Antioxidant effects of Phyllanthus niruri tea on healthy subjects
Elisângela Colpo, Carlos D. D. A. Vilanova, Romaiana P. Pereira, Luis Gustavo B. Reetz, Liliane Oliveira, Iria L. G. Farias, Aline A. Boligon, Margareth L. Athayde, João Batista T. Rocha*
1Departamento de Química, Centro de Ciências Naturais e Exatas, Universidade Federal de Santa Maria (UFSM), Santa Maria, RS, Brazil
2Laboratório de Análises Clínicas, Hospital Universitário, Universidade Federal de Santa Maria (UFSM), Santa Maria, RS, Brazil
3Departamento de Farmácia Industrial, Universidade Federal de Santa Maria (UFSM), Santa Maria, RS, Brazil
Antioxidant effects of Phyllanthus niruri tea on healthy subjects
Elisângela Colpo1, Carlos D. D. A. Vilanova1, Romaiana P. Pereira1, Luis Gustavo B. Reetz2, Liliane Oliveira2, Iria L. G. Farias2, Aline A. Boligon3, Margareth L. Athayde3, João Batista T. Rocha1*
1Departamento de Química, Centro de Ciências Naturais e Exatas, Universidade Federal de Santa Maria (UFSM), Santa Maria, RS, Brazil
2Laboratório de Análises Clínicas, Hospital Universitário, Universidade Federal de Santa Maria (UFSM), Santa Maria, RS, Brazil
3Departamento de Farmácia Industrial, Universidade Federal de Santa Maria (UFSM), Santa Maria, RS, Brazil
Objective: To investigate the potential antioxidant effects of Phyllanthus niruri (P. niruri, Euphorbiaceae) tea on healthy subjects. Methods: Five non-smoking, male healthy volunteers, 20 to 31 years old, were enrolled. Each subject was treated twice, following a randomized crossover fashion regarding the ingestion of P. niruri infusion (5 g/750 mL) (tea group) or 750 mL of water (control group). Fasting venous blood samples were collected prior to and at 1, 2 and 4 h after infusion drinking. Samples were tested for plasmatic gallic acid and ascorbic acid levels, erythrocytic catalase and superoxide dismutase activities, and intracellular DCFH fluorescence in granulocytes, monocytes and lymphocytes. Results: Catalase and superoxide dismutase activities were not altered by tea ingestion. Plasma levels of gallic acid were significantly increased at 1, 2 and 4 h after P. niruri ingestion and plasma ascorbic acid at 1 h after P. niruri ingestion.
Conclusions: Ingestion of P. niruri tea is associated with a slight increase in antioxidant markers in human blood (ascorbic acid and gallic acid), which may contribute to its pharmacological effects.
ARTICLE INFO
Article history:
Received 10 October 2014
Received in revised form 15 December 2013
Accepted 15 January 2014
Available online 20 February 2014
Antioxidants
Polyphenols
Phyllanthus niruri
Human
Ascorbic acid
Gallic acid
1. Introduction
Oxidative stress has been implicated in various pathological conditions including cardiovascular diseases, cancer and aging[1]. Positive association between the consumption of polyphenol-rich foods and beverages and the prevention of these conditions has been shown by several epidemiological studies[2]. Tea is one of the most popular beverages consumed by humans and many different types have been shown to contain polyphenols[3-5], which are known for their antioxidant properties. Moreover, the reducing power of polyphenols associated with other dietary antioxidants can protect the body tissues against oxidative stress[6-8].
Phyllanthus niruri(P. niruri, Euphorbiaceae) is a plant widely found in Brazil, locally known as quebra-pedra (stone crusher) and is popularly used for the treatment of renal pathologies, particularly urolithiasis[9,10]. The medicinal properties of this plant have been associated with some of its active components such as lignans, glycosides, alkaloids, ellagitannins, terpenes and phenylpropanoids, besides flavonoids and polyphenols, such as quercetin,rutin and gallic acid (GA)[11]. Although manyin vitroandin vivoantioxidant effects ofP. niruriextracts have been shown[11-13], which seem to be determined by its polyphenolic components[11,14,15], the bioavailability of those components and their impact on human health need further investigation.
Recently, we have shown thatP. niruriexhibits antioxidant effects inin vitroandin vivomodels of liver toxicity in mice[11]. That raises the possibility that this plant can have a broader antioxidant effect in mammals. Therefore, considering the presence of GA, one of the most readilyabsorbed polyphenolic compounds from vegetables[15], and the lack of studies investigating the effects ofP. niruritea ingestion, we focused the current study on determining the plasma levels of GA following the ingestion of the tea. Additionally, we have also investigated the potential antioxidant effects ofP. niruritea ingestion on healthy subjects.
2. Materials and methods
2.1. Preparation of P. niruri infusion
To prepare the infusion, a commercially available brand ofP. niruri(Prenda®) tea was used. The phytochemical composition of the commercial preparation was identical to that of a knownP. nirurivoucher specimen (number SMDB 13142 at the UFSM herbarium). The infusion was prepared with five packets (5 g) in 750 mL of boiling water. The infusion was allowed to cool for 15 min before being given to the subjects. The volunteers were then instructed to consume the tea within 10 min.
2.2. Participants
The study protocol n.0034.0.243.000-10 was approved by the Human Ethics Committee of the Universidade Federal de Santa Maria and informed consent was obtained from all of the participants. Five non-smoker, male subjects with no history of previous chronic diseases and not under treatment for any current chronic or acute diseases (as self declared by the volunteers), average age of (27.2±1.5) (range 20 to 30) years old, average body mass index of (24.5±2.3) (range 22.8-27.4) kg/m2, were enrolled. Exclusion criteria included smoking, high alcohol intake (above 20 g of alcohol/d), consumption of vitamins, mineral supplements or the acute or chronic use of any other licit or illicit drugs. Each subject was tested twice following a randomized crossover design regarding the ingestion of theP. niruritea or the same amount of lukewarm water. Fasting venous blood samples were collected into 10 mL heparinized syringes at 0 (before drinking), 1, 2 and 4 h after either tea or water ingestion. Blood samples were always drawn between 8:00 am and 1:00 pm. The volunteers were instructed by a nutritionist to avoid the consumption of flavonoid-rich (apples, grapes and their derivatives such as wine and juices), phytate- or tannincontaining (coffee, tea, chocolate, among others) aliments on the day before and during the blood sampling period.
2.3. Sample processing and quantification of GA by HPLC
The blood samples were stored at -20 ℃ for up to 2 months. The samples were then thawed on ice and centrifuged for 1 min at 10 000 r/min at 4 ℃. The plasma fractions (1.5 mL) were transferred to 15 mL plastic tubes containing 13.5 mL of nitrogen-saturated methanol (HPLC grade) and stored at -20 ℃ for up to 2 months. The tubes were vigorously vortexed and then centrifuged at 1 200 r/min for 10 min, at 4 ℃. The supernatants were decanted into glass tubes under a stream of nitrogen and the tubes were screw-capped right away[16]. The chromatographic analyses were carried out in isocratic conditions using a RP-C18 column (4.6 mm × 250.0 mm) packed with 5 μm diameter particles. The mobile phase consisted of methanol:acetonitrile:water (40:15:45, v/v) containing 1% (v/v) acetic acid. Aliquots (20 μL) of the clear methanolic plasma extract were injected and run at 0.8 mL/min with detection at 254 nm. The mobile phase was filtered through a 0.45 μm membrane filter and then degassed by ultrasound immediately before use. Reference GA solutions were prepared in the HPLC mobile phase at concentrations ranging from 0.006 to 0.250 mg/mL[17-18]. The retention times of the peaks from the plasma samples were compared to those obtained for the reference GA solutions and quantitated by peak integration using the external standard method. The calibration curve for GA wasy= 53 985x+1 020.6 (r=0.985 9). All chromatographic procedures were performed at room temperature and in triplicate.
2.4. Determination of plasmatic ascorbic acid (AA)
Plasma AA levels were measured as described by Jacques-Silvaet al[19]. Plasma was precipitated with one volume of 100 g/L cold trichloroacetic acid solution and centrifuged at 3 000 r/min for 5 min at 4 ℃. Aliquots (300 μL) of the supernatants were mixed with 2,4-dinitrophenylhydrazine (4.5 mg/mL), CuSO4(0.075 mg/mL) and 133 g/L trichloroacetic acid (final volume 1 mL), and incubated for 3 h at 37 ℃. Then, 1 mL of 65% H2SO4(v/v) was added to the medium. The AA content was calculated using a standard curve (1.5-4.5 μmol/L AA freshly prepared in sulfuric acid) and expressed as μ mol of AA/mL of plasma.
2.5. Erythrocyte catalase (CAT) activity
CAT activity was measured by the method of Aebi[20]. Packed erythrocytes (10 μL) were hemolyzed by adding 100 volumes of distilled water (990 μL). Then, 20 μL of the hemolysed red blood cells (RBC) were transferred to a 1 mL quartz cuvette and the reaction was started by the addition of 100 μL of freshly prepared 300 mmol/L H2O2in 50 mmol/L phosphate buffer (pH 7.0) to a final volume of 1 mL. The rate of H2O2decomposition was measured spectrophotometrically at 240 nm for 120 s. CAT activity was expressed as μmol H2O2/mL packed RBC per minute.
2.6. Erythrocyte superoxide dismutase (SOD)
SOD activity was assayed spectrophotometrically as described by Boveris and Cadenas[21]. This method is based on the capacity of SOD to inhibit the autoxidation of adrenaline to adrenochrome. Adrenaline autoxidation was monitored at 480 nm. One unit of enzyme activity is defined as the amount of enzyme required to inhibit the rate of epinephrine autoxidation by 50% at 26 ℃.
2.7. Determination of ROS by flow cytometry
Intracellular H2O2was determined using DCF-DA (Sigma Chemical Co.) as described by Hasuiet al.[22] with modifications. Intracellular peripheral blood mononuclear cells DCF-DA fluorescence was determined by flow cytometry (FACScalibur analyzer, BD Biosciences). The leukocytes were then isolated by mixing total blood with lysing solution (BDFacsTM) as indicated by the manufacturer. The cells (1×106/mL) were washed twice with PBS (pH 7.4), centrifuged at 1 800 r/min for 5 min, and resuspended in ice-cold PBS. Cells were then incubated with DCFDA (2 μmol/L final) for 30 min at 37 ℃. Excess DCFDA was removed by washing the cells once with PBS and centrifuging at 1 800 r/min for 5 min. At least 50 000 events were counted for each blood sample.
2.8. Statistical analysis
Data are expressed as mean±standard deviation (SD). Statistical analyses were performed using analysis of variance (ANOVA), followed by post hoc Duncan’s multiple range test. When appropriate, repeated measures (ANOVA) were used, followed by post hoc Duncan’s multiple range test. Results were considered significant whenP<0.05.
3. Results
GA is a compound found in plants both in a free and in a bound form. It is found in large amounts inP. nirurileaves, from which it can be extracted by hot water infusions. HPLC analysis of the commercialP. niruritea indicated the presence of GA and an unknown related compound as the major components. The quantity of GA found in the different filtrates (tea) ofP. niruriwith hot water varied from 55 to 70 mg/mL (Figure 1). Plasma levels of GA were significantly increased at 1, 2 and 4 h afterP. niruriingestion (paired comparisons of sample times 1, 2 and 4 h with baseline [before ingestion]) showing that GA was rapidly absorbed (Figure 2). No significant variation in GA levels, compared to baseline levels, was observed among control subjects [(1.32±0.15), (1.36±0.11), (1.40±0.11) and (1.36±0.15) μmol/L at 0, 1, 2 and 4 h, respectively]. The identity of GA in plasma was confirmed by the UV-VIS spectra of the corresponding standard peak of GA (Figure 3). In order to investigate whether these increases in plasma GA are associated with antioxidant effects, we determined the plasma AA levels, RBC CAT and SOD activities and peripheral blood mononuclear cell reactive oxygen species (ROS) formation by flow cytometry, before and after the ingestion ofP. niruritea.
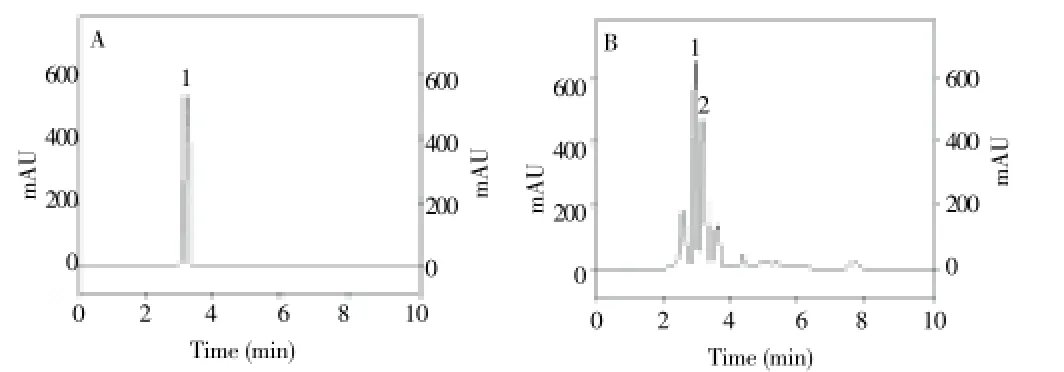
Figure 1. Chromatograms of standard gallic acid (A) and P. niruri (B). The peak 1 corresponds to gallic acid and the peak 2 is from an undetermined compound. Chromatographic conditions are described in the experimental section.
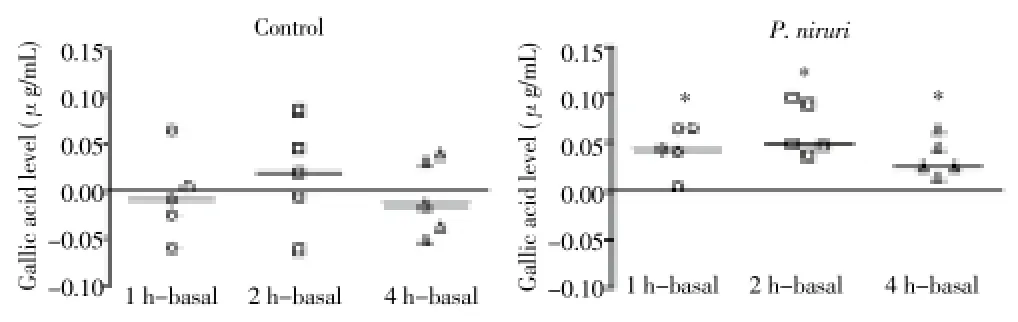
Figure 2. Gallic acid levels in plasma after P. niruri ingestion.Data are expressed as the differences between each time point minus the baseline values for each subject. *P<0.05 compared with that before treatment (Wilcoxon test).
P. niruriconsistently caused a significant transient increase in plasma AA at 1 h [paired comparisons of 1 h vs baseline (0 h),P<0.05] (Figure 4). No significant changes in plasma AA were detected at none of the tested time points among the control subjects (Figure 4). Neither RBC CAT norSOD (Table 1) activities were altered afterP. niruriintake. On the other hand, a decrease in DCF-DA fluorescence was observed at 2 h after the ingestion ofP. niruritea in peripheral blood mononuclear cells (Figure 5).

Figure 3. Peak marked refers to the increase in the area after ingestion of P. niruri tea.Peak marked (chromatograms after 1, 2 and 4 h post P. niruri ingestion) refers to the increase in the area of gallic acid after ingestion of P. niruri tea, when compared to the chromatograms after 1, 2 and 4 h post water ingestion. Peaks are indicated by arrows.

Figure 4. Plasmatic ascorbic acid after P. niruri ingestion.Data are expressed as the differences between each time point minus the baseline values for each subject. *P<0.05 compared with that before treatment (Wilcoxon test).
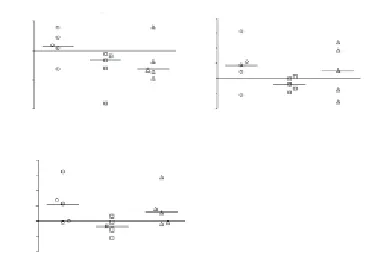
Figure 5. Reactive oxygen species (ROS) production in (A) granulocytes, (B) monocytes and (C) lymphocytes.ROS formation was measured by FACS using DCF-DA fluorescence. Data are expressed as the differences between each time point minus the baseline values for each subject. *P<0.05 compared with that before treatment (Wilcoxon test). AUF: Arbitrary units of fluorescence intensity.
4. Discussion
P. niruriis commonly used in folk medicine to treat renal disease, particularly urolithiasis, and there is a clinical evidence supporting thatP. niruriextracts can be an effective adjuvant in the treatment of renal calculus[11,23]. Of particular importance, urolithiasis has an inflammatory component, which is associated with oxidative stress[24]. Here, we detected only a modest antioxidant effect ofP. niruritea ingestion in healthy subjects, as indicated by a transitory increase in AA and by a sustained increase in plasma GA up to 4 h after tea ingestion. This increase might be related to the presence of AA inP. niruriextract[25]. Accordingly, experimental studies using rodents have shown that chronic ingestion of aqueous extracts ofP. nirurienhances the cellular antioxidant defense system, including the levels of vitamin C, glutathione, as well as SOD, CAT, and glutathione peroxidase activities[26-27]. In contrast to vitamin C, in the present study, the activities of antioxidant enzymes (SOD and CAT) were not altered by theingestion ofP. niruritea. The absence of alteration in those enzymes activities might be related to the fact thatP. niruriwas given as a single dose. Recently, Ferket alreported that supplementation of drinking water with GA in humans for 3 d caused a reduction of DNA damage in lymphocytes of healthy individuals[28]. That effect was paralleled by an increase in the activities of antioxidant enzymes, whereas the total antioxidant capacity and malondialdehyde levels in serum were not modified. Other studies have shown the modest transient increases in plasma antioxidant capacity after tea ingestion in humans[29]. Thus, although the plasma level of antioxidants can be expected to influence the intracellular antioxidant capacity of different cell types[30], this direct correlation does not always occur. Taken together, these results indicate that an overlap between extra- and intra-cellular antioxidant capacity is not always the case.
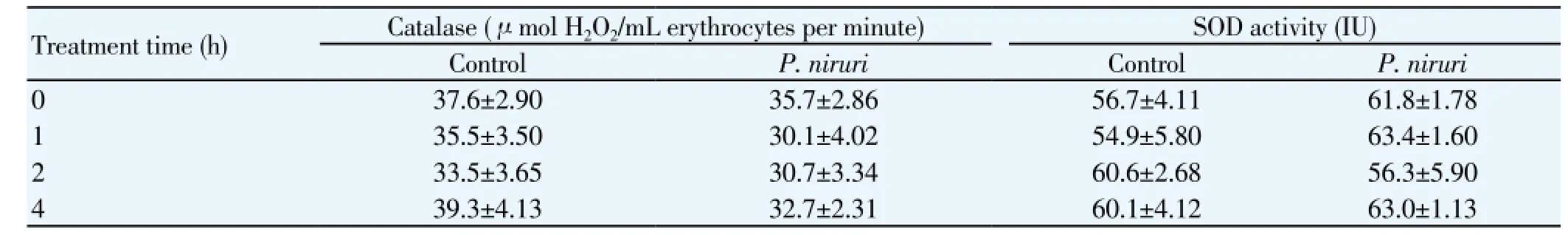
Table 1 Catalase and SOD activities after P. niruri ingestion.
Here, we have observed a significant, though modest, decrease in DFC-DA fluorescence in monocytes, but no significant alteration was detected in granulocytes or lymphocytes. Furthermore, RBC SOD and CAT activities were not altered byP. niruriingestion. Therefore, the modulation of antioxidant markers in all of these cell types is not concordant, which is in agreement with some previous studies[31-33].
Little is known about the absorption and metabolism of hydroxybenzoic acids like GA. The fact that just a few nutritional sources of GA are known and have resulted in limited interest by nutritionists. However, a few studies addressing the bioavailability of GA in humans have revealed that this compound is more absorbed than other polyphenols, though with a short mean half-live of about 1 h, indicating a very rapid elimination[15,34]. Our current results are similar to those obtained by Shahrzadet al, who found an increase in blood GA after ingestion of black tea in healthy humans[34]. In this study, plasma GA concentration increased from 1.3 to 1.6 μmol/L after ingestion of a large volume ofP. niruritea. As mentioned above, those levels of GA are within the same concentration range as previously reported (1.8-2.6 μmol/L) and vary depending on the volume, blood sampling time and formulation ingested[34,35]. This may indicate that part of the modest antioxidant effect observed herein might be associated with GA and related substances. However, here GA was determined by HPLC, and although the UV-VIS spectra of the plasmatic GA peak is coincident with that of pure GA, further studies with more accurate analytical methods should be performed to confirm the increase in plasmatic GA after ingestion ofP. niruritea. Another limitation of the present study is that only GA was quantified, despite the fact thatP. niruriextract contain a variety of polyphenolic compounds. Unfortunately, the other two peaks detected in theP. niruriinfusion, which had similar spectra and retention time compared to those found in plasma, could not be identified and quantitated due to the lack of suitable standards.
Here, it is shown an increase of GA in plasma following the ingestion (1 to 4 h) ofP. niruritea. Furthermore, we have observed a transient increase in plasma AA levels within the 1st hour after the tea ingestion, which may be associated with an increase in circulating GA. Therefore,P. niruritea ingestion seems to be associated with a modest increase in antioxidant markers in human plasma. However, further studies are required to determine the effects of chronic ingestion ofP. niruriand its potential as an antioxidant modulator of peripheral blood oxidative stress in healthy human subjects and whether or not this can be involved in the therapeutic effects of this plant.
Conflict of interest statement
The authors declare no conflicts of interest.
Acknowledgments
The financial support by CNPq is gratefully acknowledged. The authors would like to thank Marcelo Reis for revising the manuscript.
[1] Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Celular Biol 2007; 39: 44-84.
[2] Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr 2000; 130: S2073-2085.
[3] Bastos DHM, Saldanha LA, Catharino RR, Sawaya ACHF, Cunha IBS, Carvalho PO, et al. Phenolic antioxidants identified by ESIMS from Yerba maté (Ilex paraguariensis) and green tea (Camelia sinensis) extracts. Molecules 2007; 12: 423-432.
[4] Wang A, Zhou M, Lin W. Antioxidative and anti-inflammatory properties of Citrus sulcata extracts. Food Chem 2011; 124: 958-963.
[5] Castro J, Pregibon T, Chumanov K, Marcus RK. Determination of catechins and caffeine in proposed green tea standard reference materials by liquid chromatography-particle beam/electron ionization mass spectrometry (LC-PB/EIMS). Talanta 2010; 82: 1687-1695.
[6] Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010; 15: 792-814.
[7] Kontou N, Psaltopoulou T, Panagiotakos D, Dimopoulos MA, LinosA. The mediterranean diet in cancer prevention: A review. J Med Food 2011; 14: 1065-1078.
[8] Nishiura JL, Campos AH, Boim MA, Heilberg IP, Schor N. Phyllanthus niruri normalizes elevated urinary calcium levels in calcium stone forming (CSF) patients. Urol Res 2004; 32: 362-366.
[9] Barros ME, Schor N, Boim MA. Effects of an aqueous extract from Phyllanthus niruri on calcium oxalate crystallization in vitro. Urol Res 2003; 30: 374-379.
[10] Sabir SM, Rocha JBT. Water-extractable phytochemicals from Phyllanthus niruri exhibit distinct in vitro antioxidant and in vivo hepatoprotective activity against paracetamol-induced liver damage in mice. Food Chem 2008; 111: 845-851.
[11] Mahdi ES, Noor AM, Sakeena MH, Abdullah GZ, Abdulkarim M, Sattar MA. Identification of phenolic compounds and assessment of in vitro antioxidants activity of 30% ethanolic extracts derived from two Phyllanthus species indigenous to Malaysia. Afr J Pharm Pharmacol 2001; 5: 1967-1978.
[12] Amin ZA, Abdulla MA, Ali HM, Alshawsha MA, Qadir SW. Assessment of in vitro antioxidant, antibacterial and immune activation potentials of aqueous and ethanol extracts of Phyllanthus niruri. J Sci Food Agric 2012; 92: 1874-1877.
[13] Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: A review. J Pharm Pharmacol 2006; 58: 1559-1570.
[14] Souza TPD, Holzschuh MH, Lionc MI, Ortega GG, Petrovick PR. Validation of a LC method for the analysis of phenolic compounds from aqueous extract of Phyllanthus niruri aerial parts. J Pharm Biomed Anal 2002; 30: 351-356.
[15] Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005; 81: S230-242.
[16] Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. The stomach as a site for anthocyanins absorption from food. FEBS Lett 2003; 544: 210-213.
[17] Boligon AA, Pereira RP, Feltrin AC, Machado MM, Janovik V, Rocha JB, et al. Antioxidant activities of flavonol derivatives from the leaves and stem bark of Scutiabuxifolia reiss. Bioresour Technol 2009; 100: 6592-6598.
[18] Yuangang Z, Chunying L, Yujie F, Chunjian Z. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharm Biom Anal 2006; 41: 714-719.
[19] Jacques-Silva MC, Nogueira CW, Broch LC, Flores EMM, Rocha JBT. Diphenyldiselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol 2001; 88: 119-125.
[20] Aebi H. Catalase in vitro. Meth Enzym 1984; 105: 121-127.
[21] Boveris A, Cadenas E. Cellular source and steady-state levels of reactive oxygen species. In: Marcel Decker, editor. Oxygen, gene expression and cellular function. New York: Taylor & Francis; 1997, p. 1-25.
[22] Hasui M, Hirabayashi Y, Kobayashi Y. Simultaneous measurement by flow cytometry of phagocytosis and hydrogen peroxide production of neutrophils in whole blood. J Immunol Methods 1989; 117: 53-58.
[23] Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: A review. J Pharm Pharmacol 2006; 58: 1559-1570.
[24] González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr 2010; 3: S15-S27.
[25] Gami B, Kothari IL. Antioxidant & antimicrobial activity of in vivo and in vitro grown plants of Phyllanthus niruri L. Int J Pharm Bio Sci 2011; 2: 78-89.
[26] Sharma P, Parmar J, Verma P, Goyal PK. Modulatory influence of Phyllanthus niruri on oxidative stress, antioxidant defense, and chemically induced skin tumors. J Environ Pathol Tox Onc 2011; 30: S15-27.
[27] Baskaran M, Periyasamy L, Rajagopalan R. Effect of Phyllanthus niruri on alcohol and polyunsaturated fatty acid induced oxidative stress in liver. Int J Pharm Pharm Sci 2010; 2: 58-62.
[28] Ferk F, Chakraborty A, Jagerb W, Kundic M, Bichler J, Misík M, et al. Potent protection of gallic acid against DNA oxidation: Results of human and animal experiments. Mut Res 2011; 715: 61-71.
[29] Higdon JV, Frei B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 2003; 43: 89-143.
[30] Aoshiba K, Nagai A. Oxidative stress, cell death, and other damage to alveolar epithelial cells induced by cigarette smoke. Tob Induc Dis 2003; 15: 219-226.
[31] Aksoy Y, Sanal O, Metin A, Tezcan T, Ersoy F, Oğüş H, et al. Antioxidant enzymes in red blood cells and lymphocytes of ataxiatelangiectasia patients. Turk J Pediatr 2004; 46: 204-207.
[32] Ceneli O, Haznedar R, Ongun CO, Altan N. Evaluation of superoxide dismutase enzyme activity of polymorphonuclear leucocytes, erythrocytes and thrombocytes in patients with chronic myeloproliferative disorders. J Int Med Res 2009; 37: 1365-1374.
[33] Kasperczyk A, Machnik G, Dobrakowski M, Sypniewski D, Birkner E, Kasperczyk S. Gene expression and activity of antioxidant enzymes in the blood cells of workers who were occupationally exposed to lead. Toxicology 2012; 15: 79-84.
[34] Shahrzad S, Aoyagi K, Winter A, Koyama A, Bitsch I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J Nutr 2001; 131: 1207-1210.
[35] Shahrzad S, Bitsch I. Determination of gallic acid and its metabolites in human plasma and urine by high-performance liquid chromatography. J Chromatogr 1998; 705: 87-95.
*Corresponding author: João Batista T. Rocha, Departamento de Química, Centro de Ciências Naturais e Exatas, Universidade Federal de Santa Maria (UFSM), Santa Maria, RS, Brazil .
Tel: +55-3220-9462
E-mail: jbtrocha@pq.cnpq.br
Foundation project: This work was financially supported by CNPq.
 Asian Pacific Journal of Tropical Medicine2014年2期
Asian Pacific Journal of Tropical Medicine2014年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Diagnosis and multi-modality treatment of adult pulmonary plastoma: Analysis of 18 cases and review of literature
- Mathematical modeling for selecting center locations for medical and health supplies reserve in Hainan Province
- Bond strength analysis of the bone cement- stem interface of hip arthroplasties
- Comparison on serum biomarkers for anovulatory and ovulatory dysfunctional uterine bleeding in Lizu females
- Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
- Hepatic effect of NAC on sevear acute pancteatise of rats
