Growth,photosynthesis and nitrogen metabolism in soybean varieties after exclusion of the UV-B and UV-A/B components of solar radiation
Snjy Singh Broniy,Sunit Ktri*,Govind Prksh PndeyKdur N.Guruprsd
aGovernment Shri Krishnaji Rao Pawar Post Graduate College,Dewas 455001,M.P.,India
bPhotobiology Laboratory,School of Life Sciences,Khandwa Road Campus,Devi Ahilya University,Indore 452017,M.P.,India
1.Introduction
The level of ambient UV-B(280–315 nm) radiation in sunlight varies with latitude and is relatively higher in tropical regions than in temperate regions.Due to the small solar zenith angle and thin stratospheric ozone layer in the tropics,terrestrial plants encounter much higher levels of UV-B radiation than at higher latitudes[1].India lies in a low ozone belt and receives more UV-B radiation than those of temperate regions with higher latitudes[2].
Data compiled from the last two decades suggest that nearly 50% of crop plants are affected by elevated levels of solar UV-B.Studies on a number of cultivated and native plant species have shown that ambient and enhanced levels of UV-B have detrimental effects on plant growth,development and morphology,photosynthesis,and biomass production[3–7].Whether ambient UV-B has any effect on the underground organs such as roots or on the beneficial processes linked to nitrogen (N) fixation in leguminous plants is not clear.

Table 1-Origin,pedigree and morphological characteristics of Indian soybean varieties.
Nitrogen requirement of legumes can be met by inorganic N assimilation and symbiotic N2fixation; in practice,they obtain N through both processes.A reduction of nitrate to nitrite (NO2-) is catalyzed by nitrate reductase (NR; EC 1.6.6.4),an inducible enzyme whose activity depends on the availability of nitrate and light [8].At high concentrations,nitrate inhibits both nodulation and N2fixation in almost all legume species[9].Like most legumes,soybean has the potential to fix atmospheric nitrogen through symbiotic relationships with soil organisms[10].
Nitrogen fixation and assimilation are negatively affected by an elevated UV-B as confirmed by the reduced activities of nitrogenase,nitrate reductase,nitrite reductase and leghemoglobin (Lb) contents in the nodulated mung bean cultivars [11].Supplemental UV-B also led to a reduced N2fixation in the tropical leguminous crops Phaselous mungo and Vigna radiata [12].Very little is known about the effects of ambient UV-B radiation on nitrogen metabolism.The number of nodules per plant in the bean grown in conditions of ambient UV radiation was higher than that on plants deprived of UV-B[13],and Chouhan et al.[14]found that ambient UV-B reduced the number and size of nodules,total protein and Lb content in the soybean.However,Shiozaki et al.[15] found that UV(300–400 nm) applied to leaves increased the amounts of nodulation and symbiotic N2fixation in pea plants.Baroniya et al.[7] reported that UV-excluded soybean plants had higher levels of α-tocopherol,which plays an important role in translocating photoassimilates from the leaves to the roots[16].
Sensitivity to UV-B radiation varies considerably within and between plant species,but the factors underlying this diversity are not well understood [17].Considerable intraspecific variation in response to enhanced UV-B radiation in terms of growth and yield has been observed in wheat[18],maize[19],rice [20],soybean [5,17,21] and tartary buckwheat [22].Most of these studies examined the response of individual varieties to enhanced UV-B,but UV exclusion studies might provide more realistic assessments of the sensitivity of plants to current levels of UV radiation[5,7,23].
The aim of this paper was to evaluate the impact of ambient UV (280–400 nm) on the nodulation and nitrogen metabolism,particularly nitrate reductase (NR) activity,net rate of photosynthesis,Lb content and total soluble protein(in nodules) in the six varieties of soybean (Glycine max) by the exclusion of UV-B and UV-A/B.We hypothesized that the exclusion of UV radiation would increase the growth and biomass of both the aerial and below ground parts of soybean,and also affect other physiological processes by an increased rate of photosynthesis and higher nitrogen fixation resulting from increased nitrate reductase activity (NRA),and Lb content.Such changes should ultimately lead to an increased yield.
2.Material and methods
2.1.Plant materials
Seeds of soybean (G.max) varieties PK-472,Pusa-24,JS 71-05,JS-335,NRC-7 and Kalitur were obtained from the Directorate of Soybean Research,Indore.The origin and morphological characteristics of the varieties are listed in Table 1.
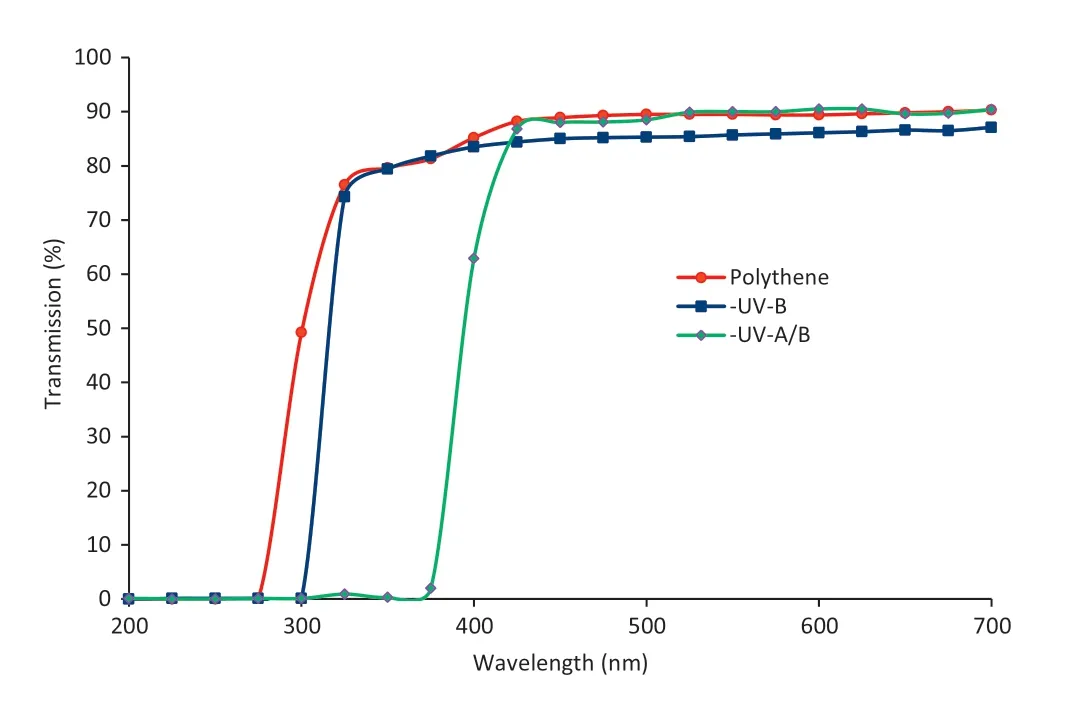
Fig.1-Transmission spectra for UV-B and UV-A/B exclusion filters on the metal cages under natural light conditions.
2.2.Experimental design of UV exclusion experiments
Field experiments under natural sunlight were conducted at the Botanical Garden,School of Life Sciences,Devi Ahilya University,Indore(22°43′N,75°49′60″E).The experiments were carried out in January–April 2012,when the average daily solar UV is around 50% higher than the average daily level in temperate regions.The seeds were surface-sterilized with 0.1% HgCl2and then inoculated with a slurry of Bradyrhizobium japonicum before sowing in 1 m rows spaced 0.3 m apart and 6 cm between plants within rows.Each plot was placed under an iron mesh cage(120 cm L × 90 cm W × 120 cm H)wrapped with UV cut-off polyester filters(Garware Polyester Ltd.,Mumbai)that selectively excluded UV-B (<315 nm) and UV-A/B (<400 nm) radiation.Control plants were grown under polythene filters transmissible to ambient solar UV-B and UV-A radiation.The transmission characteristics of the filters were measured by a Shimadzu(UV-1601) spectrophotometer (Fig.1).The transmission characteristics of the filters did not change during the experimental period and the filters did not emit fluorescence in the visible region.The cages received full solar radiation during the day without shading.Seedlings were exposed to solar radiation from the time of emergence.There was no significant temperature difference between the control and the UV-excluded chambers as the horizontal holes in the chambers allowed passive air ventilation.The experiments were conducted in a randomized block design with three replications of five plants for each treatment.
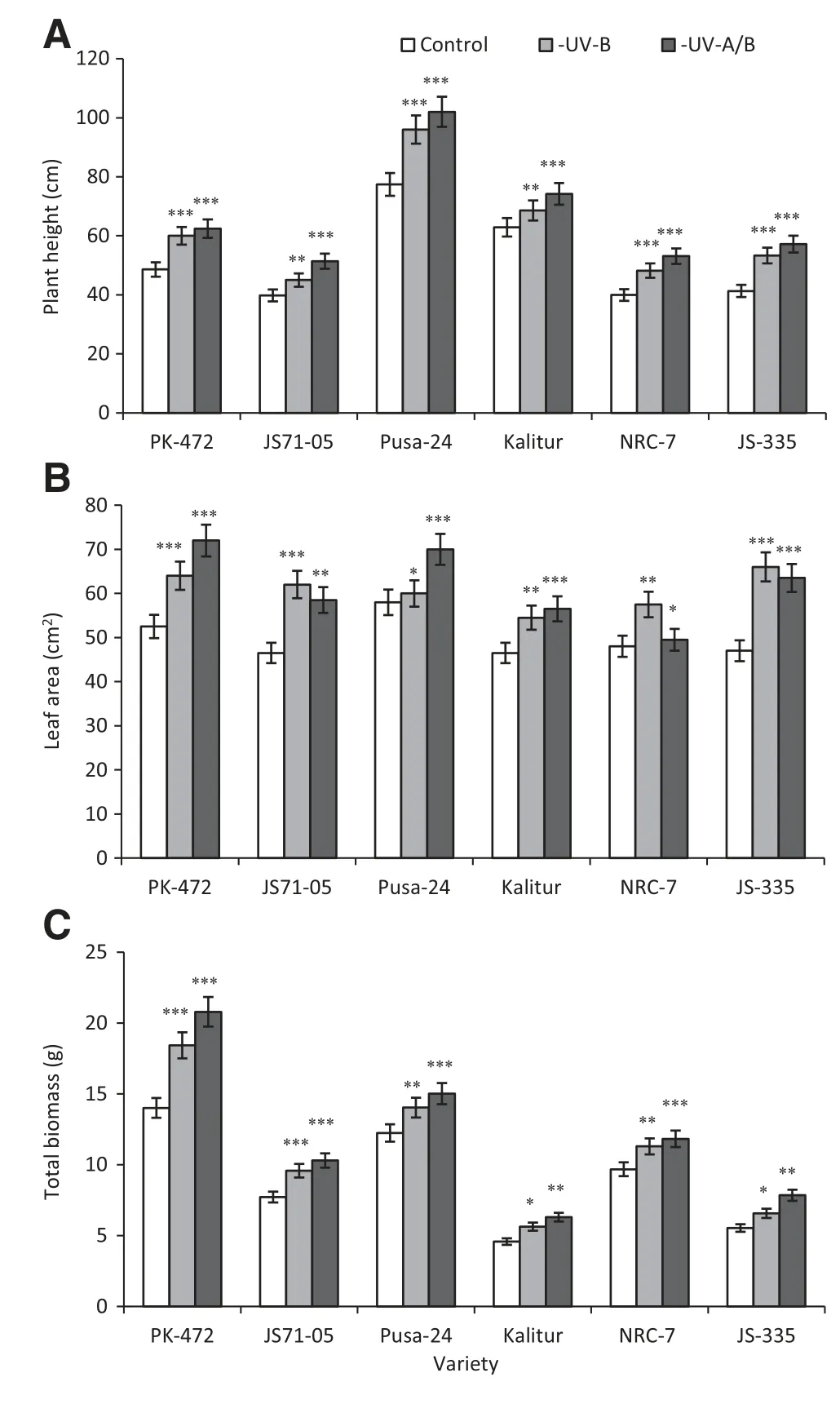
Fig.2-Effect of exclusion of solar UV-B and UV-A/B on plant height(A),leaf area(B)and total biomass(C)in soybean varieties.Vertical bars indicate±SE.Values are significantly different at(*P <0.05,**P <0.01,***P <0.001)from control(Newman-Keuls multiple comparison test).
2.3.Radiation measurement
Absolute solar irradiance without UV-B or UV-A/UV-B was measured using a radiometer(Solar light-PMA 2100,Glenside,PA,USA).The midday photosynthetic active radiation (PAR)during the experimental period was at 1378 μE m-2s-1,the loss in light intensity at midday using-UV-B filters was 10%(1240 μE m-2s-1),and 14% (1180 μE m-2s-1) under the UV-A/UV-B filter and 4.2% (1320 μE m-2s-1) under the polythene filter transmissible to UV(filter control).
2.4.Above ground growth parameters
2.4.1.Plant height and leaf area
Plant height was measured from the soil level to shoot tip in all the five plants in each replicate at crop maturity.The areas of the third trifoliate leaves were estimated by tracing the outlines on gridded graph paper and weighing the cut paper outlines.A calibration curve was prepared by weighing 0–1500 mm2sections of the graph paper.The mean of the 15 leaves was taken as the measured value for each treatment.
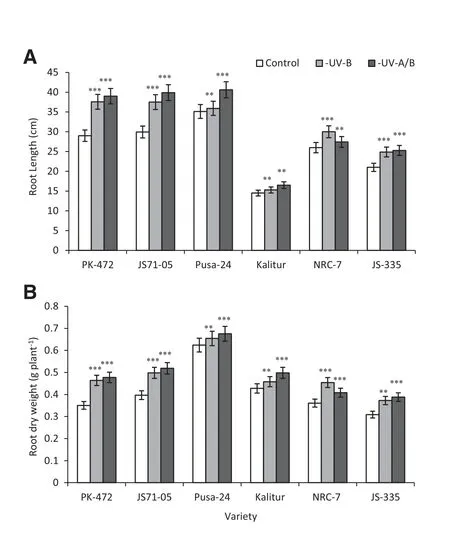
Fig.3-Effect of exclusion of solar UV-B and UV-A/B on root length(A)and root biomass(B)in soybean varieties.Vertical bars indicate±SE.Values are significantly different at(**P <0.01,***P <0.001)from control(Newman-Keuls multiple comparison test).
2.4.2.Total biomass accumulation
At maturity the 15 plants(five plants from each replicate)were randomly selected to measure the total dry weights after ovendrying at 60 °C for 72 h.
2.5.Below ground growth parameters
Below ground growth parameters,including the root length,root biomass,number of root nodules and nodule fresh weight per plant,were measured for all the six varieties at 50 days after seedling emergence(DAE).
2.5.1.Root length and root biomass
The roots were carefully removed and washed prior to measuring the lengths.The roots without the nodules were dried on a filter paper,then further dried at 60 °C for 72 h before weighing.
2.5.2.Number of nodules/fresh weight of nodules
The number of nodules on the roots were counted and recorded on a plant basis,then weighed both individually (gm/nodule)and on a per plant basis for all treatments.
2.6.Rate of photosynthesis
Net photosynthesis(Pn,μmol CO2m-2s-1)was measured on intact plants grown in normal sunlight or UV excluded sunlight under field conditions using a portable system(Li-6200,LI-COR Inc.,Lincoln,Nebraska,USA).Photosynthetic measurements were made on fully expanded leaves(3rd leaf from the apex) of the six plants in each treatment on clear days at noon; the photosynthetic photon flux density (PPFD)was 1200–1300 μmol m-2s-1,air flow (500 mol s-1),and CO2concentration(380–400 μmol L-1).
2.7.Determination of NRA
NRA(EC 1.6.6.1)in leaves were determined by the intact tissue assay method of Jaworski[24].Chopped leaf pieces(100 mg)were incubated for 1 h at 30 °C in a 10 mL reaction mixture containing 25 mmol L-1phosphate buffer,100 mmol L-1potassium nitrate,and 1.25% isopropanol.The nitrite subsequently formed was measured at 540 nm after azo coupling with sulphanilamide and naphthylenediamine dihydrochloride.NRA was expressed as nmol NO2g-1FW h-1.
2.8.Extraction and estimation of Lb content
Lb extracted from the root nodules at 50 DAE of the seedlings was measured by the method of Jun et al.[25].The nodules(1.25 g) of the plants grown under ambient UV radiation,or under exclusion of UV-B and UV-A/B were crushed in liquid nitrogen in a mortar with a pestle.The resulting powder was suspended in 25 mL of 50 mmol L-1sodium phosphate buffer(pH 7.5)containing 1 mmol L-1EDTA,1 mmol L-1PMSF,betamercaptoethanol and 10% polyvinyl pyrrolidone (PVPP),filtered through cheese cloth,and centrifuged at 20,000 × g for 20 min at 4 °C.The deep red supernatant was saturated to 50%with solid(NH4)2SO4and centrifuged at 15,000 × g for 20 min at 4 °C.The pellet was discarded and the red supernatant was saturated to 90% with (NH4)2SO4and centrifuged at 15,000 × g for 20 min at 4 °C.The red pellet was resuspended in 15 mL of 20 mmol L-1Tris–HCl(pH 8.0)containing 1 mmol L-1(NH4)2SO4.The Lb-containing fractions (50% to 90% pellets)were detected at 410 nm using a UV–visible Shimadzu spectrophotometer.
2.9.α-Tocopherol determination
α-Tocopherol was extracted from the leaves by the method of Walker and Slinger [26] and the concentrations were estimated by the method of Pearson et al.[27].The amount of α-tocopherol was calculated from a standard curve.The total soluble protein content was measured in Lb-containing fractions by the method of Lowry et al.[28].
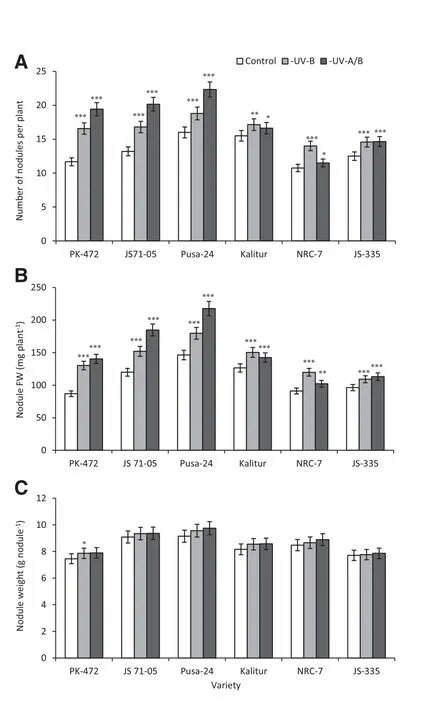
Fig.4-Effect of exclusion of solar UV-B and UV-A/B on number of nodules(A),nodule fresh weights(B) and individual nodule weights(C)in soybean varieties.Vertical bars indicate±SE.Values are significantly different at(*P <0.05,**P <0.01,***P <0.001)from control(Newman-Keuls multiple comparison test).

Fig.5-Effect of exclusion of solar UV-B and UV-A/B on rates of photosynthesis in soybean varieties.Vertical bars indicate±SE for the mean.All values are significantly different(P <0.001)from the controls(Newman-Keuls multiple comparison test).
2.10.Statistical analyses
All the data are presented as means of the 15 plants,expressed as means ±SE,and analyzed by the analysis of variance(ANOVA)followed by post hoc Newman–Keuls multiple comparison tests(*P <0.05,**P <0.01,***P <0.001)using Prism 4 software for Windows,Graf Pad Software,Inc,La Jolla,CA,USA.
3.Results
3.1.Growth parameters of the aerial parts
The plant height,the leaf area(third trifoliate leaves)and the total biomass accumulation were enhanced by the exclusion of UV-B as well as by the exclusion of UV-A/B from all of the six soybean varieties.A maximum enhancement of the plant height after the exclusion of UV-B was obtained in JS-335(29%)and a minimum enhancement was recorded for Kalitur (9%).The plant height was further increased after the elimination of UV-A/B,with the maximum increase occurring in JS-335 (39%)and the minimum enhancement in Kalitur(18%)(Fig.2-A).
The same trend was observed for the increases in the leaf area and the total plant biomass accumulation for all of the six varieties(Fig.2-B and C).
3.2.Growth parameters of the below ground parts
The exposure of the plants to ambient UV-B and UV-A radiation resulted in less root growth/root biomass for all of the six varieties.The root length of the plants grown under UV-A/B at 50 DAE showed maximum increases in PK-472,JS 71-05 and JS-335(34%,33%and 20%,respectively)(Fig.3-A).Similar trends were observed in the promotion of root biomass after the exclusion of UV-B and UV-A/B(Fig.3-B).
The exclusion of UV-B and UV-A/B increased the number of nodules and fresh weights of nodules in all of the varieties(Fig.4-A,B).Maximum increases were recorded for PK-472,NRC-7 and JS 71-05(42%,30%and 27%,respectively)relative to the respective controls.The exclusion of UV-A/B from solar radiation further increased the number of nodules on PK-472,JS 71-05 and Pusa-24(67%,53%and 40%,respectively)relative to the controls (Fig.4-A).A similar trend was obtained for the increased fresh weights of nodules in all varieties by the exclusion of UV-B and UV-A/B(Fig.4-B),but individual nodule weights were not significantly altered after the exclusion of UV-B or UV-A/B(Fig.4-C).
3.3.Rate of photosynthesis
Significant increases in the rates of photosynthesis were observed after the exclusion of solar UV-B and UV-A/B in all of the six varieties.However the extents of increase were greater in PK-472,Pusa-24 and JS 71-05 compared to the others(Fig.5).The increased net rates of photosynthesis were higher after the exclusion of UV-A/B than UV-B relative to the respective controls (Fig.5).
3.4.NRA
The mean NRA in the soybean leaves on the plants grown in ambient conditions ranged from 2.68 to 20.31 nmol g-1FW h-1.The UV-A/B exclusion significantly decreased the NRA in the leaves of PK-472,Pusa-24 and JS 71-05.A lower effect occurred in the plants subjected only to UV-B exclusion (Fig.6).Maximum reductions in NRA after the exclusion of UV-B were shown by PK-472,Kalitur and NRC-7 (33%,28% and 20%,respectively)compared to the respective controls.
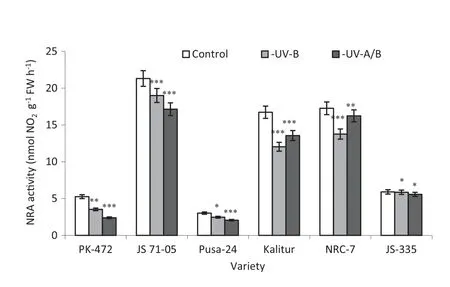
Fig.6-Effect of exclusion of solar UV-B and UV-A/B on nitrate reductase(NRA)activity in soybean varieties.Vertical bars indicate±SE for mean.Values are significantly different at(*P <0.05,**P <0.01,***P <0.001)from control(Newman-Keuls multiple comparison test).
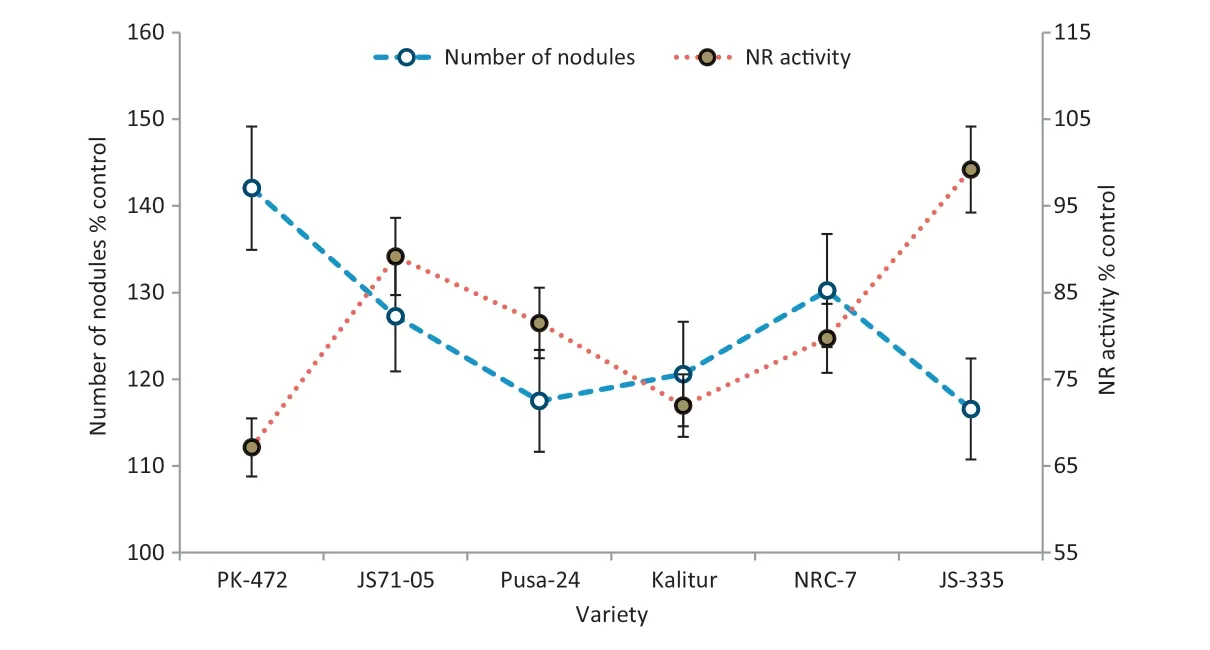
Fig.7-Negative correlations between number of nodules and NRA in soybean varieties after exclusion of solar UV-B.Vertical bars indicate ±SE of means.
There was a significant negative correlation between the number of nodules and NRA in the soybean varieties after the UV-B (-0.847 at P = 0.008**) and UV-A/B (-0.763 at P = 0.028*)exclusion.NRA was much lower in the varieties with the highest increases in the number of nodules after a UV exclusion(Figs.7,8).
3.5.Total soluble protein in the nodules and Lb
Biochemical analyses of the nodules from all six varieties indicated higher amounts of Lb and total soluble proteins.The amounts of the total soluble proteins in the nodules were increased by 25% (-UV-B) and 33% (-UV-A/B) in PK-472,and 9% (-UV-B) and 15% (-UV-A/B) in JS-335 compared to the controls(Fig.9-A).Lb is a protein that plays an important role in the fixation of nitrogen in the nodules.The UV exclusion increased the Lb content in the root nodules by 36% (-UV-B),and 56%(-UV-A/B)(Fig.9-B)in PK-472 with the corresponding values of 10%and 22%for JS-335(Fig.9-B).
3.6.α-Tocopherol
The UV-B and UV-A/B exclusion resulted in significant increases in the levels of α-tocopherol in all varieties.Reductions in the levels of α-tocopherol caused by the exclusion of UV-B were further increased by the exclusion of UV-A/B(Fig.10).

Fig.8-Negative correlations between number of nodules and NRA in soybean varieties after exclusion of solar UV-A/B.Vertical bars indicate ±SE of means.
4.Discussion
The results of this study indicate the positive effects of UV exclusion on the aerial and the below ground parts of the six soybean varieties.Differences between the varieties were also apparent.The exclusion of solar UV-A/B led to larger effects than the exclusion of solar UV-B in the varieties PK-472,JS-7105,JS-335 and Pusa-24.The varieties NRC-7 and Kalitur responded more in response to the UV-B exclusion than in response to the UV-A/B exclusion for traits such as leaf area,root length,root fresh weight,nodule number per plant,nodule fresh weight and NRA.
Conflicting data have been published regarding the effects of UV-B on the underground parts or non-UV exposed parts,namely the roots,number of root nodules,weight of nodules and the amount of nitrogen fixation.Shiozaki et al.[15] found that the growth of pea plants was enhanced by the near UV(300–400 nm)radiation.Nodulation and symbiotic nitrogen fixation were also enhanced twofold and eightfold,respectively.Similarly,Tezuka et al.[29]found that UV-A(320–400 nm)sourced from UV lamps promoted nodulation on roots and increased symbiotic N2fixation in soybean.Ambient UV-B affected the biomass partitioned to tubers and increased root diameter and root fresh weight of radish [30].Pinto et al.[13] found that bean plants grown in a greenhouse,where UV-B levels are low compared to outside levels had almost 60%more nodules per plant and 2.5-fold increases in nodulation compared to the plants grown outside.However,moderate and elevated UV-B exposure had no effect on the number of nodules,nodule mass and nodule size,although nitrogen concentration was markedly reduced in the roots of soybean and common bean[31].The responses in the root biomass as presented here are consistent with the report of increased root length in a Carex species subjected to a reduced UV radiation [32].Similarly,our results indicated that the absence of solar UV components significantly enhanced the number of nodules and the fresh weight of nodules along with the increased root length and root mass; however,the weight per nodule was not significantly changed.Thus the greater N fixation was attributed to the increased number of nodules.
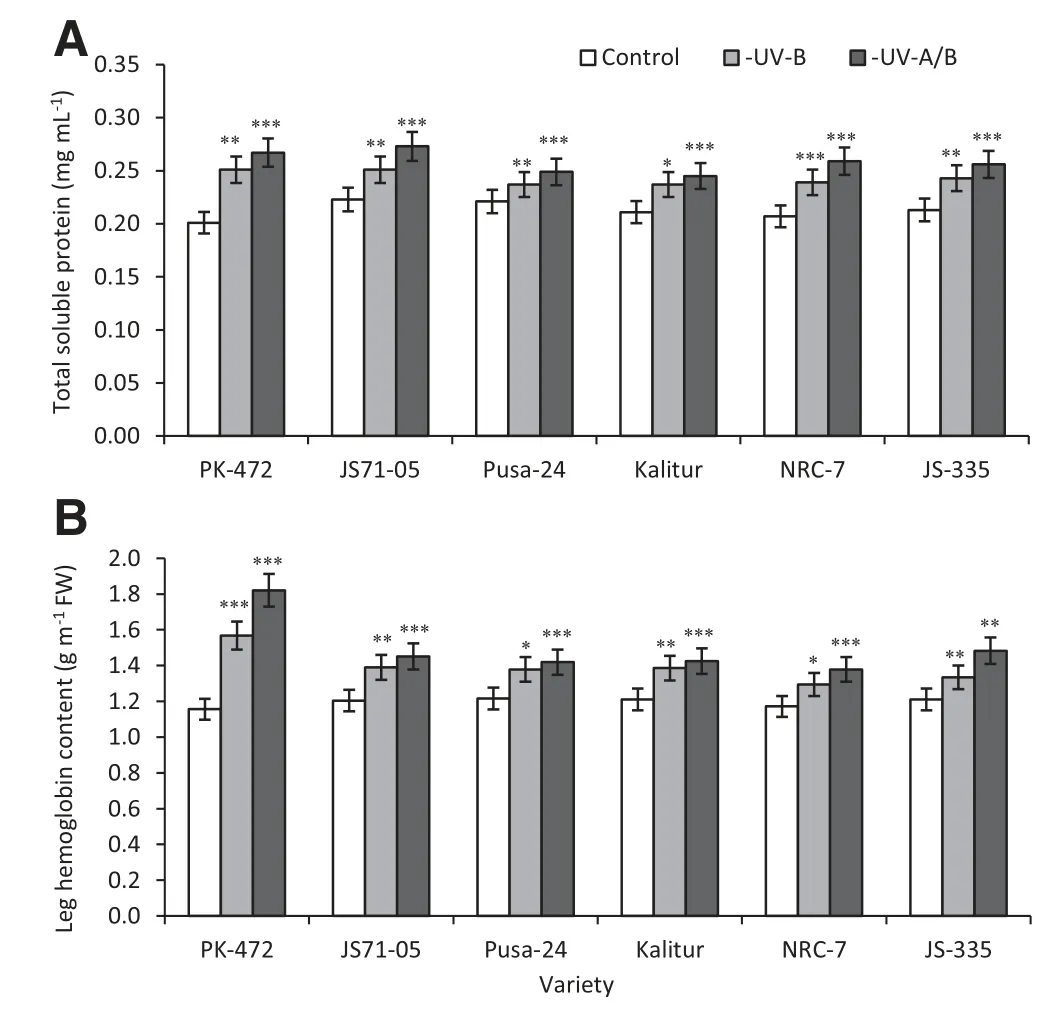
Fig.9-Effect of exclusion of solar UV-B and UV-A/B on total soluble protein(A)and leghemoglobin(B)contents in root nodules of soybean varieties.Values are significantly different at(*P <0.05,**P <0.01,***P <0.001)from controls(Newman-Keuls multiple comparison test).
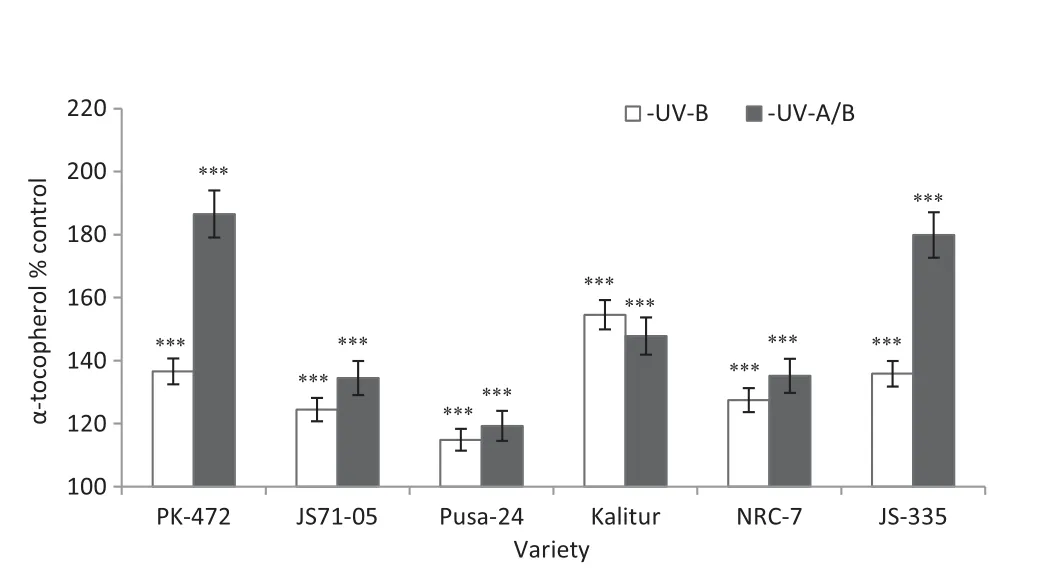
Fig.10-Effect of exclusion of solar UV-B and UV-A/B on α-tocopherol levels in third trifoliate leaves of soybean varieties.Values are significantly different at(***P <0.001)from respective controls(Newman-Keuls multiple comparison test).
The number of studies on the effect of ambient UV irradiance on the N metabolism of plants is limited.Moreover,the enhancement of UV radiation with UV-B lamps has been more commonly used in experimental work than the exclusion of UV radiation with filters.In the present study,UV-A/B exclusion led to a significant reduction in the NRA in the leaves of soybean varieties when compared to control plants.Previous studies showed that UV-A/B exclusion in the field reduced NRA in the leaves of silver birch (Betula pendula Roth.) seedlings[33],and enhanced UV-B,reduced growth and decreased NRA in dragon spruce (Picea asperata) needles [34] and in crop seedlings[35,36].
NR catalytic flux is controlled by substrate availability and level and activity of functional NR.Nitrate reduction capacity is regulated in relation to the overall plant metabolic level by metabolic sensors and signal transduction pathways [8].Nitrate reductase that is located at the junction of two energy-consuming pathways,nitrate assimilation and carbon fixation,results in a controlled response to environmental changes that affect photosynthesis [37].Biological N2fixation can fulfill the N demand of leguminous crops such as soybean,resulting in significant increases in plant total N accumulation and higher N concentrations in the seeds [38] compared to N-fertilized plants.However,in terms of N acquisition,these benefits are accompanied by increased respiration costs of 14%or more of the current photosynthesis when compared to N-fertilized soybean[39].Nitrate assimilation results in costs of up to 2.5 g C g-1N assimilated,whereas N2fixation costs 5.2–18.8 g C g-1N[40].Therefore,N2fixation will be limited by the availability of photosynthate unless there is a simultaneous increase in the rate of photosynthesis[38,41].
The rates of photosynthesis also respond to factors other than leaf N concentration,such as environmental conditions and changes in the source:sink ratio of the plant[42,43].There are reports showing that a decrease in the sink:source ratio caused by a removal of pods at the reproductive stage in soybean,decreases the rate of photosynthesis[42,44].Moreover,changes in the sink:source ratio may lead to an absence of nodules and decrease photosynthetic response to elevated CO2[43].
The higher contents of Lb increase the efficiency of the plants in terms of the capacity to fix atmospheric nitrogen[45].The present data on the exclusion of solar UV radiation indicates an effect on the activity of nitrogen fixation by enhanced synthesis of Lb.Increased growth of the aerial parts seems to be deriving more nitrogen to support growth and enhanced protein synthesis.Increased number of nodules,Lb content and net rates of photosynthesis after the exclusion of UV-B and UV-A/B reflect the increase in sink:source ratio.α-Tocopherol,plays an important role in translocating photoassimilates from the leaves to the roots[16].In the present study α-tocopherol levels were higher in UV excluded plants,enabling them to translocate more photoassimilates from the leaves to the roots (Fig.10).Recently,Kaschuk et al.[39] found N-independent effects of Rhizobia on photosynthesis,whereby the rates of photosynthesis were stimulated by the photosynthate (C) sink strength of symbiosis.Since the C costs of N2fixation are higher than those of the NO3-uptake[40],our data support the assumption that higher C sink strength of N2fixation increases the rate of photosynthesis in UV excluded plants.Higher N2fixation by Rhizobia nitrogenase was reported in UV excluded soybean plants [46] suggesting that the plants with more nodules achieved higher rates of photosynthesis because they had a larger demand for photosynthate.

Fig.11-Impact of UV radiation on photosynthesis,UAS,α-tocopherol,leghemoglobin,nodulation,NRA and growth in soybean plants.
We found a significant negative correlation between the NRA and the number of nodules per plant after the exclusion of UV-B(r = –0.847 at P = 0.008**) and UV-A/B (r = –0.763 at P = 0.028*)(Figs.7,8).
The plants growing under ambient levels of UV-A/B radiation are constantly under stress,and a reduced photosynthesis(Fig.5)may result in a reduced transport of photosynthates to the roots.The rate of N2fixation in a legume depends upon the amount of carbon product the host plant can provide and therefore any reduction in photosynthates decreases the nodule activity [12].Abu-Shakra et al.[41]observed that nodulated soybeans with higher nitrogenase activity had prolonged photosynthetic activity.They suggested that longer photosynthetic activity is related to higher N availability in the leaves as a consequence of higher N2fixation.
In the present study the varieties NRC-7 and Kalitur performed differently from the other four in showing lower increases in the number of nodules and larger increases in NRA following the exclusion of UV-A/B.On the other hand JS-7105,JS-335 and Pusa-24 showed lower number of nodules and larger increases in NRA by the exclusion of UV-B.These variations in the responses of the nodulated legumes to ambient UV-B and UV-A could be attributed to genotypic differences in their sensitivity.
In conclusion,the control plants(+UV-A/UV-B)with reduced photosynthesis,Lb content and reduced primary metabolism seem to fulfill their N demand through the assimilation of NO3-by NR and thus NRA in the control plants is higher to compensate for the reduced symbiotic N2fixation.These results indicate that exclusion of UV increased the growth of nodules as well as primary metabolism,and the higher rate of photosynthesis associated with enhanced levels of α-tochopherol permitted the translocation of photoassimilates from the leaves to the roots,to support better growth of nodules and Lb content for increased symbiotic nitrogen fixation(Fig.11).
The work was financially supported by grants from the UGC and Department of Higher Education M.P.(TRF) to Sanjay Singh Baroniya,and the Department of Science Technology (WOS-A Scheme SR/WOS-A/LS101/2009)to S.Kataria.Dr.V.S.Bhatia,DRS Indore,kindly provided the seeds of the soybean varieties.
[1] S.Madronich,R.L.Mckenzie,M.M.Caldwell,L.O.Bjorn,Changes in UV-radiation reaching the earth's surface,AMBIO 24 (1995) 143–152.
[2] A.P.Mitra,Global change and Indian experience,Impact of Global Climatic Changes on Photosynthesis and Plant Productivity,Proc.the Indo-US Workshop,1991,pp.8–12.
[3] S.Koti,K.Raja Reddy,V.R.Reddy,V.G.Kakani,D.Zhao,Interactive effects of carbon dioxide,temperature,and ultraviolet-B radiation on soybean(Glycine max L.)flower and pollen morphology,pollen production,germination,and tube lengths,J.Exp.Bot.56(2005) 725–736.
[4] K.N.Guruprasad,S.Bhattacharjee,S.Kataria,S.Yadav,A.Tiwari,S.S.Baroniya,A.Rajiv,P.Mohanty,Growth enhancement of soybean(Glycine max)upon exclusion of UV-B and UV-A components of solar radiation characterization of photosynthetic parameters in leaves,Photosynth.Res.94(2007)299–306.
[5] S.S.Baroniya,S.Kataria,G.P.Pandey,K.N.Guruprasad,Intraspecific variation in sensitivity to ambient ultraviolet-B radiation in growth and yield characteristics of eight soybean cultivars grown under field conditions,Braz.J.Plant Physiol.23 (2011) 197–202.
[6] P.Dehariya,S.Kataria,K.N.Guruprasad,G.P.Pandey,Photosynthesis and yield in cotton (Gossypium hirsutum L.)var.Vikram after exclusion of ambient solar UV-B/A,Acta Physiol.Plant.34(2012) 1133–1144.
[7] S.S.Baroniya,S.Kataria,K.N.Guruprasad,G.P.Pandey,Intraspecific variations in antioxidant defense responses and sensitivity of soybean varieties to ambient UV radiation,Acta Physiol.Plant.35(2013) 1521–1530.
[8] W.H.Campbell,Nitrate reductase structure,function and regulation: bridging the gap between biochemistry and physiology,Annu.Rev.Plant Physiol.Plant Mol.Biol.50(1999)277–303.
[9] M.Becana,J.I.Sprent,Nitrogen fixation and nitrate reduction in the root nodules of legumes,Physiol.Plant.70 (1987)757–765.
[10] H.Stefan,N.Christian,Biomass production and N2fixation of five Mucuna pruriens varieties and their effect on maize yield in the forest zone of Cameroon,J.Plant Nutr.Soil Sci.165(2002) 101–109.
[11] K.K.Choudhary,S.B.Agrawal,Cultivar specificity of tropical mung bean (Vigna radiata L.) to elevated ultraviolet-B:changes in antioxidative defense system,nitrogen metabolism and accumulation of jasmonic and salicylic acids,Environ.Exp.Bot.99(2014)122–132.
[12] A.Singh,Increased UV-B radiation reduces N2fixation in tropical leguminous crops,Environ.Pollut.3(1997) 289–291.
[13] M.E.Pinto,G.E.Edwards,A.A.Riquelme,M.S.B.Ku,Enhancement of nodulation in bean (Phaseolus vulgaris) by UV-B irradiation,Funct.Plant Biol.29(2002) 1189–1196.
[14] S.Chouhan,K.Chauhan,S.Kataria,K.N.Guruprasad,Enhancement in leghemoglobin content of root nodules by exclusion of UV-A and UV-B radiation in soybean,J.Plant Biol.51(2008) 132–138.
[15] N.Shiozaki,I.Hattori,R.Gojo,T.Tezuka,Activation of growth and nodulation in a symbiotic system between pea plants and leguminous bacteria by near UV radiation,J.Photochem.Photobiol.B Biol.50(1999) 33–37.
[16] D.Hofius,M.R.Hajirezaei,M.Geiger,H.Tschiersch,M.Melzer,U.Sonnewald,RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants,Plant Physiol.135 (2004) 1256–1268.
[17] Y.Li,Y.Q.Zu,J.J.Chen,H.Y.Chen,Intraspecific responses in crop growth and yield of 20 soybean cultivars to enhanced ultraviolet-B radiation under field conditions,Field Crops Res.78 (2002) 1–8.
[18] Y.Li,L.L.He,Y.Q.Zu,Intraspecific variation in sensitivity to ultraviolet-B radiation in endogenous hormones and photosynthetic characteristics of 10 wheat cultivars grown under field conditions,S.Afr.J.Bot.76(2010)493–498.
[19] C.M.Correia,E.L.V.Areal,M.S.Torres-Pereira,J.M.G.Torres-Pereira,Intraspecific variation in sensitivity to ultraviolet-B radiation in maize grown under field conditions II.Physiological and biochemical aspects,Field Crops Res.62(1999)97–105.
[20] T.Kumagai,J.Hidema,H.S.Kang,T.Sato,Effects of supplemental UVB radiation on the growth and yield of two cultivars of Japanese low land rice (Oryza sativa L.) under the field in a cool rice growing region of Japan,Agric.Ecosyst.Environ.83(2001) 201–208.
[21] S.Koti,K.R.Reddy,V.G.Kakani,D.Zhao,V.R.Reddy,Soybean(Glycine max) pollen germination characteristics,flower and pollen morphology in response to enhanced ultraviolet-B radiation,Ann.Bot.94(2004) 855–864.
[22] Y.Yao,Z.Xuan,Y.He,S.Lutts,H.Korpelainen,C.Li,Principal component analysis of intraspecific responses of tartary buckwheat to UV-B radiation under field conditions,Environ.Exp.Bot.61(2007) 237–245.
[23] S.Kataria,K.N.Guruprasad,Solar UV-B and UV-A/B exclusion effects on intraspecific variations in crop growth and yield of wheat varieties,Field Crops Res.125 (2012) 8–13.
[24] E.G.Jaworski,Nitrate reductase assay in intact plant tissue,Biochem.Biophys.Res.Commun.43(1971) 1274–1279.
[25] H.Jun,G.Sarath,R.W.Wagner,Detection and purification of leghemoglobins from soybean root nodules,Plant Sci.100(1994) 31–40.
[26] B.L.Walker,S.J.Slinger,Effect of processing on the tocopherol content of rapeseed oils,Can.Inst.Food Sci.Technol.J.8(1975) 179–180.
[27] C.K.Pearson,R.R.Davies,M.M.Barnes,Separation of alpha-tocotrienol from alpha-tocopherol by polyethylene celite column chromatography,Chem.Ind.8 (1970)275–276.
[28] O.H.Lowry,N.J.Roseburg,A.L.Farr,R.J.Randall,Protein measurement with the folin phenol reagent,J.Biol.Chem.193 (1951) 265–275.
[29] T.Tezuka,N.Shiozaki,F.Yamaguchi,Y.Ando,Responses of soybean plants to nodulation and N2fixation by near UV radiation,Environ.Sci.6 (1998) 107–112.
[30] J.A.Zavalla,J.F.Botto,Impact of solar UV-B radiation on seedling emergence,chlorophyll fluorescence and growth and yield of radish(Raphanus sativus),Funct.Plant Biol.29(2002) 797–804.
[31] S.B.Chimphango,C.F.Musil,F.D.Dakora,Effects of UV-B radiation on plant growth,symbiotic function and concentration of metabolites in three tropical grain legumes,Funct.Plant Biol.30(2003)309–318.
[32] R.Rinnan,M.M.Keinanen,A.Kasurinen,J.Asikainen,T.K.Kekki,T.Holopainen,H.Ro-Poulasen,T.N.Mikkelsens,A.Michelsen,Ambient ultraviolet radiation in the Arctic reduces root biomass and alters microbial community composition but has no effects on microbial biomass,Glob.Change Biol.11(2005)564–574.
[33] M.Krywult,M.Turunen,M.L.Sutinen,K.Derome,Y.Norokorpi,Nitrate reductase activity in some subarctic species and UV influence in the foliage of Betula pendula Roth.seedlings,Sci.Total Environ.284(2002)149–155.
[34] X.Yao,Q.Liu,Responses in growth,physiology and nitrogen nutrition of dragon spruce (Picea asperata) seedlings of different ages to enhanced ultraviolet-B,Acta Physiol.Plant.29 (2007) 217–224.
[35] S.Quaggiotti,A.R.Trentin,F.DallaVecchia,R.Ghisi,Response of maize nitrate reductase to UV-B radiation,Plant Sci.167(2004) 107–116.
[36] K.Rajendiran,M.P.Ramanujam,Interactive effects of UV-B irradiation and triadimefon on nodulation and nitrogen metabolism in Vigna radiata plants,Biol.Plant.50(2006)709–712.
[37] C.L.Cheng,G.N.Acedeo,M.Cristinsin,M.A.Conkling,Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription,Proc.Natl.Acad.Sci.U.S.A.89(1992)1861–1864.
[38] J.Imsande,Enhanced nitrogen fixation increases net photosynthetic output and seed yield of hydroponically grown soybean,J.Exp.Bot.39 (1988) 1313–1321.
[39] G.Kaschuk,T.W.Kuyper,P.A.Leffelaar,M.Hungria,K.E.Giller,Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses?Soil Biol.Biochem.41(2009)1233–1244.
[40] F.R.Minchin,J.F.Witty,Respiratory/carbon costs of symbiotic nitrogen fixation in legumes,in: H.Lambers,M.Ribas-Carbo(Eds.),Plant Respiration,Springer,Dordrecht,the Netherlands,2005,pp.195–205.
[41] S.S.Abu-Shakra,D.A.Phillips,R.C.Huffaker,Nitrogen fixation and delayed leaf senescence in soybeans,Science 199 (1978)973–975.
[42] S.J.Crafts-Brandner,D.B.Egli,Sink removal and leaf senescence in soybean,Plant Physiol.85(1987)662–666.
[43] E.A.Ainsworth,A.Rogers,R.Nelson,S.P.Long,Testing the“source-sink”hypothesis of down-regulation of photosynthesis in elevated(CO2)in the field with single gene substitutions in Glycine max,Agric.For.Meteorol.122(2004)85–94.
[44] V.A.Wittenbach,Effects of pod removal on leaf photosynthesis and soluble protein composition of field-grown soybeans,Plant Physiol.73(1983)121–124.
[45] P.Gurumoorthi,S.S.Kumar,V.Vadivel,K.Janardhanan,Studies on agro botanical characters of different accessions of velvet bean collected from Western Ghats,South India,Trop.Subtrop.Agroecosyst.2(2003) 105–115.
[46] P.Singh,Impact of exclusion of solar UV components on photosynthesis,nitrogen fixation and growth of soybeanPh.D.Dissertation Devi Ahilya Vishwavidyalaya,Indore,India,2012.112–119.
- The Crop Journal的其它文章
- Maize forage aptitude: Combining ability of inbred lines and stability of hybrids
- Molecular detection of Xanthomonas oryzae pv.oryzae,Xanthomonas oryzae pv.oryzicola,and Burkholderia glumae in infected rice seeds and leaves
- Differences between soybean genotypes in physiological response to sequential soil drying and rewetting
- Mapping and validation of a dominant salt tolerance gene in the cultivated soybean(Glycine max) variety Tiefeng 8
- Genetic background effects on QTL and QTL × environment interaction for yield and its component traits as revealed by reciprocal introgression lines in rice
- Brief Guide for Authors

