Iron-chelating and anti-lipid peroxidation properties of 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1) in longterm iron loading β-thalassemic mice
Kanokwan Kulprachakarn, Nittaya Chansiw, Kanjana Pangjit, Chada Phisalaphong, Suthat Fucharoen, Robert C. Hider, Sineenart Santitherakul, Somdet Srichairatanakool*
1Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
2College of Medicine and Public Health, Ubon Ratchathani University, Ubon Ratchathani, Thailand
3Institute of Research and Development, Government Pharmaceuticals Organization, Ministry of Public Health, Thailand
4Thalassemia Research Center, Institute of Molecular Biosciences, Mahidol University Salaya Campus, Nakornprathom, Thailand
5Institute of Pharmaceutical Science, King's College London, Franklin-Wilkins Building, London, United Kingdom
6Medical Science Research Equipment Center, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
Iron-chelating and anti-lipid peroxidation properties of 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1) in longterm iron loading β-thalassemic mice
Kanokwan Kulprachakarn1, Nittaya Chansiw1, Kanjana Pangjit2, Chada Phisalaphong3, Suthat Fucharoen4, Robert C. Hider5, Sineenart Santitherakul6, Somdet Srichairatanakool1*
1Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
2College of Medicine and Public Health, Ubon Ratchathani University, Ubon Ratchathani, Thailand
3Institute of Research and Development, Government Pharmaceuticals Organization, Ministry of Public Health, Thailand
4Thalassemia Research Center, Institute of Molecular Biosciences, Mahidol University Salaya Campus, Nakornprathom, Thailand
5Institute of Pharmaceutical Science, King's College London, Franklin-Wilkins Building, London, United Kingdom
6Medical Science Research Equipment Center, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
PEER REVIEW
Peer reviewer
Dr. Yongmin Ma, College of Pharmaceutical Sciences, Zhejiang Chinese Medical University, 548 Binwen Road, Binjiang District, Hangzhou, Zhejiang Province, P R China.
Tel: +86 571 86633046
Fax: +86 571 86613606
E-mail: yongmin.ma@zcmu.edu.cn
Comments
This manuscript has evaluated that CM1 on the iron removal capacity and lipid peroxidation in long-term iron loaded β-thalassaemia mice before translating the compound to the β-thalassaemia patients, which is very common in southeast Asia. The results are interesting.
Details on Page 667
Objective:To evaluate the iron-chelating properties and free-radical scavenging activities of 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1) treatment in chronic iron-loaded β-thalassemic (BKO) mice.
Iron-chelating, Iron overload, β-thalassemia, Iron chelator, Non-transferrin bound iron, Lipid peroxidation
1. Introduction
Iron is a component of many metalloproteins and plays a crucial role in a range of vital biochemical activities, such as oxygen sensing and transport, electron transfer, and catalysis[1]. When present in excess, cellular iron overload leads to toxicity and cell death via free radical formation and lipid peroxidation[2]. Non-transferrin bound iron (NTBI),and labile plasma iron (LPI) are toxic forms of the iron that appear in plasma when the transferrin saturation increases. Changes in the labile iron pool (LIP) can be considered a cytosolic equivalent of plasma NTBI influence on intracellular ferritin (Ft) levels[3]. Thus, elevated levels of the LIP lead to an increased accumulation of Ft iron and in extreme cases to the formation of hemosiderin[4]. Iron chelation therapy is required to prevent iron-mediated injury to cells and to reduce the levels of NTBI, LPI, LIP and plasma Ft[5,6].
At present, the treatment of iron overload diseases especially in β-thalassemia patients commonly involves the administration of deferiprone (DFP), desferioxamine (DFO) and deferasirox (DFX)[7-9]. Effectiveness, cost, compliance, quality of life and side effects of the chelators are all relevant considerations. Many adverse effects of these chelators include: nausea, vomiting, gastrointestinal tract disturbance, leukocytopenia, thrombocytopenia, arthopathy, zinc deficiency and agranulocytosis from DFP, skin redness, local irritation, mild pain at the applied sites from DFO, renal toxicity, Fanconi syndrome, formation of rashes and gastrointestinal tract disturbance from DFX[10].
We have been studying the properties of a specific novel orally active iron chelator, 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1). Our previous studies have illustrated that the CM1 (MW=256, Kpart=0.53) is an effective bidentate chelator and is slightly more lipophilic than the DFP (MW=139, Kpart=0.11)[11]. Preliminary results have established that CM1 is relative non-toxic in acute studies and can reduce the levels of malondialdehyde (MDA), LIP and reactive oxygen species in both mouse primary hepatocytes and human hepatocellular carcinoma (HepG2) cells[12,13]. Furthermore, CM1 was found not to be toxic to the peripheral blood mononuclear cells and liver cells of β-thalassemia mice under normal and iron overload conditions after 240 d exposure[14]. Srichairatanakoolet al.[15] reported that CM1 removed excess iron in the blood compartment and tissues of iron loaded wild type C57BL/6 mice. These preliminary studies have now been extended to include β-thalassemic mice.
2. Materials and methods
2.1. Animals
The heterozygous β-thalassemia knockout (BKO,muβth-3/+) mice strain C57BL/6 aged between 6-10 weeks and having a body weight (20±5) g were kindly supplied by the Thalassemia Research Center, Institute of Molecular Biosciences, Mahidol University, Thailand[16]. The animals were housed in polyethylene cages and maintained in a clean airconditioned room under the controlled conditions of 12-h day/12-h night cycle at (25±3) °C and at 40%-70% humidity. The study protocol that was used has been approved by the Animal Ethical Committee of the Medical Faculty, Chiang Mai University, Thailand (Reference Number -3/2554).
2.2. Iron overload in mice and chelation treatment
The mice were fed a normal pellet diet (N diet) and an N diet supplemented with 0.2% (w/w) ferrocene (Fe diet) to induce iron overload, over 240 d[17]. The iron-loaded mice were randomly subdivided into 5 groups. The study group was fed with the Fe diet along with treatments of deionized water placebo, DFP [50 mg/(kg.day)] and CM1 [50 and 100 mg/(kg.day)] orally for 180 d (5 mice in each group)[18]. The control group was fed with N diet throughout the study. Blood samples were collected from the tail vein and collected into Na-heparin tubes. Plasma was separated immediately and kept frozen -20 °C for further analysis.
2.3. Quantification of plasmaNTBI
Plasma NTBI was quantified based on nitrilotriacetic acid (NTA) chelation/HPLC technique with slight modifications[19]. Briefly, plasma was incubated with a weak chelator; NTA solution (80 mmol/L, at final concentration, pH 7.0) for 30 min at room temperature to produce the Fe3+-(NTA)2complex from NTBI. Subsequently, the complex was filtered through a membrane (Nano-Sep®, 10-kDa cutoff, polysulfone type; Pall Life Sciences, Ann Arbor, MI, USA) at 12 000 r/min for 60 min and analyzed using a non-metallic HPLC system. NTBI was fractionated on a glass analytical column (ChromSep-ODS1, 100 mm×3.0 mm, 5 μm), eluted with a mobile phase solvent (3 mmol/L CP22 in 20% acetonitrile/MOPS pH 7.0) at a flow rate of 1.0 mL/min and the optical density (OD) was monitored at 450 nm using a flow cell detector (SpecMonitor 2300; LDC Milton-Roy Inc., Riviera Beach, FL, USA). Data analysis was conducted with BDS software (BarSpec Ltd., Rehovot, Israel). NTBI concentration represented by Fe3+-(CP22)3peak area was calculated with a calibration curve constructed from Fe3+-(NTA)2in 80 mmol/L NTA (0-32 μmol/L).
2.4. Quantification ofLPI
In principle, redox-active LPI can convert non-fluorescent dihydrorhodamine (DHR) to oxidized form rhodamine (R), resulting in an increase of fluorescence intensity (FI)[20]. In the assay, plasma was incubated with/without 5 mmol/L DFP at 37 °C for 30 min and then the DHR solution containing ascorbic acid was added. Kinetics of increasing FI was followed immediately for 40 min, with readings every 2 min at 37 °C using a 96-well plate spectrofluorometer (λexcitation485 nm, λemission538 nm). The slope of the FI was plotted against a reaction time of between 15-40 min. A calibration curve was constructed from the standard ferrous ammonium sulfate solution (0-20 μmol/L). Difference in rate of DHR oxidation represents a component of redox active LPI. TheLPI concentration was calculated from the calibration curve relating the differences in slope with/without DFP versus the standard iron concentration as described[15].
2.5. Measurement of plasma ferritin concentration
Ft concentration was determined by sandwich ELISA method as described by the manufacturer (Abnova, United Kingdom). Briefly, plasma was reacted with the solid phase-immobilized anti-Ft antibodies at room temperature for 60 min. After the removal of unbound proteins by being washed four times, anti-Ft antibodies conjugated with horseradish peroxidase was added to the plasma solution to form a complex with the previously bound Ft and it was then incubated at room temperature for 10 min. Following the washing, the enzyme bound to the immunosorbent was assayed by the addition of a chromogenic substrate, 3,3’,5,5’-tetramethylbenzidine and incubated in the dark for 10 min. The quantity of the bound enzyme was proportional to the concentration of Ft in the sample. Thus, the absorbance was determined at 450 nm.
2.6. Assessment of plasma lipid peroxidation
MDA was adopted as an index of lipid peroxidation and was determined in plasma using the HPLC-based thiobarbituric acid reactive substance (TBARS) method[21]. Plasma was mixed with the reaction mixture; 10% (w/v) trichloroacetic acid containing 50 mg/L butylated hydroxytoluene and heated at 90 °C for 30 min. After centrifugation (10 000 r/mim, 10 min), the supernatant was mixed with the chromogenic solution containing 0.44 mol/L H3PO4and 0.6% (w/v) thiobarbituric acid (TBA). The mixture was heated to 90 °C for 30 min to produce a pink-colored product represented as TBARS. In the HPLC analysis[21] the product was subsequently fractionated on the column (ZORBAX Eclipse XDB-C18, 150 mm×4.6 mm, 5 μm, Agilent Technologies), eluted with a mobile-phase solvent of 50 mmol/L KH2PO4pH 7.0 : methanol (65:35, v/v) at a flow rate of 1.0 mL/min and detected at 532 nm. Finally, TBARS concentrations were determined from the standard curve constructed by varied concentrations of 1,1,3,3-tetramethoxypropane (0-100 μmol/L).
2.7. Statistical analysis
Data were presented as mean±SEM. Statistical significance was determined using One-way analysis of variance (ANOVA), in whichP<0.05 was considered significant.
3. Results
3.1. PlasmaNTBIconcentration
Low levels of NTBI were detected in the plasma of N diet-fed BKO mice (2.1±0.78) μmol/L. In contrast, the NTBI concentration was significantly increased in the plasma of the BKO mice fed with Fe diet for 240 d (28.9±4.33) μmol/ L, indicating iron overload (Figure 1). As expected, the increase of NTBI concentration was reduced as a result of treatment with DFP (50 mg/kg) and CM1 (50 mg/kg) (P<0.005). Surprisingly, the levels of plasma NTBI in the mice after intervention with CM1 (100 mg/kg) were found to be higher than the value resulting with 50 mg/kg.
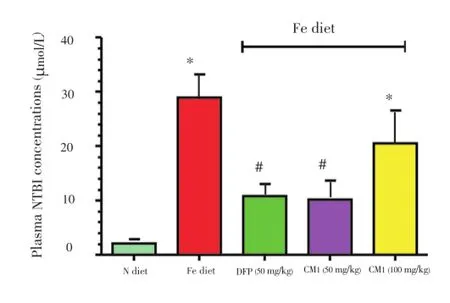
Figure 1.Plasma NTBI concentrations of the BKO mice fed with N diet, Fe diet, and the Fe diet following intervention with DFP (50 mg/ kg) and CM1 (50 and 100 mg/kg) for 6 months.Data were expressed as mean±SEM (n=5).*P<0.05 compared with the N diet group;#P<0.005 compared with the Fe diet group on the intervention.
3.2. PlasmaLPIlevel
Plasma LPI was not detected in N diet-fed BKO mice (Figure 2). However, the Fe diet induced the formation of LPI in plasma of mice, when mice were fed with the high Fe diet, generating a value of 15 μmol/L after 8 months. After chelation with DFP and CM1 at concentration of 50 mg/kg for 6 months, both chelators were found to be effective in lowering the plasma LPI levels in Fe-fed BKO mice [(10.20±1.21) and (10.30±3.71) μmol/L, respectively]. There was no significant difference in the LPI levels for the different doses of CM1.
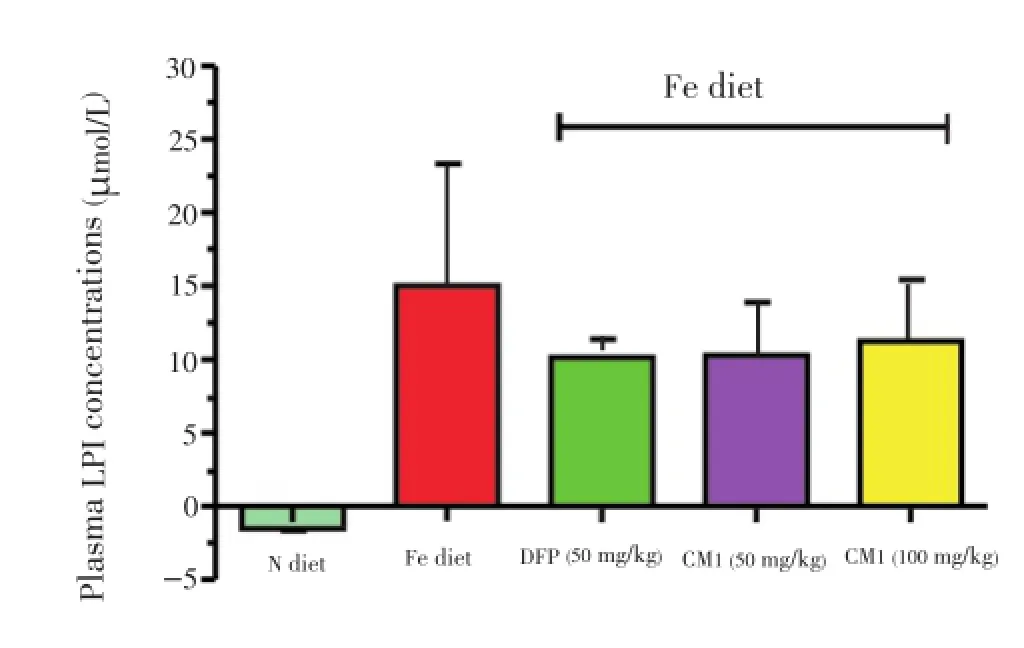
Figure 2.Plasma LPI concentrations of the BKO mice fed with N diet, Fe diet, and the Fe diet following intervention with DFP (50 mg/ kg) and CM1 (50 and 100 mg/kg) for 6 months.Data were expressed as mean±SEM (n=5).
3.3. Plasma ferritin (Ft) content
The plasma Ft content of BKO mice fed with Fe diet over 240 d [(16.90±0.18) μg/mL] was found to be significantly higher than that of the N diet-fed BKO mice [(1.90±0.46) μg/ mL]. However, the concentration of plasma Ft remained unchanged after six months of intervention with both DFP (50 mg/kg) and CM1 (50 and 100 mg/kg) (Table 1).

Table 1 Plasma Ft concentrations of BKO mice.
3.4. PlasmaMDAconcentration
Coincidently with the increase of NTBI, LPI and Ft levels in the plasma, long-term feeding with Fe diet mice led to a marked increase in plasma MDA concentration [(3.70±1.70) μmol/L] as compared with N diet-fed mice [(0.80±0.04) μmol/L] (Figure 3). DFP and CM1 treatments (50 mg/kg) over 180 d reduced the increase of plasma MDA. Interestingly, CM1 (100 mg/kg) was again found to be less effective than 50 mg/kg.

Figure 3. Plasma MDA concentrations of BKO mice fed with N diet, Fe diet, and Fe diet following intervention with DFP (50 mg/kg) and CM1 (50 and 100 mg/kg) for 6 months.Data were expressed as mean±SEM (n=5).
4. Discussion
Iron is an essential cofactor in a variety of cellular processes. However, iron in excess is toxic because of its propensity to induce the formation of dangerous free radicals, leading to mitochondrial dysfunctions and cell death via oxidative damage of biomolecules and lipid peroxidation[22-24]. Enhanced lipid peroxidation and hepatocellular injury have been proposed as an initial step by which iron causes cellular injury[25,26]. NTBI is the circulating forms of iron that are not tightly bound to plasma transferrin of thalassemia and sickle cell anemia patients[27,28]. LPI represents a component of NTBI that is redox-active and capable of permeating into organs and inducing tissue iron overload[29]. NTBI is cleared rapidly from plasma by the liver via the transmembrane protein Zrt- and Irt-like protein 14 (Zip14)[30] and is likely to play an important role in hepatocyte iron loading in hereditary hemochromatosis and other iron overload conditions especially in β-thalassemia. Both NTBI and LPI appear primarily in heavily transfused β-thalassemia patients[5,31,32].
In order to investigate problems associated with iron overload tissue, several animal models have been developed. Thus duodenal iron absorption by β-thalassemic mice are found to be modestly increased due to low hepcidin level and enhanced mucosal iron uptake, leading to a sustained iron overload[33-35]. Liver 25-amino acid hepcidin controls influx of dietary iron from duodenum to plasma and efflux of heme-derived iron from macrophages through degradation of the cellular iron exporter called ferroportin[36]. Interestingly, patients with myelodysplastic syndrome and iron overload showed an increase in serum levels of hepcidin related to their iron and oxidative stress status after 3-month treatment with DFX[37]. In addition, serum level of growth differentiation factor 15 which involves in hepcidin regulation can be induced in normal subjects after iron chelation treatment[38]. Another useful animal model is that of ferrocene-loaded rats, which has been used to examine the efficacy of iron chelators[39,40].
In this study we decided to iron load β-thalassemic mice with ferrocene. Long-term ferrocene administration induced iron overload in our experimental mice as determined by the significant increase of NTBI, LPI and Ft levels in the plasma. The iron loaded mice behaved normally for the duration of the study and showed the same weight gain as control animals. Chelation treatment with either DFP or CM1 led to the reduction of NTBI and LPI levels in β-thalassemic mice. At the higher concentration of 100 mg/kg, CM1 was found to be less effective than when present at a concentration of 50 mg/kg. This is surprising, but may be related to the toxicity of CM1 at the higher dose. Both DFP and CM1 were found to be effective at reducing MDA concentrations and this activity is almost certainly associated with their iron chelating ability. Interestingly, CM1 again was found to be more effective at 50 mg/kg than at 100 mg/kg. In contrast to the findings related to NTBI,serum Ft levels were found to be unaffected by the presence of chelators. This finding reflects the observation that in some cases in β-thalassemia patients treated with iron chelators, the plasma Ft levels are largely unchanged or are only reduced after prolonged iron chelator treatment.
In conclusions, CM1 is an effective orally iron chelator and demonstrates marked iron chelating properties under biological conditions. It also reduces iron-induced lipid peroxidation in chronic iron overloaded β-thalassemic mice. Whether these beneficial effects can be translated to the treatment of thalassemia patients remains to be established.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This work was supported by the Royal Golden Jubilee PhD Program, Thailand Research Fund (Grant No. PHD/0333/2551); Faculty of Medicine Research Fund, Chiang Mai University, Thailand; the Office of Higher Education Commission and Mahidol University under the National Research University Initiative, Thailand Research Fund through Professor Suthat Fucharoen, MD.; and a Research Chair Grant from the National Science and Technology Development Agency (NSTDA) and Mahidol University through Professor Suthat Fucharoen, MD.
Comments
Background
Iron overload leads to toxicity and cell death as free iron can be induced by Fenton reaction to form free radicals. Current clinical drugs for treating iron overload in β-thalassaemia include DFP, DFX and DFO, all having various side effects.
Research frontiers
The present research work describes that the iron chelator CM1 can effectively reduce non-transferrin-bound iron, LPI and MDA level in the plasma of iron overloaded mice, but not ferritin content.
Related reports
The data presented in this article showed that CM1 (100 mg/kg) has no effect on the reduction of NTBI, LPI and MDA of plasma in iron overload mice whereas in other papers, increase of CM1 dose leads to a gradual iron removal in mouse primary hepatocytes and human hepatocellular carcinoma cells (Kulprachakarnet al.2013).
Innovations and breakthroughs
Data has showed that CM1 at 50 mg/kg is effective to reduce NTBI, LPI and MDA level in the plasma of iron overload but not at 100 mg/kg. At both doses, CM1 does not change serum ferritin level.
Applications
The article describes that CM1 is an effective iron chelator to remove iron in β-thalassaemia mice and reduce ironinduced lipid peroxidation. Combined with other studies on CM1, the compound may be applicable to β-thalassaemia patients.
Peer review
This manuscript has evaluated that CM1 on the iron removal capacity and lipid peroxidation in long-term iron loaded β-thalassaemia mice before translating the compound to the β-thalassaemia patients, which is very common in southeast Asia. The results are interesting.
[1] Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol 2008; 88(1): 7-15.
[2] Britton RS, Ramm GA, Olynyk J, Singh R, O’Neill R, Bacon BR. Pathophysiology of iron toxicity. Adv Exp Med Biol 1994; 356: 239-253.
[3] Brissot P, Ropert M, Le Lan C, Loreal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 2012; 1820(3): 403-410.
[4] Cabantchik ZI. Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front Pharmacol 2014; 5: 45.
[5] Taher AT, Musallam KM, Inati A. Iron overload: consequences, assessment, and monitoring. Hemoglobin 2009; 33(Suppl 1): S46-S57.
[6] Prus E, Fibach E. Effect of iron chelators on labile iron and oxidative status of thalassaemic erythroid cells. Acta Haematol 2010; 123(1): 14-20.
[7] Cappellini MD, Taher A. Deferasirox (Exjade) for the treatment of iron overload. Acta Haematol 2009; 122(2-3): 165-173.
[8] Galanello R, Campus S. Deferiprone chelation therapy for thalassemia major. Acta Haematol 2009; 122(2-3): 155-164.
[9] Viprakasit V, Lee-Lee C, Chong QT, Lin KH, Khuhapinant A. Iron chelation therapy in the management of thalassemia: the Asian perspectives. Int J Hematol 2009; 90(4): 435-445.
[10] Neufeld EJ. Update on iron chelators in thalassemia. Hematology Am Soc Hematol Educ Program 2010; 2010: 451-455.
[11] Srichairatanakool S, Pangjit K, Phisalaphong C. Characterization and investigation of chelating activity of a novel iron chelator: 1-(N-acetyl-6-aminohexyl)-3-hydroxypyridin-4-one.).Thailand patent No. 0901000799. 2009.
[12] Kulprachakarn K, Pangit K, Phisalaphong C, Fucharoen S, Hider RC, Srichairatanakool S. Combination treatments of 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1) with deferiprone and desferrioxamine reduced labile iron pool and protected oxidative stress in iron-loaded cultured hepatocytes. Vitam Miner 2013; 2: 1-8.
[13] Pangjit K, Banjerdpongchai R, Phisalaphong C, Fucharoen S, Srichairatanakool S. Efficacy of 1-(N-acetyl-6-aminohexyl)-3-hydroxypyridin- 4-one (CM1) in treatment of iron-loaded hepatocyte cultures. Adv Biosci Biotechnol 2012; 3: 1060-1067.
[14] Chansiw N, Pangjit K, Phisalaphong C, Fucharoen S, Evans P, Porter JB, et al. Toxicity study of a novel oral iron chelator: 1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one (CM1) in transgenic b-thalassemia mice. Vitam Miner 2013; 2: 1-6.
[15] Srichairatanakool S, Pangjit K, Phisalaphong C, Fucharoen S. Evaluation of a novel oral iron chelator 1-(N-acetyl-6-ami nohexyl)-3-hydroxypyridin-4-one (CM1) for treatment of iron overload in mice. Adv Biosci Biotechnol 2013; 4: 153-163.
[16] Jamsai D, Zaibak F, Vadolas J, Voullaire L, Fowler KJ, Gazeas S, et al. A humanized BAC transgenic/knockout mouse model for HbE/beta-thalassemia. Genomics 2006; 88(3): 309-315.
[17] Thephinlap C, Phisalaphong C, Lailerd N, Chattipakorn N, Winichagoon P, Vadolas J, et al. Reversal of cardiac iron loading and dysfunction in thalassemic mice by curcuminoids. Med Chem 2011; 7(1): 62-69.
[18] Ounjaijean S, Thephinlap C, Khansuwan U, Phisalapong C, Fucharoen S, Porter JB, et al. Effect of green tea on iron status and oxidative stress in iron-loaded rats. Med Chem 2008; 4(4): 365-370.
[19] Singh S, Hider RC, Porter JB. A direct method for quantification of non-transferrin-bound iron. Anal Biochem 1990; 186(2): 320-323.
[20] Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 2003; 102(7): 2670-2677.
[21] Grotto D, Santa Maria LD, Boeira S, Valentini J, Charão MF, Moro AM, et al. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal 2007; 43(2): 619-624.
[22] Siah CW, Trinder D, Olynyk JK. Iron overload. Clin Chim Acta 2005; 358(1-2): 24-36.
[23] Urrutia PJ, Mena NP, Nunez MT. The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front Pharmacol 2014; 5: 38.
[24] Lu W, Zhao M, Rajbhandary S, Xie F, Chai X, Mu J, et al. Free iron catalyzes oxidative damage to hematopoietic cells/ mesenchymal stem cells in vitro and suppresses hematopoiesis in iron overload patients. Eur J Haematol 2013; 91(3): 249-261.
[25] Ramm GA, Ruddell RG. Iron homeostasis, hepatocellular injury, and fibrogenesis in hemochromatosis: the role of inflammation in a noninflammatory liver disease. Semin Liver Dis 2010; 30(3): 271-287.
[26] Delima RD, Chua AC, Tirnitz-Parker JE, Gan EK, Croft KD, Graham RM, et al. Disruption of hemochromatosis protein and transferrin receptor 2 causes iron-induced liver injury in mice. Hepatology 2012; 56(2): 585-593.
[27] Piga A, Longo F, Duca L, Roggero S, Vinciguerra T, Calabrese R, et al. High nontransferrin bound iron levels and heart disease in thalassemia major. Am J Hematol 2009; 84(1): 29-33.
[28] Porter JB. Pathophysiology of transfusional iron overload: contrasting patterns in thalassemia major and sickle cell disease. Hemoglobin 2009; 33(Suppl 1): S37-S45.
[29] Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol 2005; 18(2): 277-287.
[30] Pinilla-Tenas JJ, Sparkman BK, Shawki A, Illing AC, Mitchell CJ, Zhao N, et al. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am J Physiol Cell Physiol 2011; 301(4): C862-C871.
[31] Inati A, Musallam KM, Cappellini MD, Duca L, Taher AT. Nontransferrin-bound iron in transfused patients with sickle cell disease. Int J Lab Hematol 2011; 33(2): 133-137.
[32] Zanninelli G, Breuer W, Cabantchik ZI. Daily labile plasma iron as an indicator of chelator activity in thalassaemia major patients. Br J Haematol 2009; 147(5): 744-751.
[33] Ramos P, Melchiori L, Gardenghi S, Van-Roijen N, Grady RW, Ginzburg Y, et al. Iron metabolism and ineffective erythropoiesis in beta-thalassemia mouse models. Ann N Y Acad Sci 2010; 1202: 24-30.
[34] Gardenghi S, Marongiu MF, Ramos P, Guy E, Breda L, Chadburn A, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by downregulation of hepcidin and up-regulation of ferroportin. Blood 2007; 109(11): 5027-5035.
[35] Gardenghi S, Ramos P, Follenzi A, Rao N, Rachmilewitz EA, Giardina PJ, et al. Hepcidin and Hfe in iron overload in betathalassemia. Ann N Y Acad Sci 2010; 1202: 221-225.
[36] Camaschella C. Iron and hepcidin: a story of recycling and balance. Hematology Am Soc Hematol Educ Program 2013; 2013: 1-8.
[37] Ghoti H, Fibach E, Westerman M, Gordana O, Ganz T, Rachmilewitz EA. Increased serum hepcidin levels during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndrome. Br J Haematol 2011; 153(1): 118-120.
[38] Lakhal S, Talbot NP, Crosby A, Stoepker C, Townsend AR, Robbins PA, et al. Regulation of growth differentiation factor 15 expression by intracellular iron. Blood 2009; 113(7): 1555-1563.
[39] Florence A, Ward RJ, Peters TJ, Crichton RR. Studies of in vivo iron mobilization by chelators in the ferrocene-loaded rat. Biochem Pharmacol 1992; 44(6): 1023-1027.
[40] Liu D, He H, Yin D, Que A, Tang L, Liao Z, et al. Mechanism of chronic dietary iron overload-induced liver damage in mice. Mol Med Rep 2013; 7(4): 1173-1179.
10.12980/APJTB.4.2014APJTB-2014-0155
*Corresponding author: Somdet Srichairatanakool, Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Tel: +66 53 945322
Fax: +66 53 894031
E-mail: ssrichai@med.cmu.ac.th
Foundation Project: Supported by the Royal Golden Jubilee PhD Program, Thailand Research Fund (Grant No. PHD/0333/2551).
Article history:
Received 25 May 2014
Received in revised form 10 Jun, 2nd revised form 18 Jun, 3rd revised form 24 Jun 2014
Accepted 12 Jul 2014
Available online 28 Aug 2014
Methods:The BKO mice were fed with a ferrocene-rich diet and were orally administered with CM1 [50 mg/(kg.day)] for 6 months. Blood levels of non-transferrin bound iron, labile plasma iron, ferritin (Ft) and malondialdehyde were determined.
Results:The BKO mice were fed with an iron diet for 8 months which resulted in iron overload. Interestingly, the mice showed a decrease in the non-transferrin bound iron, labile plasma iron and malondialdehyde levels, but not the Ft levels after continuous CM1 treatment.
Conclusions:CM1 could be an effective oral iron chelator that can reduce iron overload and lipid peroxidation in chronic iron overload β-thalassemic mice.
 Asian Pacific Journal of Tropical Biomedicine2014年8期
Asian Pacific Journal of Tropical Biomedicine2014年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- An overview of travel-associated central nervous system infectious diseases: risk assessment, general considerations and future directions
- Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates along the Thai-Myanmar borders
- Impact of antibacterial drugs on human serum paraoxonase-1 (hPON1) activity: an in vitro study
- High levels of Zinc-α-2-Glycoprotein among Omani AIDS patients on combined antiretroviral therapy
- Toxicity effects of water extracts of Holothuria atra Jaeger in mice
- Prevention of renal dysfunction by nutraceuticals prepared from oil rich plant foods
