Differences between soybean genotypes in physiological response to sequential soil drying and rewetting
Md Mokter Hossin,Xueyi Liu,Xusheng Qi,Hon-Ming Lm,Jinhu Zhng,*
aSchool of Life Sciences and Center for Soybean Research of the State Key Laboratory of Agrobiotechnology,The Chinese University of Hong Kong,Shatin,Hong Kong,China
bInstitute of Economic Crops,Shanxi Academy of Agricultural Sciences,Fenyang 032200,China
cInstitute of Crop Science,Gansu Academy of Agricultural Sciences,Lanzhou 730070,China
1.Introduction
Drought is a critical environmental factor that imposes water stress on crops,and a major constraint on plant growth and productivity[1].It is the most damaging abiotic stress affecting modern agriculture[2].Most cultivated crops are comparatively susceptible to even mild water stress.Scarcity of water may become more severe in the future with changing global climate.A lack of sufficient moisture leading to drought stress is a common phenomenon in rainfed areas,brought about by infrequent rain and poor irrigation[3].Economic yield reduction due to drought stress at various growth stages has been reported in many field crops,such as soybean [4],maize [5],barley[6],rice[7],common bean[8],and potato[9].
The response of drought stress to plants is a highly complex trait involving multiple genetic,morphological,physiological,and biochemical mechanisms[10,11].Species tolerant to drought generally differ morphologically and/or physiologically and possess mechanisms allowing better production under limited water supply [12].Drought-tolerance mechanisms involve maximization of water uptake by deep,dense root systems and minimization of water loss by stomatal closure and reduction of leaf area[13].
Plants can sense water shortage around their roots and respond instantaneously by sending chemical signals to shoots to initiate various adaptive responses including reducing leaf expansion and increasing stomatal closure[14,15].During water shortage,roots produce chemical signals such as increased abscisic acid (ABA) concentration and pH of xylem sap transported to the leaf through the transpiration stream,and regulate stomatal opening and leaf growth[14,16–18].Even mild water stress may increase xylem sap pH,owing to reduced nitrate uptake causing an increase in apoplastic pH [19,20].Increased xylem pH has been suggested to act as a drought signal [21].However,increased pH was observed in some species experiencing water deficit and reduced pH in others[22].In another study,soil water deficit plants did not show a drought-induced increase in xylem pH[23].
Plants have evolved mechanisms that allow them to perceive external stresses and rapidly regulate their physiology and metabolism to cope with them [2].Leaf conductance can be reduced in the absence of visible reduction of leaf water potential [24,25].The net photosynthetic and transpiration rates of water-stressed plants decrease [26–28],and instantaneous water use efficiency (WUEi) reflects the ability of plants to produce biomass per unit of water transpired[29].In this context,WUEi can be considered as an adaptive indicator of soil drying conditions.The adaptive response of proline accumulation is commonly observed in plants under drought stress[30].Proline may act in osmotic adjustment[31]and also as an antioxidant [32].
Soybean (Glycine max L.Merr.) is one the major sources of protein for human and animal nutrition as well as a key source of vegetable oil.It is considered as a potential crop for production of biodiesel.However,it requires adequate soil moisture throughout its growth period to attain its yield potential[33].
Depending on genotypic characteristics,soybean uses 450–700 mm of water during the growing season[34].Soybean is considered susceptible to drought stress,especially in the critical period of its ontogeny[35].It is accordingly desirable to identify drought tolerant soybean genotypes able to grow well with limited water supplies.Different physiological mechanisms in leaves and roots are important in regulating the growth of soybean genotypes under progressive soil drying.A droughttolerant soybean genotype may escape water stress effects by increasing root depth in soil,reducing leaf area expansion,closing stomata,and maintaining higher relative water content and consequently water potential and turgor pressure.
Unirrigated soybeans showed greater root length than irrigated plants,especially in the subsoil [36].Significant correlations have been found in soybean between drought resistance and various root traits such as dry weight,total length,and volume and number of lateral roots[37,38].Rooting depth was greater in drought-tolerant than in droughtsusceptible clones of Coffea canephora [12].In Hibiscus rosa-sinensis,relative water content,turgor potential,transpiration,stomatal conductance,and water use efficiency decreased under drought stress[39].
However,to our knowledge,leaf expansion rate,gas exchange,water relations,proline,total chlorophyll content,root xylem sap pH,and root traits have not been investigated concomitantly in both drought-tolerant and drought-susceptible soybean genotypes under progressive soil drying followed by rewetting.The present study was designed to improve our understanding of the manner in which drought-tolerant genotypes cope with sequential soil drying,affording a better opportunity to select drought-tolerant soybean genotypes for cultivation in dry areas.In a preliminary experiment,we studied the drought tolerance of eight soybean genotypes under four weeks of water restriction.Two genotypes performed better under water-limited conditions than the others,based on their leaf water status,stomatal conductance,and root length,while one of the genotypes showed markedly poor performance.We focused on these three soybean genotypes for detailed physiological study under progressive water-limited conditions.In the present study,these three genotypes,Jindou 21 (C12),Union(C08),and Mengjin 1 (W05) were used for detailed evaluation of their physiological responses to water restriction and subsequent rewetting.
2.Materials and methods
2.1.Plant materials and growing conditions
Seeds of soybean genotypes were obtained from the Center for Soybean Research of the State Key Laboratory of Agrobiotechnology,The Chinese University of Hong Kong.Seedlings were grown in a plastic tray containing soil mixture(soil and peat moss) in the greenhouse.Five days after germination,seedlings were transplanted into PVC tube(50 cm length and 5 cm inner diameter)filled with soil mixture(sandy loam soil and peat moss at 1:1 volume ratio fertilized with NPK at 14:14:14).Fertilizer granules were added at 5 g L-1of soil mixture.Plants were grown under natural sunlight in the greenhouse with average daytime temperature 25 ± 2 °C and relative humidity 60–70%.The light intensity in the greenhouse was recorded daily at noon and the average was 140–160 μmol m-2s-1.Soybean plants were watered daily with similar water volumes (30 mL plant-1) until the second trifoliate leaves emerged,after which water restriction treatment was imposed.Half of the seedlings of each genotype were kept for regular watering as control plants and the remaining half were allowed to dry during the experimental period.Control plants were irrigated daily in the evening.For a preliminary study,eight soybean genotypes were used to evaluate drought tolerance performance.Three genotypes,Jindou 21 (C12),Mengjin 1 (W05) as drought-tolerant and Union(C08)as drought-susceptible,were selected for detailed study of drought tolerance responses under progressive soil drying and rewetting.
2.2.Measurement of net photosynthetic rate,stomatal conductance,and transpiration rate
After the onset of water restriction,net photosynthetic rate(Pn),stomatal conductance (gs) and transpiration rate (Tr) of fully expanded youngest leaves were determined at three-day intervals until day 12 of the dry cycle and after rewetting,using a LI-6400 Portable Photosynthetic System (Li-COR,Inc.,USA).Measurement was performed from 1000–1300 h under a photosynthetic flux density (PPFD) of 1000 μmol m-2s-1.The air humidity in the leaf chamber was about 50%,with CO2concentration of 350–400 μmol mol-1and ambient air temperature 28 ± 2 °C.Instantaneous water use efficiency(WUEi)was calculated as Pn/Tr.
2.3.Determination of leaf relative water content
Relative water content (%RWC) of fully expanded leaves from the top was measured in both well-watered and waterrestricted plants.To minimize solute leakage and cut surface effects,the entire leaf was used for this purpose.The leaf petiole was carefully cut,leaf fresh weight was recorded,and it was placed in a water-containing plastic tube in a closed container.The container air was saturated by keeping wet tissue paper around the inner wall to maintain high relative humidity.The turgid weight was taken after 24 h and dry weight after oven drying for 48 h at 65 °C.Leaf relative water content was calculated by the following equation:
Relative water content (%RWC)= (Fresh weight-dry weight)/(Turgid weight-dry weight)×100.
2.4.Measurement of leaf water status
Leaf water potential was measured using a pressure chamber(Soilmoisture Equipment Corp.,Santa Barbara,USA).Briefly,a leaf petiole was sealed in the pressure chamber and the chamber was gradually pressurized until the meniscus of the xylem sap became visible at the cut surface,at which time the pressure reading was immediately recorded.For measuring osmotic potential,a middle leaflet was excised following the measurement of water potential and frozen in liquid nitrogen(at-80 °C)in a sealed plastic vial for 24 h,after which the leaf sample was thawed at room temperature and tissue sap was squeezed out with a glass rod.A 10-μL aliquot of cell sap was immediately used to measure osmotic potential using a vapor-pressure osmometer (Wiscor 5600,Logan,USA).The turgor pressure of the leaf was determined as the difference between water and osmotic potentials: ψp= ψw-ψs.
2.5.Measurement of leaf area expansion
Leaf area (LA) was measured with a portable area meter(LI-3000A;Li-Cor,Inc.USA).After imposition of water restriction,newly emerging leaves(middle leaflets of third trifoliate leaves)were tagged for measuring LA daily.Leaf length (LL) and leaf width (LW)were measured daily and the relationship between the product of LL × LW and LA was determined for each genotype from individual leaf measurement of 15 leaves.The regression of LA on LL × LW was calculated as LA = k × LL × LW,where k is the slope of the linear function.
2.6.Measurement of weight loss,RWC,and osmotic potential of excised leaves
Water loss from detached youngest mature leaves was determined according to the method described by Okamoto et al.[40].Leaves from well-watered plants of three soybean genotypes were excised and subjected to sun drying on a sheet of paper under ambient conditions.Measurement was performed on a clear,sunny day at 34 ± 2 °C temperature and 56%relative humidity.Percent weight loss due to water loss from detached leaves was recorded at 10-min intervals with an analytical balance (Shimadzu AUW220D,Shimadzu Corporation,Tokyo,Japan).Leaf samples from each time course were collected for the measurement of%RWC and osmotic potential following the above mentioned methods.
2.7.Determination of leaf proline and total chlorophyll content
Leaf proline content was determined according to the method described by Bates et al.[41].Briefly,fully expanded leaves from both treatments of each genotype were collected at 1100 h.Leaf samples of 0.5 g fresh weight (FW) were ground in a mortar after addition of 5 mL of a 3% (w/v) aqueous sulfosalicylic acid solution.The homogenate was filtered through Whatman No.2 filter paper and the clear filtrate was used in the assay.Acid ninhydrin and glacial acetic acid(1 mL each) were added to 1 mL of filtrate.The closed test tubes with the reaction mixture were kept in a boiling water bath for 1 h at 100 °C,and the reaction was terminated at room temperature.The reaction mixture was then extracted with 2 mL toluene,mixed vigorously with a test tube stirrer for 10–15 s.The chromophore containing toluene was aspirated from the aqueous phase,and the absorbance was read at 520 nm using toluene as blank.The proline concentration was determined from a standard curve and calculated on a fresh weight basis as follows:
Proline (μmol g-1of FW of leaf )=[(μg proline mL-1×mL toluene)/115.5 μg μmol-1]/[(g sample)/5].
Leaf chlorophyll(Chl)content was determined according to the method described by Moran [42].Briefly,50 mg of fresh leaf was cut and kept in a 1.5-mL plastic microtube followed by addition of 0.8 mL of N,N-dimethylformamide (DMF).The tube was then kept overnight in the dark at 4 °C.After incubation and mixing,the optical density of the solution was determined at 603,664,and 647 nm wavelengths using a microplate spectrophotometer(Spectra MAX250,Carlifornia,USA).The leaf chlorophyll content was calculated as Chlt=8.24A664+ 23.97A647-16.64 A603,where Chltis the chlorophyll content in μg mL-1of the DMF subjected to measurement[42].
2.8.Determination of root xylem sap pH
Root xylem sap was collected according to the method described by Zhang and Davies[43]with some modifications.Briefly,root xylem sap was collected by exudation of soybean plants under minor pressure application using a pressure chamber(Soilmoisture Equipment Corp.,Santa Barbara,USA).Plants were initially cut about 15 cm above the soil surface to release the tension in the xylem.Then the stump with the whole root system (including attached soil) was carefully separated from the PVC tube and immediately sealed in a pressure chamber,after which the shoot was re-cut about 2 cm above the base.The chamber was gradually pressurized with compressed nitrogen gas until xylem sap appeared at the cut surface.Initial sap was wiped off with tissue paper to remove contaminants arising from cut cells.The system was then over-pressurized to yield xylem sap.The exuded xylem sap was collected and its voltage was determined within 1–2 min by touching the sap with a microelectrode (MI-410 Micro-combination pH electrode,Microelectrodes,Inc.,USA).Thereafter,pH was calculated from a standard curve previously constructed from the voltages of different buffered solutions.
2.9.Measurement of shoot length,number of internode,root length,root fresh mass,dry mass,root number,and soil water content
On day 12 of the water restriction,well-watered and water-restricted plants were harvested and shoot length and number of internodes was recorded.For the measurement of root length,fresh mass,and number,half of the PVC tube was removed and roots were separated carefully from the soil and washed gently with tap water.Water was wiped from the root surface before weighing,followed by measurement of root length.Root dry mass was recorded after oven drying at 6 °C for 48 h.Roots were counted at 20 cm depth in the soil profile and soil samples were collected from the same location.Soil was oven dried at 105 °C for 72 h and the water content of the soil was determined and expressed as percentage on an oven-dry weight basis.
2.10.Statistical analysis
Data were subjected to one-way analysis of variance(ANOVA)followed by post hoc multiple comparisons using the Tukey test to identify significant differences among the three genotypes.The results presented are means with standard deviations of three to six replicates.The maximum accepted P-value for significance was 0.05.
3.Results
3.1.Drought tolerance performance of soybean genotypes
In a preliminary trial,the drought tolerance of eight soybean genotypes was evaluated within four weeks of withholding water supply,based on their leaf stomatal conductance(gs),%relative water content(RWC),and root length.The gsof some,but not all,genotypes declined quickly as water restriction progressed (Fig.1).Fig.1 showed that genotypes C12,W01,and W05 closed their stomata earlier than other genotypes.With respect to%RWC,genotype C08 showed very low%RWC compared to all other genotypes after four weeks of water restriction(Fig.2).Similarly,root growth of C01,C08,C27,and W08 under water restriction for four weeks were significantly shorter than those of the other genotypes (Fig.3).In view of these responses,C12 and W05 were considered to be droughttolerant genotypes and C08 a drought-susceptible genotype.For further detailed study of drought stress response under progressive water restriction and subsequent rewetting,we focused on the three representative soybean genotypes C08,C12,and W05.
3.2.Water retention ability of detached leaf
The ability of water retention of excised leaves of three soybean genotypes was evaluated.Fully expanded leaves of soybean genotypes were excised from plants and subjected to sun drying in the greenhouse.Percent weight loss from excised leaves of the drought-susceptible genotype was significantly higher than that of the drought tolerant genotypes 10 min following excision (Fig.4-a).The %RWC of excised leaves of the susceptible genotype was substantially lower than that of the tolerant genotypes (Fig.4-b).As water was eliminated faster from excised leaves of C08,the osmotic potential became more negative (Fig.4-c).In contrast,the drought-tolerant soybean genotypes (C12 and W05) exhibited significantly slower % weight loss reduction,%RWC,and osmotic potential.
3.3.Effect of drought stress on soil water content and leaf expansion rates of soybean genotypes
Drought stress was imposed on soybean genotypes(C12,C08,and W05)by withholding of water supply for 12 days,followed by rewetting.After water withholding began,the soil water contents at 20 cm depth in the soil profile in PVC tubes decreased rapidly.Thereafter,the soil water content rapidly returned to the original level after rewetting for all genotypes(Fig.5).
Though leaf expansion rates decreased in soybean genotypes after imposition of water restriction,there were different drought-stress responses among the drought-tolerant and drought-susceptible genotypes.The leaf expansion rates of the drought-susceptible genotype(C08)declined significantly compared to those of the well-watered plants(Fig.6)one day after imposition of water restriction,whereas the drought-tolerant genotypes (C12 and W05) maintained higher leaf expansion rates than C08(Fig.6).

Fig.1-Stomatal conductance of leaves of eight soybean genotypes after four weeks of withholding water supply.ww and wr indicate well-watered and water-restricted(by soil drying)status of soybean genotypes,respectively.Vertical bars indicate mean ± SD(n = 5).
3.4.Effect of drought stress and rewetting on leaf gas exchange and leaf total chlorophyll content
Leaf gas exchange parameters of all genotypes used in the experiment showed similar response patterns during progressive water restriction and rewetting cycles (Fig.7-a,b,c).In general,leaf gas exchange parameters declined with soil water content under water restriction.However,the net photosynthetic rate (Pn) of the drought-tolerant genotypes remained higher than that of the drought-susceptible genotype as water stress progressed(Fig.7-a).Leaf transpiration rate (Tr) and stomatal conductance (gs) of tolerant genotypes diverged significantly faster than those of the susceptible genotype on days 3 and 6 of the drought-stress cycle(Fig.7-b,c).After rewetting on day 12 of the stress cycle,gas exchange parameters of tolerant genotypes exhibited rapid recovery than susceptible genotype,indicating the greater dynamism of stomata of the tolerant genotypes than of stomata of the susceptible one.In addition,the drought-induced stomatal closure in tolerant genotypes resulted in an increase in the leaf instantaneous water use efficiency (WUEi),which remained elevated during the entire period of dehydration (Fig.8).In contrast,the WUEi of the drought-susceptible genotype remained below that of the control plants until one week into the drought-stress cycle.
Total chlorophyll (Chlt) content of leaves of all soybean genotypes was lower than that of well-watered plants under sequential water restriction (well-watered data not shown)but a declining trend in Chltcontent of C08 was pronounced and continued until day 12 of the dry cycle (Fig.9),whereas C12 and W05 maintained greater leaf Chltcontent during the entire period of drought stress cycles.On day 12,the highest Chlt(0.959 μg mL-1)content was recorded in W05,followed by C12 (0.93 μg mL-1) and the lowest Chlt(0.876 μg mL-1) was recorded in C08(Fig.9).
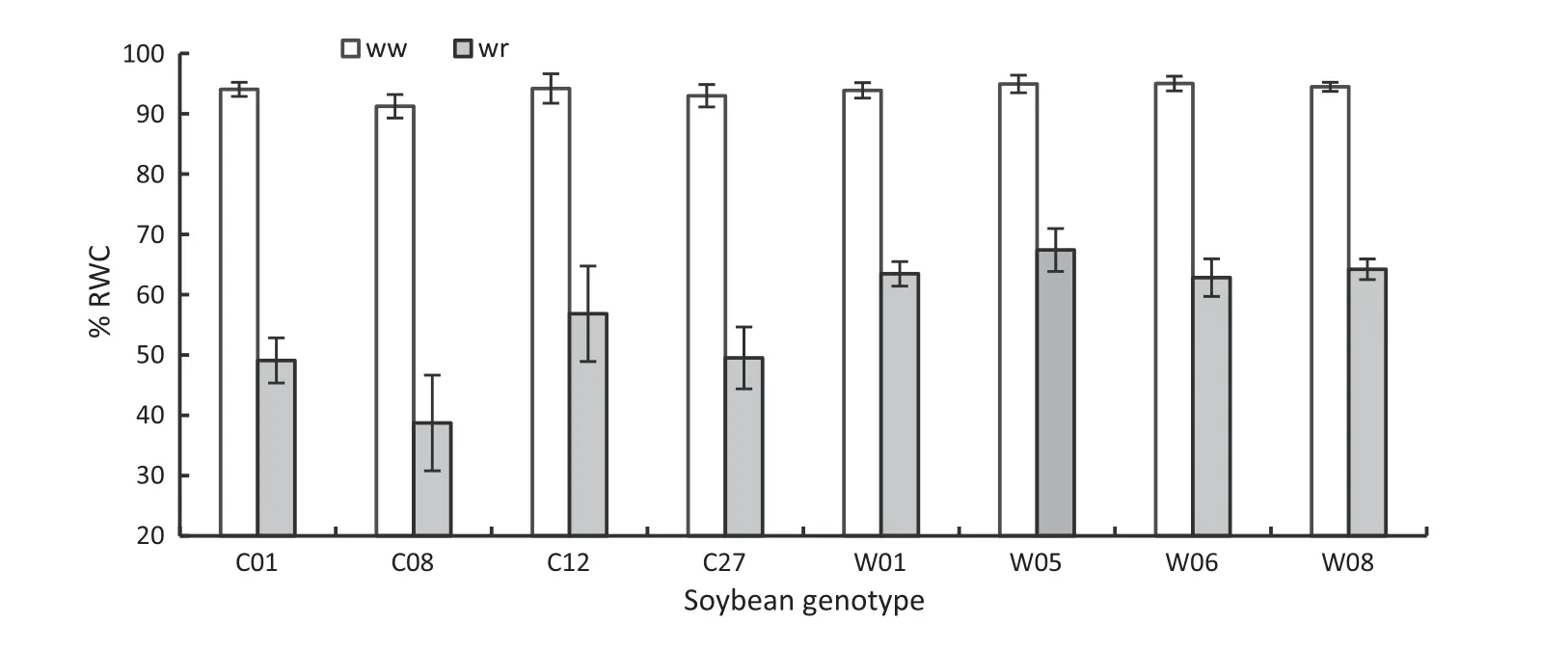
Fig.2-Relative water content(%RWC)of leaves of eight soybean genotypes after four weeks of withholding water supply.ww and wr indicate well-watered and water-restricted status of soybean genotypes,respectively.Vertical bars indicate mean ± SD(n = 5).
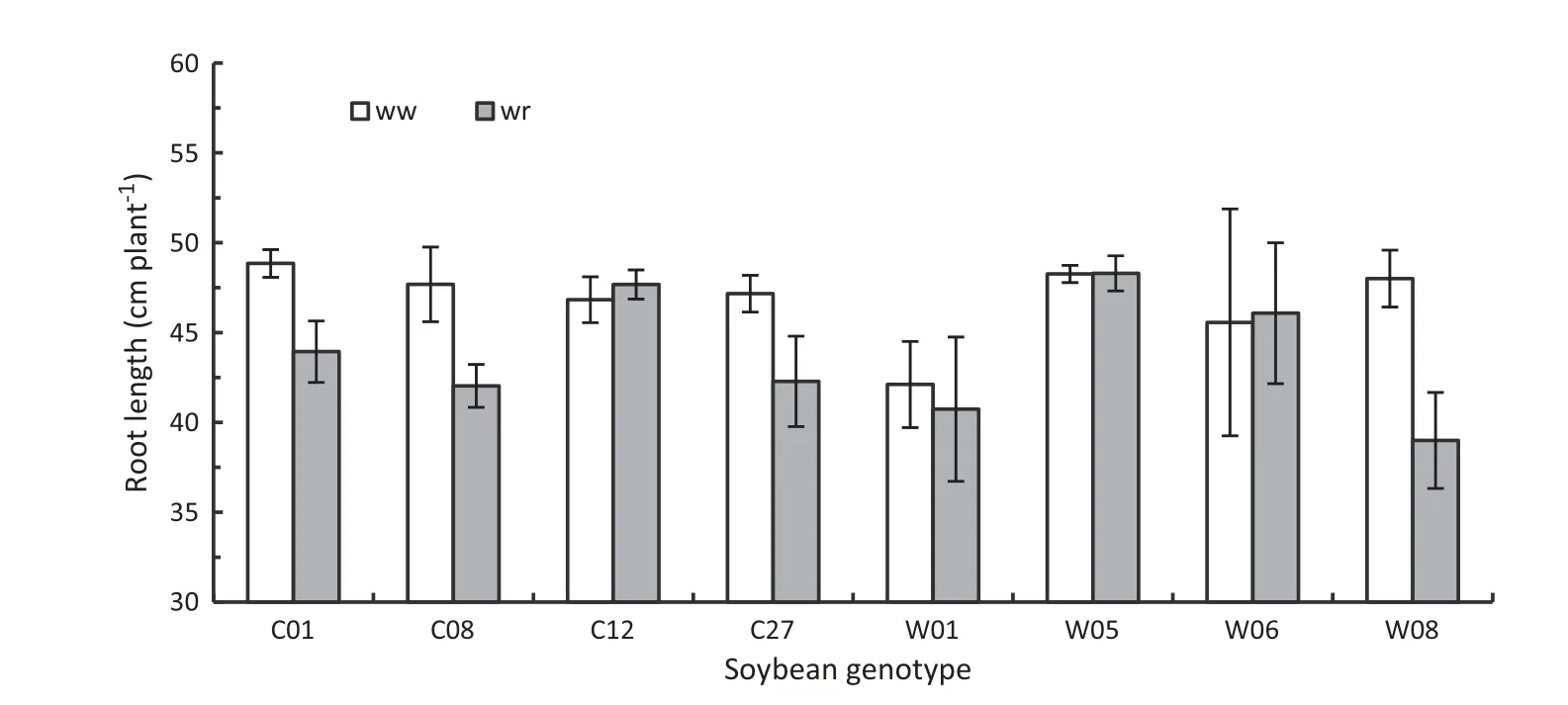
Fig.3-Root lengths of soybean genotypes after four weeks of withholding water supply.ww and wr indicate well-watered and water-restricted status of soybean genotypes,respectively.Vertical bars indicates mean ± SD(n = 4).
3.5.Effect of drought stress and rewetting on water relation parameters
When water stress was intensifying along the soil column,leaf relative water content (%RWC) declined significantly compared to well-watered plants in all genotypes after 3 days,but this declining tendency was more pronounced in the susceptible genotype than in the tolerant genotypes(Fig.10).%RWC of W05 and C12 remained greater throughout the period of withholding water.On day 12 of the stress cycle,%RWC of C08 was significantly reduced to 72.5% of those of the control plants,while %RWC of W05 and C12 were 77.5%and 76.2% of the control plants.When the soil was rewetted,%RWC of the drought-tolerant genotypes exhibited a more rapid recovery and reached values almost equal to those of well-watered plants(Fig.10).
Leaf water potential was measured before water withholding,and the values varied from-0.40 to-0.55 MPa in wellwatered plants (Fig.11-a).Upon imposition of water restriction,these water potentials(ψw)decreased,and after six days of water withholding,the leaf water potential of the drought-tolerant W05 genotype remained higher than those of the other two genotypes.The water potential of the drought-susceptible genotype(C08)declined sharply throughout the entire water restriction period.At the end of the drought cycle,the values of the water potentials of C12,C08,and W05 genotypes were-2.16,-2.28,and-1.85 MPa,respectively (Fig.11-a).When the soil was rewetted,the ψwof drought-tolerant genotypes increased faster and reached-0.89 MPa,a value nearly equal to that of well-watered plants,whereas that the drought-susceptible genotype was-1.13 MPa (Fig.11-a).
Osmotic potential (ψs) was determined from the same leaf after freezing at-80 °C and thawing at room temperature.As dehydration progressed,the values of leaf ψsdeclined more rapidly in the C12 than in the C08 genotype (Fig.11-b).At the end of the drought period,the ψsvalues in the C12,C08 and W05 genotypes were-2.43,-2.32,and-2.07 MPa,respectively(Fig.11-b).Turgor potential(ψp)of leaves was calculated as the difference between ψwand ψs.The result showed that ψpin the drought-susceptible genotype markedly declined after three days of water restriction and remained significantly lower until day 12 of the stress cycle,whereas drought-tolerant genotypes retained leaf turgor pressure significantly higher than that of C08 until day 12 and returned to the control level after rewetting(Fig.11-c).The turgor pressure of C08 did not recover fully even two days after rewetting(Fig.11-c).
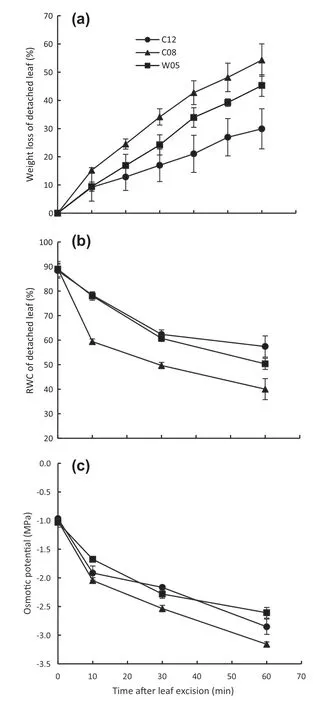
Fig.4-Weight loss(a),relative water content(RWC)(b),and osmotic potential(c)of detached leaves of drought-tolerant(C12 and W05)and drought-susceptible(C08)soybean genotypes.Values are mean ± SD,(n = 4 for a,3-5 for b,and 3 for c).
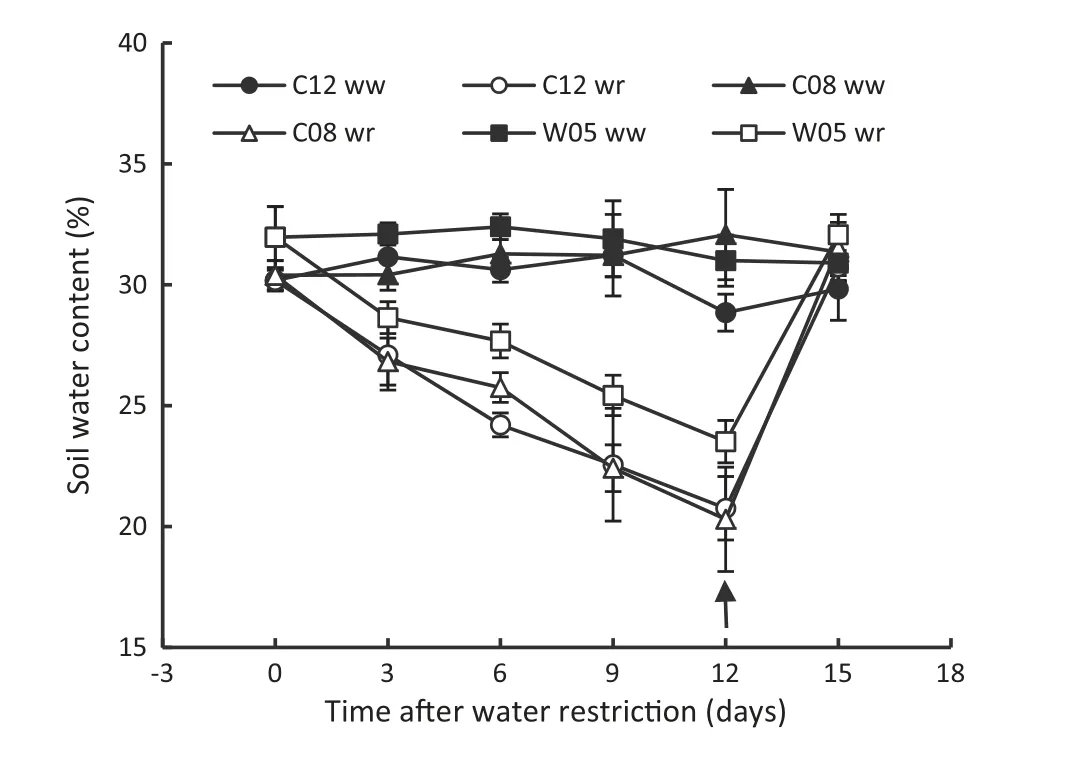
Fig.5-Soil water contents at a depth of 20 cm of soil profile in the PVC tube.Water restriction resumed on day 0 and rewetting was applied on day 12,indicated by the arrow.ww and wr indicate well-watered and water-restricted status of soybean genotypes,respectively.Data are mean ± SD,(n = 3).
3.6.Effect of drought stress on leaf proline content and root xylem sap pH
Leaf proline content of all soybean genotypes was determined at 3-day intervals after water withholding.The leaf proline content of the drought-tolerant genotypes(C12 and W05)was unchanged as water restriction progressed until day 6,while the proline content of drought-susceptible genotype (C08)markedly increased after withholding of water (Fig.12).The proline level of the tolerant genotypes declined sharply and reached almost the well-watered level by one day after rewetting,but this declining pattern in the susceptible genotype was noticeably slower(Fig.12).
Root xylem sap pH was measured at 3-day intervals after water withholding.Change in soil water availability with time induced increases in root xylem sap pH in all soybean genotypes (Fig.13-b).The pH of xylem sap of water-restricted C12 plants was significantly higher than that of the other two genotypes throughout the water restriction cycle.However,the pH of the drought-susceptible genotype(C08)always remained lower than that of the tolerant genotype C12.The subsequent rewetting led to almost complete recovery of the pH value in the xylem sap of the drought-tolerant genotypes,whereas the pH value of the susceptible genotype remained higher(Fig.13-b).
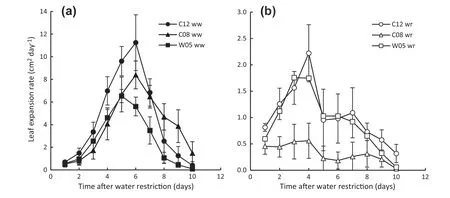
Fig.6-Leaf expansion rate of drought-tolerant(C12 and W05)and drought-susceptible (C08)soybean genotypes in well-watered(a) and during water-restriction(b).Water restriction resumed on day 0.Data are mean ± SD,(n = 6).
3.7.Effect of drought stress on root and shoot growth traits
Progressive water restriction significantly reduced shoot length and root fresh and dry mass of all soybean genotypes(Table 1).However,the drought-susceptible genotype responded most sharply to water restriction,and shoot length,root fresh and dry mass were respectively 44.30%,17.37%,and 30.68% of the corresponding values for well-watered plants.In the droughttolerant genotypes,those traits decreased less than those in the susceptible genotype,reaching 56.70%,24.27%,and 41.84%,respectively (for C12) and 59.14%,20.83%,and 45.78%,respectively(for W05)(Table 1).
Among the three genotypes,drought stress did not affect the number of internodes in C12,but did affect those in C08 and W05.The drought-susceptible C08 showed an internode number higher than that of W05 and 58.12% of those in well-watered plants.
There was no significant difference among the three soybean genotypes in root number due to water restriction at a 20-cm depth in soil column (Table 1).The maximum number of roots was observed at a depth of 15–20 cm in soil profile.Accordingly,root numbers for all genotypes were recorded at 20 cm depth on day 12 of the stress cycle.However,genotype C12 showed the largest root number compared to C08 and W05.Drought stress did not affect root length in C12,but did affect the root length of C08 and W05.The highest root length reduction,in C08,was 65.26% of the control(well-watered)plants(Table 1 and Fig.14).
4.Discussion
Drought tolerance is an important character permitting soybean growth under water-limited conditions.Among the genotypes used in this study,C12 (Jindou 21) has been widely adopted in semi-arid regions in northwest China,with a total accumulated cultivation area over 3.75 million ha [44,45].C08 (Union) is a soybean from the USA used as a sensitive line and W05(Mengjin 1)is a drought-tolerant wild soybean.A small-scale field study of C12,C08 and W05 was performed at Dunhuang,China.Results showed that C12 produced the highest yield(7.67 g plant-1)and C08 the lowest(2.92 g plant-1),and that W05 was late to produce seed[44].Seed maturities of C12 and C08 were reached at similar times,whereas W05 took slightly longer than the cultivated genotypes,as found in the greenhouse (data not shown).The criteria for drought tolerance and susceptibility were primarily yield performance under water-limited conditions.
In the present study,these soybean genotypes were used to investigate drought responses based on changes in leaf gas exchange and water relation traits and root and shoot growth under progressive water restriction and rewetting under greenhouse conditions.
The results from the detached leaf experiment showed that percent weight loss due to water loss from detached leaf was higher in the drought-susceptible genotype,whereas leaves from drought-tolerant genotypes showed much slower weight loss(Fig.4-a).We assumed that water loss from excised leaves occurred via stomata and cuticular transpiration.Ristic and Jenks [46] postulated that a maize line with lower rates of detached leaf water loss had thicker epidermal cell wall and thicker cuticle than a maize line with higher rates of detached leaf water loss.We also estimated%RWC and osmotic potential of the excised leaves and found that drought-tolerant genotypes maintained higher %RWC and osmotic potential during 1 h of dehydration after excision from plants (Fig.4-b,c).Our results suggest that genotypes able to close their stomata very rapidly after leaf detachment from the plant can retain higher%RWC in water-limited environments.

Fig.7-Net photosynthetic rate(a),transpiration rate(b),and stomatal conductance(c)of drought-tolerant(C12 and W05)and drought-susceptible(C08)soybean genotypes after imposition of water restriction.Water restriction began on day 0,and rewetting on day 12 is indicated by the arrow.ww and wr indicate well-watered and water-restricted status of soybean genotypes,respectively.Values of points aremean ± SD(n = 5).
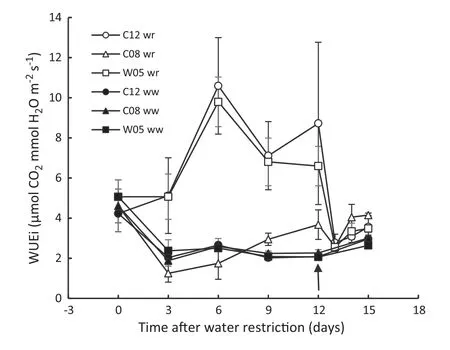
Fig.8-Instantaneous water use efficiency(WUEi)of drought-tolerant(C12 and W05)and drought-susceptible(C08)soybean genotypes after imposition of water restriction.Water restriction began on day 0,and rewetting on day 12 is indicated by the arrow.ww and wr indicate well-watered and water-restricted status of soybean genotypes,respectively.Values of points are mean ± SD(n = 5).

Fig.9-Leaf total chlorophyll content of drought-tolerant(C12 and W05)and drought-susceptible (C08)soybean genotypes after imposition of water restriction.Water restriction began on day 0,and rewetting on day 12 is indicated by the arrow.wr indicates water-restricted status of soybean genotypes.Values of points are mean ± SD(n = 6).
Three soybean genotypes were subjected to progressive water restriction for twelve days and then the soil was rewetted,and leaf gas exchange and water relation parameters were evaluated at three-day intervals.Usually plants can avoid water limited conditions by minimizing water loss by closing the stomata.Moreover,plants can sense water shortage around their roots and respond quickly by sending chemical signals to the shoot to induce various adaptive responses,including increased stomatal closure[14,15].Flexas et al.[47] reported that stomatal conductance was an indicator of the severity and pattern of water stress development.Stomatal response was important for maintaining the water status of woody plants[48].

Fig.10-Relative water contents(RWC) of drought-tolerant(C12 and W05)and drought-susceptible (C08)soybean genotypes after imposition of water restriction.Water restriction began on day 0,and rewetting on day 12 is indicated by the arrow.ww and wr indicate well-watered and water-restricted status of soybean genotypes,respectively.Values of points are mean ± SD(n = 4).
In our study,a reduction of gsdue to progressive water restriction was observed and this reduction of gsled to reduced Trand Pnin all soybean genotypes on day 3 of the stress cycle(Fig.7-a,b,c).Interestingly it was observed from our results that the perception of water shortage was rapid in drought-tolerant genotypes and that they showed reduced gs and Trmore rapidly than the susceptible genotype.In the preliminary trial with eight soybean genotypes under four weeks of withholding water supply,gsof genotypes C12 and W05 responded more rapidly in response to water limit condition than that of C08(Fig.1).Those genotypes were used in this study under progressive water restriction followed by rewetting and exhibited similar tendencies with respect to gs.It was clear in the present study that the decrease of Trduring progressive water restriction was due mainly to decreased gs.A similar response was observed in studies of Liu and Stutzel[49]and Liu et al.[38].Vassileva et al.[50] reported that drought-tolerant wheat genotype leaves displayed lower gsand higher Pnduring dehydration.It is noteworthy that all of these gas-exchange traits of tolerant genotypes recovered quickly to their original levels after rehydration.Rewetting of dry soil led to full recovery of both gsand Pnin Acacia confusa[24].
Pnof the drought-tolerant genotypes remained larger than that of the susceptible genotype during the entire period of water restriction,leading to increased instantaneous water use efficiency (WUEi) of tolerant genotypes.However,the WUEi of the susceptible genotype remained even lower than that of well-watered plants(Fig.8).Liu et al.[38]reported higher WUEi in soybean during soil drying.Water stress induced substantial stomatal closure,resulting in greater reduction in Trthan Pnand in higher WUEi[51].
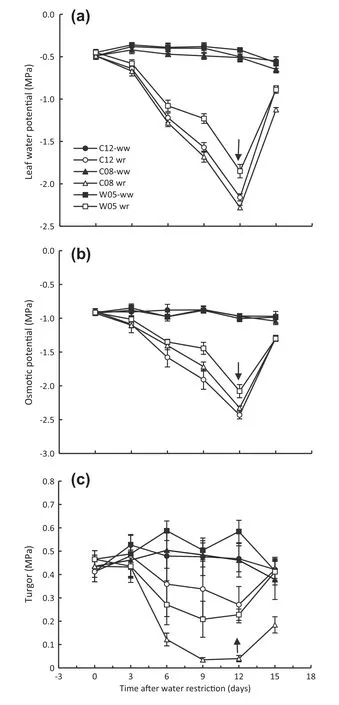
Fig.11-Leaf water potential(a),osmotic potential(b),and turgor pressure(c)of drought-tolerant(C12 and W05)and drought-susceptible (C08)soybean genotypes after imposition of water restriction.Water restriction began on day 0,and rewetting on day 12 is indicated by the arrow.ww and wr indicate well-watered and water-restricted status of soybean genotypes,respectively.Values of points are mean ± SD(n = 5).
The earlier closer of stomata restricted water loss from the plant and helped plants to maintain higher % RWC.Fig.10 illustrates that drought-tolerant genotypes maintained higher values of%RWC until day 12 of the stress cycle and recovered faster after rewetting to the control level than did the susceptible genotype.The preliminary study of %RWC of leaves of eight soybean genotypes after four weeks of withholding water also showed that drought stress significantly reduced leaf %RWC for all genotypes,but that the rate of reduction was lower for tolerant genotypes (C12 and W05)than for the drought-susceptible genotype (C08) (Fig.2).The lower gsand higher %RWC assisted the tolerant genotypes to maintain higher leaf water potential until day 12 of the dehydration period and achieve a rapid increase after rewetting (Fig.11-a).The rapid recovery of ψwafter rewetting accompanied by increased gshighlights the role of leaf water status in stomatal control[52].
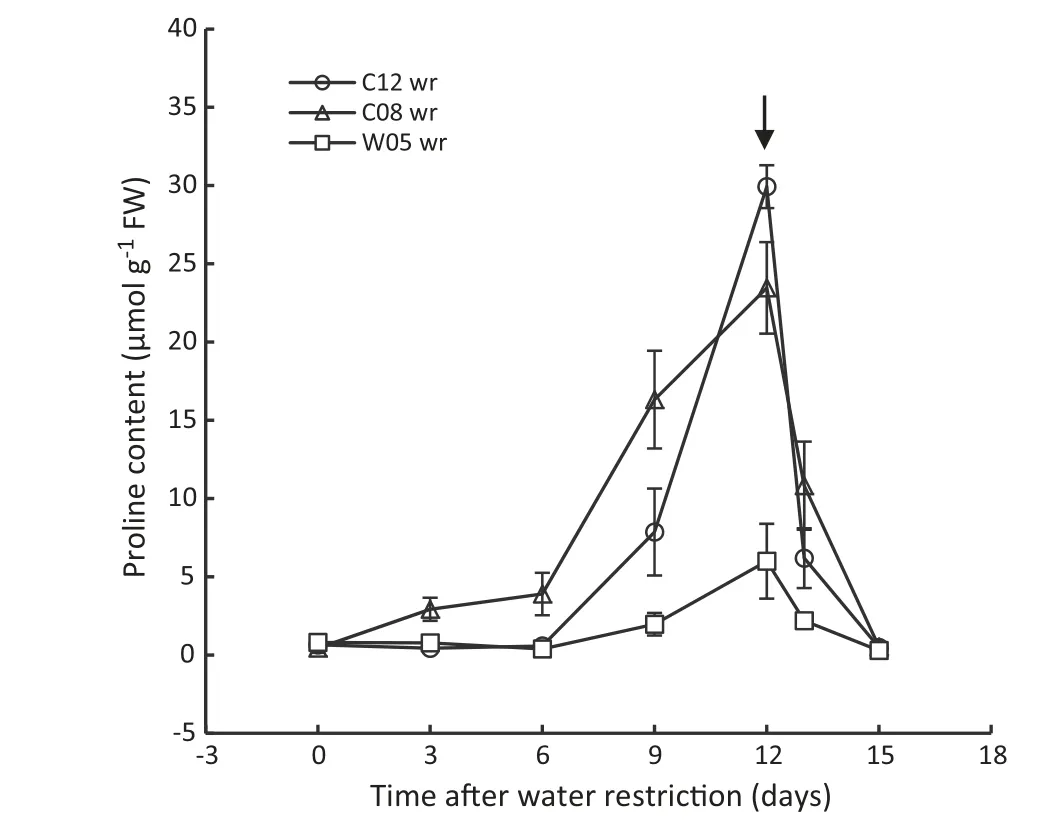
Fig.12-Leaf proline content of drought-tolerant(C12 and W05)and drought-susceptible(C08)soybean genotypes after the imposition of water restriction.Water restriction began on day 0,and rewetting on day 12 is indicated by the arrow.wr indicates water-restricted status of soybean genotypes.Values of points are mean ± SD(n = 4).
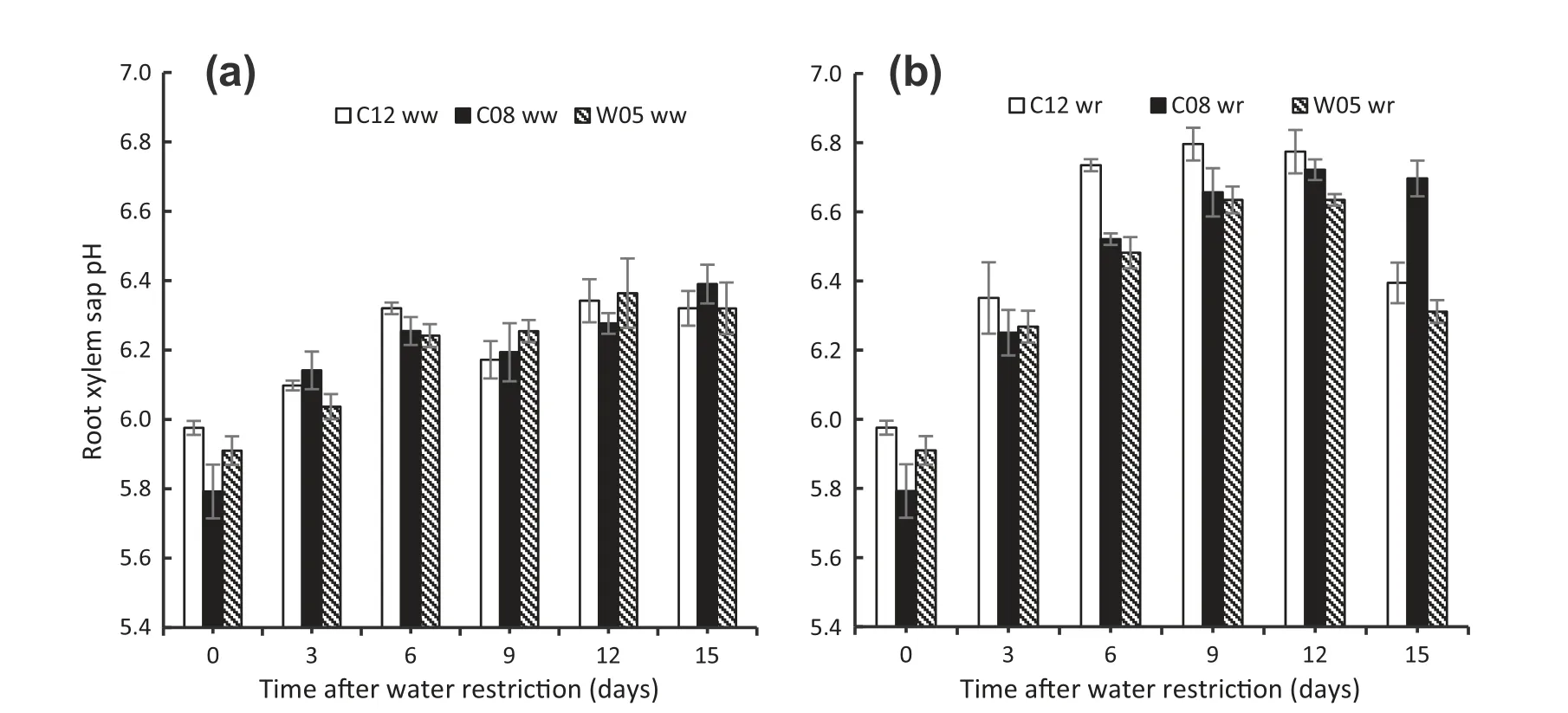
Fig.13-Root xylem sap pH of drought-tolerant(C12 and W05)and drought-susceptible (C08)soybean genotypes during well-watered (a) and water-restricted (b)conditions.Water restriction began on day 0 and rewetting occurred on day 12.ww and wr indicate well-watered and water-restricted status of soybean genotypes,respectively.Vertical bars indicate mean ± SD(n = 4).
A variety of physiological mechanisms may allow a species to tolerate prolonged periods of water shortage.One is the accumulation of osmotically active solutes into cells,maintaining cell turgor and plant growth during soil drying.In genotype C12,the decline in leaf osmotic potential under prolonged dehydration period may be due to active accumulation of solute and the maintenance of comparatively high leaf turgor pressure.In contrast,C08 could maintain osmotic potential only as low as-2.32 MPa,and its turgor potential declined significantly(Fig.11-b,c).The difference in osmoregulation may be associated with the root growth of genotype C12.Root length was higher in the tolerant genotypes than in the susceptible genotype (Table 1 and Fig.14).
In all plants,roots play an important role in survival during drought stress.Growing roots adapt to water deficit via a combination of osmotic and cell wall changes[53].Water deficit may induce many other changes in plant cells,including changes in cell wall polysaccharide composition and gene expression[54–56].
Given that the tolerant genotypes maintained higher water status in leaves during water restriction,the leaf expansion of those genotypes was inferred to be greater than that of the susceptible genotype.It was observed that the rate of leaf area expansion of all genotypes declined markedly owing to water restriction,in comparison with well-watered plants,but that the tolerant genotypes maintained a greater leaf area expansion rate than the susceptible genotype (Fig.6).This result indicated that tolerant genotypes could take up as well as retain more water than the susceptible genotype and thus maintain better growth during water restriction.Guo et al.[57]reported that drought-tolerant poplar clones showed better growth under water stress.Other researchers reported that drought stress successively induced cessation of leaf and shoot growth [58].Zhang and Davies [43] postulated that leaf expansion and stomatal behavior respond directly to soil drying before the occurrence of any detectable shoot water deficit.

Table 1-Shoot length,internode number,root number,root length,root fresh mass,and root dry mass of three soybean genotypes under well-watered(ww)and water-restricted(wr)conditions at 12 days after imposition of water restriction.
Other studies have shown that plants reduced their Chl contents under water stress [59–61].But our study showed that the Chl content of the drought-susceptible genotype was markedly reduced as water restriction progressed,whereas the Chl content of drought-tolerant genotypes remained almost unchanged (Fig.9).It may accordingly be argued that the presence of higher Chl content in leaves of the droughttolerant genotypes led to higher photosynthetic rates during water restriction than those in the susceptible genotype.Similar findings were reported by Guo et al.[57],who found that drought-tolerant poplar clones maintained higher Chl during water stress.Li et al.[62]suggested that the decrease in Chl of a drought-tolerant genotype was much lower than that of a drought-susceptible genotype.

Fig.14-Photograph showing root length of drought-tolerant(C12 and W05)and drought-susceptible(C08)soybean genotypes under well-watered and water-restricted conditions on day 12 of a drought cycle.Bars indicate 10 cm.
The tendency of free proline accumulation in plants under any abiotic stress condition is an indicator of a mechanism of avoidance of those stresses.Proline is a reliable indicator of environmental stress imposed on plants [63].In this experiment we found that progressive water restriction increased the concentration of proline in leaves of soybean genotypes.A rapid accumulation of proline was observed in the droughtsusceptible genotype (C08) after withholding of water(Fig.12).This phenomenon suggested that C08 suffers from water shortage earlier than do tolerant genotypes.Interestingly,the leaf proline levels of both tolerant genotypes were similar to the well-watered level until day 6 of the stress cycle,after which the proline content of C12 markedly increased and reached a peak on day 12 (Fig.12).The lower proline content after water restriction suggested that the wild-type drought-tolerant W05 retained more water in the leaves.Some authors have reported higher proline content in drought-tolerant crop species such as bean [64] and poplar[57].
As dehydration progressed,root-originating signals such as abscisic acid (ABA) and pH are transported to leaves through xylem,eventually reducing water loss via stomatal closure [18].We found that the pH of root xylem sap of all three genotypes increased as water restriction progressed(Fig.13-b).The drought-tolerant C12 genotype exhibited a sharper increase in xylem sap pH and approached the control level after rewetting.This result can be explained by the importance of promoting the closure of opened stomata and also inhibiting the opening of closed stomata[14,65].
Root number at a certain depth of soil profile (20 cm and below) was not affected by drought stress in our study(Table 1).It might be expected that genotypic differences in drought tolerance were not associated with the number of roots at 20 cm depth.The much deeper root system of drought-tolerant coffee clones enabled them to gain better access to water towards the bottom of pots and thereafter maintain a more favorable internal water status than drought-susceptible clones[12].In our results,root length of tolerant genotypes was not reduced significantly with water restriction,whereas the susceptible genotype showed markedly reduced root length compared to well-watered plants(Table 1 and Fig.14).In the preliminary trial investigating the root length of different soybean genotypes under four weeks of water-limiting conditions,it was found that root lengths of drought-tolerant genotypes were not influenced by water shortage but were reduced in drought-susceptible genotypes(Fig.3).Based on the drought responses of leaf gas exchange,water relations,and root physiological traits of soybean genotypes to progressive water restriction,it can be concluded that drought-tolerant genotypes cope with water stress better than drought-susceptible genotype by regulating stomatal openings in response to chemical signals from roots,maintaining higher %RWC in leaves,and maintaining Pnprobably by increasing their root length.
We thank Fuk-Ling Wong,Dr.Weifeng Xu,Dr.Ken Lau,and Helen Tsai for technical support.This study was supported by the Hong Kong RGC Collaborative Research Fund(CUHK3/CRF/11G)to Prof.H.-M.Lam.and J.H.Zhang.
[1] P.Rampino,S.Pataleo,C.Gerardi,G.Mita,C.Perrotta,Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes,Plant Cell Environ.29(2006) 2143–2152.
[2] J.Zhang,W.Jia,J.Yang,A.M.Ismail,Role of ABA in integrating plant responses to drought and salt stresses,Field Crop Res.97 (2006) 111–119.
[3] F.Z.Wang,Q.B.Wang,S.Y.Kwon,S.S.Kwak,W.A.Su,Enhance drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase,J.Plant Physiol.162 (2005) 465–472.
[4] N.H.Samarah,R.E.Mullen,S.R.Cianzio,P.Scott,Dehydrin-like proteins in soybean seeds in response to drought stress during seed filling,Crop Sci.46(2006)2141–2150.
[5] P.Monneveux,C.Sanchez,D.Beck,G.O.Edmeades,Drought tolerance improvement in tropical maize source populations:evidence of progress,Crop Sci.46(2006) 180–191.
[6] N.H.Samarah,Effect of drought stress on growth and yield of barley,Agron.Sustain.Dev.25(2005) 145–149.
[7] H.R.Lafitte,G.Yongsheng,S.Yan,Z.K.Li,Whole plant responses,key processes,and adaptation to drought stress:the case of rice,J.Exp.Bot.58(2007) 169–175.
[8] J.P.Martinez,H.Silva,J.F.Ledent,M.Pinto,Effect of drought stress on the osmotic adjustment,cell wall elasticity and cell volume of six cultivars of common beans(Phaseolus vulgaris L.),Eur.J.Agron.26(2007) 30–38.
[9] J.Kawakami,K.Iwama,Y.Jitsuyama,Soil water stress and the growth and yield of potato plants grown from microtubers and conventional seed tubers,Field Crop Res.95(2006) 89–96.
[10] J.C.Cushman,H.J.Bohnert,Genomic approaches to plant stress tolerance,Plant Biol.3(2000) 117–124.
[11] M.Mattana,E.Biazzi,R.Consonni,F.Locatelli,C.Vannini,S.Provera,I.Coraggio,Overexpression of Osmyb4 enhances compatible solute accumulation and increases stress tolerance of Arabidopsis thaliana,Physiol.Plant.125 (2005)212–223.
[12] H.A.Pinheiro,F.M.Damatta,A.R.M.Chaves,M.E.Loureiro,C.Ducatti,Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora,Ann.Bot.96(2005) 101–108.
[13] P.J.Kramer,J.S.Boyer,Water Relations of Plant and Soils,Academic Press,San Diego,1995.1–308.
[14] D.J.Davies,W.J.J.Zhang,Root signals and the regulation of growth and development of plants in drying soil,Annu.Rev.Plant Physiol.Plant Mol.Biol.42(1991) 55–76.
[15] M.B.Jackson,Are plant hormones involved in root-to-shoot communication? Adv.Bot.Res.19(1993)103–187.
[16] W.J.Davies,F.Tardieu,C.L.Trejo,How do chemical signals work in plants that grow in drying soil? Plant Physiol.104(1994) 309–314.
[17] D.S.Thompson,S.Wilkinson,M.A.Bacon,W.J.Davies,Multiple signals and mechanisms that regulate leaf growth and stomatal behavior during water deficit,Physiol.Plant.100 (1997) 303–313.
[18] D.P.Schachtman,J.Q.D.Goodger,Chemical root to shoot signaling under drought,Trends Plant Sci.13 (2008) 281–287.
[19] T.Gollan,U.Schurr,E.D.Schulze,Stomatal response to drying soil in relation to changes in the xylem sap concentration of Helianthus annuus: I.The concentration of cations,anions,amino acids in,and pH of,the xylem sap,Plant Cell Environ.15(1992) 551–559.
[20] U.Schurr,T.Gollan,E.D.Schulze,Stomatal response to soil drying in relation to changes in the xylem sap composition of Helianthus annuus: II.Stomatal sensitivity to abscisic acid imported from the xylem sap,Plant Cell Environ.15(1992)561–567.
[21] A.G.Netting,pH,abscisic acid and integration of metabolism in plants under stressed and non-stressed conditions:cellular responses to stress and their implication for plant water relations,J.Exp.Bot.51(2000) 147–158.
[22] W.Jia,W.J.Davies,Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals,Plant Physiol.143 (2007) 68–77.
[23] S.Wilkinson,M.A.Z.Bacon,W.J.Davies,Nitrate signaling to stomata and growing leaves: interactions with soil drying,ABA,and xylem sap pH in maize,J.Exp.Bot.58(2007)1705–1716.
[24] J.Liang,J.Zhang,M.H.Wong,Can stomatal closure caused by xylem ABA explain the inhibition of leaf photosynthesis under soil drying? Photosynth.Res.51(1997) 149–159.
[25] A.Bahrun,C.R.Jensen,F.Asch,V.O.Mogensen,Drought-induced changes in xylem pH,ionic composition,and ABA concentration act as early signals in field grown maize (Zea mays L.),J.Exp.Bot.53(2002) 251–263.
[26] I.F.Ike,Effect of water deficits on transpiration,photosynthesis and leaf conductance in cassava,Physiol.Plant.55(1982) (1982) 411–414.
[27] J.Galmes,A.Abadia,H.Medrano,J.Flexas,Photosynthesis and photoprotection responses to water stress in the wild-extinct plant Lysimachia minoricensis,Environ.Exp.Bot.60 (2007) 308–317.
[28] H.Sircelj,M.Tausz,D.Grill,F.Batic,Detecting different levels of drought stress in apple trees (Malus domestica Borkh.) with selected biochemical and physiological parameters,Sci.Hortic.113 (2007) 362–369.
[29] S.V.Caemmeter,S.Farquhar,Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves,Planta 153 (1981) 376–387.
[30] F.Ain-Lhout,M.Zunzunegui,M.C.D.Barradas,R.Tirado,A.Clavijo,F.G.Novo,Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit,Plant Soil 230 (2001) 175–183.
[31] P.D.Hare,W.A.Cress,J.Van Staden,Dissecting the roles of osmolyte accumulation during stress,Plant Cell Environ.21(1998) 535–553.
[32] A.R.Reddy,K.V.Chaitanya,M.Vivekanandan,Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants,J.Plant Physiol.161(2004) 1189–1202.
[33] S.Silvente,P.S.Anatoly,M.Lara,Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress,PLoS One (2012),http://dx.doi.org/10.1371/journal.pone.0038554.
[34] E.Dogan,H.Kirnak,O.Copur,Deficit irrigations during soybean reproductive stages and CROPGRO-soybean simulations under semi-arid climatic condition,Field Crop Res.103 (2007) 154–159.
[35] F.L.Liu,C.R.Jensen,M.N.Andersen,Pot set related to photosynthetic rate and endogenous ABA in soybean subject to different water regimes and exogenous ABA and BA at early reproductive stages,Ann.Bot.94(2004) 405–411.
[36] M.G.Huck,K.Ishihara,C.M.Peterson,T.Ushijima,Soybean adaptation to water stress at selected stages of growth,Plant Physiol.73(1983) 422–427.
[37] D.J.Read,E.M.Bartlett,The physiology of drought resistance in soybean plant (Glycine max):I.The relationship between drought resistance and growth,J.Appl.Ecol.9(1972)487–489.
[38] F.Liu,M.N.Andersen,S.E.Jacobsen,C.R.Jensen,Stomatal control and water use efficiency of soybean (Glycine max L.Merr.) during progressive soil drying,Environ.Exp.Bot.54(2005) 33–40.
[39] J.N.Egilla,F.T.Davies,T.W.Boutton,Drought stress influences leaf water content,photosynthesis,and water-use efficiency of Hibiscus rosa-sinensis at three potassium concentrations,Phytosynthetica 43(2005) 135–140.
[40] M.Okamoto,F.C.Peterson,A.Defries,S.Y.Park,E.Nambara,B.F.Volkman,S.R.Cutler,Activation of dimeric ABA receptors elicits guard cell closure,ABA-regulated gene expression,and drought tolerance,Proc.Natl.Acad.Sci.U.S.A.110 (2013)12132–12137.
[41] L.S.Bates,R.P.Waldren,I.D.Teare,Rapid determination of free proline for water-stress studies,Plant Soil 39(1973)205–207.
[42] R.Moran,Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide,Plant Physiol.69(1982) 1376–1381.
[43] J.Zhang,W.J.Davies,Sequential response of whole plant water relations to prolonged soil drying and the involvement of xylem sap ABA in the regulation of stomatal behavior of sunflower plants,New Phytol.113 (1989) 167–174.
[44] R.Chang,L.Qiu,Evaluation and utilization of soybean germplasm in China,in: H.M.Lam,R.Chang,G.Shao,Z.Liu(Eds.),Research on Tolerance to Stresses in Chinese Soybean,China Agricultural Press,Beijing,2009.
[45] Y.S.Ku,K.W.Au-Yeung,Y.L.Yung,M.W.Li,C.Q.Wen,X.Liu,H.M.Lam,Drought stress and tolerance in soybean,in: J.E.Board (Ed.),A Comprehensive Survey of International Soybean Research-Genetics,Physiology,Agronomy and Nitrogen Relationships,In Tech Education and Publishing,Austria,2013,pp.209–237.
[46] Z.Ristic,M.A.Jenks,Leaf cuticle and water loss in maize lines differing in dehydration avoidance,J.Plant Physiol.159(2002)645–651.
[47] J.Flexas,J.Bota,F.Loreto,G.Comic,T.D.Sharkey,Diffusive and metabolic limitation of photosynthesis under drought and salinity in C3plants,Plant Biol.6 (2004)269–279.
[48] T.T.Kozlowski,S.G.Pallardy,Acclimation and adaptive responses of woody plants to environmental stresses,Bot.Rev.68(2002) 270–334.
[49] F.Liu,H.Stutzel,Leaf expansion,stomatal conductance,and transpiration of vegetable amaranth (Amaranthus sp.) in response to soil drying,J.Am.Soc.Hortic.Sci.127 (2002)878–883.
[50] V.Vassileva,C.Signarbieux,I.Anders,U.Feller,Genotypic variation in drought stress response and subsequent recovery of wheat(Triticum aestivum L.),J.Plant Res.124(2011)147–154.
[51] A.Pou,J.Flexas,M.Alsina Mdel,J.Bota,C.Carambula,F.de Herralde,J.Galmes,C.Lovisolo,M.Jimenez,M.Ribas-Carbo,D.Rusjan,F.Secchi,M.Tomas,Z.Zsofi,H.Medrano,Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110(V.berlandieri × V.rupestris),Physiol.Plant.134 (2008) 313–323.
[52] E.E.Fuchs,N.J.Livingston,Hydraulic control of stomatal conductance in Douglas fir [Pseudotsuga menziesii(Mirb.)Franco] and alder[Alnus rubra (Bong)]seedlings,Plant Cell Environ.19(1996) 1091–1098.
[53] Y.Wu,D.J.Cosgrove,Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins,J.Exp.Bot.51(2000) 1543–1553.
[54] N.M.Iraki,R.A.Bressan,P.M.Hasegawa,N.C.Carpita,Alteration of physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress,Plant Physiol.91(1989) 39–47.
[55] H.Zhong,A.Lauchi,Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity,J.Exp.Bot.44(1993) 773–778.
[56] R.A.Creelman,J.E.Mullet,Water deficit modulates gene expression in growing zones of soybean seedlings.Analysis of differentially expressed cDNAs,a new β-tubulin gene,and expression of genes encoding cell wall proteins,Plant Mol.Biol.17(1991) 591–608.
[57] X.Y.Guo,X.S.Zhang,Z.Y.Huang,Drought tolerance in three hybrid poplar clones submitted to different watering regimes,J.Plant Ecol.3(2010) 79–87.
[58] M.B.Bogeat-Triboulot,M.Brosche,J.Renaut,L.Jouve,D.L.Thiec,P.Fayyaz,B.Vinocur,E.Witters,K.Laukens,T.Teichmann,A.Altman,J.F.Hausman,A.Polle,J.Kangasjärvi,E.Dreyer,Gradual soil water depletion results in reversible changes of gene expression,protein profiles,ecophysiology,and growth performance in Populus euphratica,a poplar growing in arid regions,Plant Physiol.143(2007) 876–892.
[59] Y.Y.Cui,D.M.Pandey,E.J.Hahn,K.Y.Paek,Effect of drought on physiological aspects of Crassulacean acid metabolism in Doritaenopsis,Plant Sci.167 (2004) 1219–1226.
[60] Y.Lei,C.Yin,C.Li,Differences in some morphological,physiological,and biochemical responses to drought stress in two contrasting populations of Populus przewalskii,Physiol.Plant.127 (2006) 182–191.
[61] M.Pagter,C.Bragato,H.Brix,Tolerance and physiological responses of Phragmites australis to water deficit,Aquat.Bot.81 (2005) 285–299.
[62] R.H.Li,P.G.Guo,M.Baum,S.Grando,S.Ceccarelli,Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley,Agric.Sci.China 5(2006) 751–757.
[63] W.Claussen,Proline as a measure of stress in tomato plants,Plant Sci.168 (2005) 241–248.
[64] I.Turkan,M.Bor,F.Ozdemir,H.Koca,Differential responses of lipid peroxidation and antioxidants in the leaves of drought tolerant P.acutifolius Gray and drought-sensitive P.vulgaris L.subjected to polyethylene glycol mediated water stress,Plant Sci.168 (2005) 223–231.
[65] J.Zhang,W.Jia,D.Zhang,Re-export and metabolism of xylem-delivered ABA in attached maize leaves under different transpirational fluxes and xylem ABA concentrations,J.Exp.Bot.48 (1997) 1557–1564.
- The Crop Journal的其它文章
- Maize forage aptitude: Combining ability of inbred lines and stability of hybrids
- Molecular detection of Xanthomonas oryzae pv.oryzae,Xanthomonas oryzae pv.oryzicola,and Burkholderia glumae in infected rice seeds and leaves
- Growth,photosynthesis and nitrogen metabolism in soybean varieties after exclusion of the UV-B and UV-A/B components of solar radiation
- Mapping and validation of a dominant salt tolerance gene in the cultivated soybean(Glycine max) variety Tiefeng 8
- Genetic background effects on QTL and QTL × environment interaction for yield and its component traits as revealed by reciprocal introgression lines in rice
- Brief Guide for Authors

