture contained 1× PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 2.5 UTaqpolymerase (Fermentus, USA), and 1 μM of each primer. For primary and secondary PCR, 30 cycles of 94 ℃ for 1 min, 60 ℃ for 1 min and 72 ℃ for 1 minwere used. Of the PCR results, 10 μL were separated by agarose gel electrophoresis (1.5% agarose) and visualized after staining in a 1 μg/mL ethidium bromide solution for 10 minutes.
3. Results
Among the 2 540 participants, 2 001 were adults and 539 were children. Twelve children (7 males and 5 females, gender ratio 1.4: 1.0) were found to haveC. cayetanensisinfection. The positive cases were all primaryschoolchildren from Ban Pang Kae, Thung Chang District and Ban Huai Kon, Chalerm Phrakiat District, Nan Province. By age group, prevalence ranged between 5-12 years, the children had no experience of prolonged fever, vomiting or weight loss, but some had episodes of upper respiratory tract infections.
C. cayetanensis-positive faecal samples can be detected by direct smear with normal saline solution. Numerous spherical organisms, containing 8-12 refractile globular granules; turned brown and revealed a distinct, thick wall in 1% iodine solution. These are identified asC. cayetanensisoocysts (Figure 1). The sizes of the oocysts varied from 8-10 μm in diameter. With modified acid-fast stain, these round bodies remain unstained, but are tinged glassy-green, with a pink background. Some sporulated oocysts are confirmed by the observation of two characteristic sporocysts within each oocyst after modified acid-fast staining (Figure 2).
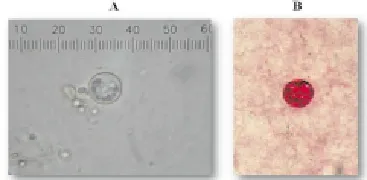
Figure 1. Organisms identified as C. cayetanensis.

Figure 2. Modified acid-fast staining revealed sporulated oocysts.
The findings from physical examinations were mostly unremarkable. Infected schoolchildren showed no obvious clinical features or diarrhea except for somewhat loose faeces (41.7%), abdominal pain (8.3%), and pale conjunctiva with low hematocrit (8.3%) and no eosinophilia. Some (66.7%) had mixed infections of hookworms with the intensity ranged between 23-897 EPG and non-pathologic protozoa, such asE. coli, E. nana,andT. hominis. In the molecular study, samples were found positive forC. cayetanensisby nested PCR. The infected schoolchildren were all treated with trimethoprim-sulfamethoxazole (TMP-SMZ) (160 mg/800 mg) 1 tablet/day for a week forC. cayetanensisinfection and albendazole (200 mg) 2 tablets single dose for hookworm infection. Faecal sample were collected and examined repeatedly as treatment follow-up, however no parasites were found.
4. Discussion
Cyclosporiasis is generally associated with diarrhea among children in developing countries while travelers’ diarrhea and food- and water-borne disease outbreaks, usually occur in developed counties[27]. This corresponds with the finding ofC. cayetanensisamong hill-tribe children in rural areas of northern Thailand, where sanitation and personal hygiene are poor. Cases of cyclosporiasis are frequently missed, despite the increase in data on this parasite, mainly because the parasite can be difficult to detect in human faecal samples[7].
Diagnosis in many cases is made by microscopic faecal examination and appropriate staining techniques, such as modified acid-fast stain, which yields greater sensitivity in the diagnosis ofCyclosporaorganisms. They are generally refractile, and 8-10 μm in diameter under wet-mount, which can be difficult for those unfamiliar with them. Hence, rapid DMSO modified acid-fast stain is recommended since the organisms uptake the stain and appear red or pink with variable intensity, with 10- to 20-dot granular inclusions. The method is more suitable for routine investigation in remote area than other established methods for positive diagnosis ofC. cayetanensis, including the recovery of oocysts in intestinal fluid, small bowel biopsy or even amplification by polymerase chain reaction, which required special equipments, trained personnel, labourintensive, and higher cost. The environmentally resistant form of the organisms, the oocyst, is shed in faeces, and subsequently sporulates into the infectious form after 5-13 days’ incubation at ambient temperature[2,10,11]. Repeatedexaminations of the same faeces over consecutive days with special stains should be considered to increase the detection ofCyclosporasporocysts, which possess a more typical morphology, and easier to recognize than unsporulated oocysts. Regarding molecular studies,CyclosporaDNA has been successfully amplified from food samples using nested PCR. In the regimen, primary PCR products, 639-bp fragments, are recommended for DNA sequencing[26]. In the present study, the primers were used for stool samples under modified conditions. The results showedC. cayetanensis-positive amplified-product bands at 294 bp of secondary PCR products. But few bands were seen at 639 bp from primary PCR products. Therefore, the amounts of primary PCR products were inadequate for sequencing. Low sensitivity for this nested PCR techniques with stool samples has been reported elsewhere[28]. It is hoped that more research will be conducted and published on molecular diagnostic techniques in stool samples to elucidate this organism further.
This study also described the clinical features of the infected schoolchildren who appeared normal. This increase concerns about indirect human-to-human spread of the parasite via oocysts. To the best of our knowledge, this is the first report of infection among asymptomatic children who were undertaking their normal activities and daily lives in Thailand Information about cyclosporiasis remains scanty in this country. There was no evidence of diarrhea in any of theC. cayetanensis–positive cases in this study, nor of other main symptoms noted in previous reports (eg., nausea, fatigue, abdominal cramp, fever, headache)[29]. However, some clinical features were presented in some caseseg. loose faecal excretion with mucous in 5 of 12 cases (41.7%) even most of them (4 out of 5 cyclosporiasis cases) were mixed-infected with non-pathogenic protozoa such as E. coli and E. nana. Meanwhile, only one case each of sporadic abdominal pain corresponding with meal times, and pale conjunctiva with low hematocrit, were found. These presentation were probably not caused by Cyclospora infection, particularly one with mixed Cyclospora-hookworm infection (897 EPG), which was classified as a moderate infection by the number of eggs found (range 100-999) in an entire microscopic fields[30,31]. This could have caused the anemia in addition to the child’s malnutrition[32]. The other parasites in mixed infections; E. coli, E. nana, and T. hominis, were non-pathogenic and asymptomatic.
In conclusion, this study appears that the clinical features of the infected schoolchildren ofC. cayetanensisgave no obvious signs of which might possibly serve as disease carriers whereby children can be exposed to an environment contaminated with food-and water-borne transmitted oocysts, and can hence become infected withC. cayetanensis.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This study was partially supported by a grant from the Faculty of Tropical Medicine, Mahidol University. Thanks to staff of the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University for collecting subjects’ faeces. Thanks also to Dr. Yaowalak Sukthana for permission to use molecular-laboratory facilities, Mrs. Panida Muangkhum for assistance enlisting villagers’ cooperation in the study and Mr. Paul Adams for reading manuscript. Lastly, an acknowledgement in fond memory of the late Dr. Chuthatip Siriphant for her kind confirmation and comments on theC. cayetanensisorganisms.
[1] Ashford RW. Occurrence of an undescribed coccidian in man in Papua New Guinea. Ann Trop Med Parasitol 1979; 73: 497-500.
[2] Ortega YR, Sterling CR, Gilman RH, Cama VA, Diaz F. Cyclospora species: A new protozoan pathogen of humans. N Engl J Med 1993; 328: 1308-1312.
[3] Puente S, Morente A, Garcia-Benavas T, Subirat M, Gascon J, Gonzalez-Lahoz JM. Cyclosporiasis: a point source outbreak acquired in Guatemala. J Travel Med 2006; 13: 334-337.
[4] Mansfield LS, Gaiadhar AA. Cyclospora cayetanensis, a food-and waterborne coccidian parasite. Vet Parasitol 2004; 126: 73-90.
[5] Tiirk M, Tiirker M, Ak M, Karaavak B, Kava T. Cyclosporiasis associated with diarrhoea in an immunocompetent patient in Turkey. J Med Microbiol 2004; 53: 255-257.
[6] Helmy MM, Rashed LA, Abdel-Fattah HS. Co-infection with Cryptosporidium parvum and Cyclospora cayetanensis in immunocompromised patients. J Egyp Soc Parasitol 2006; 36: 613-627.
[7] Koru O, Araz E, Inci A, Tanvuksel M. Co-infection of Giardia intestinalis and Cyclospora cayetanensis in an immunocompetent patient with prolonged diarrhea: case report. J Microbiol 2006; 44: 360-362.
[8] Sancak B, Akvon Y, Eraiiven S. Cyclospora infection in fiveimmunocompetent patients in a Turkish university hospital. J Med Microbiol 2006; 55: 459-462.
[9] Bouree P, Lancon A, Bisaro F, Bonnot G. Six human cyclosporiasis: with general review. J Egyp Soc Parasitol 2007; 37: 349-360.
[10] Masucci L, Graffeo R, Siciliano M, Eranceschelli A, Bugli F, Fadda G. First Italian case of cyclosporiasis in an immunocompetent woman: local acquired infection. New Microbiol 2008; 31: 281-284.
[11] Naito T, Mizue S, Misawa S, Nakamura A, Isonuma H, Kondo S, et al. Cyclospora infection in an immunocompetent patient in Japan. Jap J Inf Dis 2009; 62: 57-58.
[12] Yu JR, Sohn WM. A case of human cyclosporiasis causing traveler’s diarrhea after visiting Indonesia. J Kor Med Sci 2003; 18: 738-741.
[13] Kansouzidou A, Charitidou C, Varnis T, Vavatsi N, Kamaria F. Cyclospra cayetanensis in a patient with travelers’ diarrhea: case report and review. J Travel Med 2004; 11: 61-63.
[14] Brennan MK, MacPherson DW, Palmer J, Keystone JS. Cyclosporiasis: A new cause of diarrhea. Cmaj 1996; 155: 1293-1296.
[15] Visvesvara GS, Moura H, Kovacs-Nace E, Wallace S, Eberhard ML. Uniform staining of Cyclospora oocysts in fecal smears by a modified safranin technique with microwave heating. J Clin Microbiol 1997; 35: 730-733.
[16] Morakote N, Siriprasert P, Piangjai S, Vitayasai P, Tookyang B, Uparanukraw P. Microsporidium and Cyclospora in human stools in Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health 1995; 26: 799-800.
[17] Manatsathit S, Tansupasawasdikul S, Wanachiwanawin D, Setawarin S, Suwanagool P, Prakasvejakit S, et al. Causes of chronic diarrhea in patients with AIDS in Thailand: a prospective clinical and microbiological study. J Gastroenterol 1996; 31: 533-537.
[18] Chokephaibulkit K, Wanachiwanawin D, Tosasuk K, Vanprapa N, Chearskul S. A report case of Cyclospora and Cryptosporidium mixed infection in a HIV-negative child in Thailand. J Med Assoc Thai 2001; 84: 589-592.
[19] Wiwanitkit V. Intestinal parasite infestation in HIV infected patients. Curr HIV Res 2006; 4: 87-96.
[20] Viriyavejakul P, Nintasen R, Punsawad C, Chaisri U, Punpoowong B, Riganti M. High prevalence of Microsporidium infection in HIV-infected patients. Southeast Asian J Trop Med Public Health 2009; 40: 223-228.
[21] Saksirisampant W, Prownebon J, Saksirisampant P, Mungthin M, Siripatanapipong S, Leelayoova S. Intestinal parasitic infections: prevalences in HIV/AIDS patients in a Thai AIDS-care centre. Ann Trop Med Parasitol 2009; 103: 573-581.
[22] Maipanich W, Waikagul J, Watthanakulpanich D, Muennoo C, Sanguankiat S, Pubampen S, et al. Intestinal parasitic infections among inhabitants of the North, West-central and Eastern border areas of Thailand. J Trop Med Parasitol 2004; 27: 51-58.
[23] Ash LR, Orihel TC. Parasites: A guide to laboratory procedures and identification. Chicago: American Society of Clinical Pathologists (ASCP) Press; 1987, p. 18-19.
[24] Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 1972; 14: 397-400.
[25] Bronsdon MA. Rapid DMSO modified acid-fast stain of Cryptosporidium oocysts in stool specimens. J Clin Microbiol 1984; 19: 952-953.
[26] Ho AY, Lopez AS, Eberhart MG, Levenson R, Finkel BS, da Silva AJ, et al. Outbreak of cyclosporiasis associated with imported raspberries, Philadelphia, Pennsylvania, 2000. Emerg Inf Dis 2002; 8: 783-788.
[27] Karanja RM, Gatei W, Wamae N. Cyclosporiasis: an emerging public health concern around the world and in Africa. Afri Health Sci 2007; 7: 62-67.
[28] Pieniazek NJ, Slemenda SB, da Silva AJ, Alfano EM, Arrowood MJ. PCR confirmation of infection with Cyclospora cayetanensis. Emerg Inf Dis 1996; 2: 357-359.
[29] Milord F, Lampron-Goulet E, St-Amour M, Levac E, Ramsay D. Cyclospora cayetanensis: a description of clinical aspects of an outbreak in Quebec, Canada. Epidemiol Inf 2011; 27: 1-7.
[30] Kobayashi A. Collected papers on the control of soil-transmitted helminthiases by the APCO Research Group. Vol. Ⅰ. Tokyo: The Asian Parasite Control Organization; 1980.
[31] Watthanakulpanich D, Waikagul J, Maipanich W, Nuamtanong S, Sanguankiat S, Pubampen S, et al. Haplorchis taichui as a possible etiologic agent of irritable bowel syndrome-like symptoms. Korean J Parasitol 2010; 48: 225-229.
[32] Watthanakulpanich D, Maipanich W, Pubampen S, Sa-nguankiat S, Pooudouang S, Chantaranipapong Y, et al. Impact of hookworm deworming on anemia and nutritional status among children in Thailand. Southeast Asian J Trop Med Public Health 2011; 42: 782-792.
*Corresponding author: D Watthanakulpanich, Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand.
Tel: +662 643 5600
E-mail: dorn.wat@mahidol.ac.th
Schoolchildren
Carriers
Traveler’s diarrhea
 Asian Pacific Journal of Tropical Medicine2014年2期
Asian Pacific Journal of Tropical Medicine2014年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Diagnosis and multi-modality treatment of adult pulmonary plastoma: Analysis of 18 cases and review of literature
- Mathematical modeling for selecting center locations for medical and health supplies reserve in Hainan Province
- Bond strength analysis of the bone cement- stem interface of hip arthroplasties
- Comparison on serum biomarkers for anovulatory and ovulatory dysfunctional uterine bleeding in Lizu females
- Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
- Hepatic effect of NAC on sevear acute pancteatise of rats
