P53蛋白过表达可预测雌激素受体阳性,早期绝经后乳腺癌对芳香化酶抑制剂治疗的敏感性
贾晓青 洪琪 程竞仪 李剑伟 王玉洁 莫淼 邵志敏
沈镇宙1 柳光宇1
1.复旦大学附属肿瘤医院乳腺外科,复旦大学上海医学院肿瘤学系,上海 200032;2.复旦大学附属肿瘤医院核医学科,复旦大学上海医学院肿瘤学系,上海 200032;3.复旦大学附属肿瘤医院临床统计中心,复旦大学上海医学院肿瘤学系,上海 200032
P53蛋白过表达可预测雌激素受体阳性,早期绝经后乳腺癌对芳香化酶抑制剂治疗的敏感性
贾晓青1 洪琪1 程竞仪2 李剑伟1 王玉洁1 莫淼3 邵志敏1
沈镇宙1 柳光宇1
1.复旦大学附属肿瘤医院乳腺外科,复旦大学上海医学院肿瘤学系,上海 200032;2.复旦大学附属肿瘤医院核医学科,复旦大学上海医学院肿瘤学系,上海 200032;3.复旦大学附属肿瘤医院临床统计中心,复旦大学上海医学院肿瘤学系,上海 200032
背景与目的:抑癌基因P53在肿瘤中的研究一直是热点,包括乳腺癌。对于P53基因和乳腺癌预后的研究很多,多数研究表明P53蛋白表达是乳腺癌的不良预后因素之一。而目前对于P53蛋白表达在雌激素受体(estrogen receptor,ER)阳性,早期绝经后乳腺癌对芳香化酶抑制剂(aromotase inhibitors,AI)的敏感性的作用尚无明确结论。本研究旨在探讨P53蛋白表达在ER阳性,早期绝经后乳腺癌对AI治疗敏感性的预测作用。方法:收集2000年1月—2006年12月间复旦大学附属肿瘤医院乳腺外科住院的患者资料共293例。所有患者均接受AI治疗。中位随访时间为72个月(6~140个月)。分析不同P53表达状态对无病生存率(disease free survival,DFS)的影响。应用统计学方法进行生存分析及Cox回归模型进行单因素和多因素分析。结果:P53阳性和P53阴性患者的5年生存率分别为78%和89%。多因素回归分析显示P53表达状态(HR=1.729,95%CI:1.038~2.880,P=0.035)、病理分期(HR=2.270,95%CI:1.399~3.681,P=0.001)、组织学分级(HR=2.328,95%CI:1.312~4.133,P=0.004)及年龄(HR=1.988,95%CI:1.511~2.617,P<0.005)是接受AI治疗的早期乳腺癌复发转移的独立危险因素。结论:P53蛋白表达可预测ER阳性,早期绝经后乳腺癌对AI治疗的敏感性;其可能成为决定绝经后激素受体阳性乳腺癌患者治疗策略的关键因素。
P53;乳腺癌;预后;内分泌耐药
芳香化酶抑制剂(aromotase inhibitors,AI)是绝经后雌激素受体(estrogen receptor,ER)阳性乳腺癌患者的标准治疗,ATAC临床试验结果表明对绝经后患者的治疗效果,AI优于他莫昔芬[1]。然而在初始使用AI治疗有效后,仍有一部分患者不可避免地出现耐药,从而导致预后变差。原发或继发(获得性)耐药的机制包括ER共调节蛋白,ER通路与其他生长因子信号通路之间存在交叉[2-3]。由于雌激素信号通路网络活性调节的分子机制的认识不断加深,临床急需克服内分泌耐药的新方法。
最近许多研究发现人类表皮生长因子受体-2(human epidermal growth factor receptor-2,HER-2)表达与总生存(overall survival,OS)呈显著正相关,且其扩增可能导致他莫昔芬耐药[4-6]。确定更为准确、简便、重复性好的预后标志物如编码P53基因的P53蛋白,将对乳腺癌的治疗决策起非常重要的作用。近1/3的乳腺癌含有P53基因突变,导致高组织学分级和迅速进展[7]。Yamashita等[8]分析了506例浸润性导管癌患者组织的HER-2、P53及Ki-67表达情况,发现HER-2与P53同时表达与乳腺癌预后显著相关。
研究表明P53改变与蒽环类、环磷酰胺、甲氨蝶呤及氟尿嘧啶等化疗药物耐药相关[9-15]。然而,鲜有研究证实P53表达状态与内分泌治疗的相关性[16-20]。Kai等[21]发现P53过表达可以显著预测激素敏感性复发转移乳腺癌对第3代AI的反应性。对于P53表达在早期激素敏感乳腺癌中的作用鲜有报道。本研究旨在了解P53蛋白表达在早期绝经后激素受体阳性乳腺癌对AI治疗敏感性的预测作用。
1 资料和方法
1.1 研究对象
选择2000年1月—2006年12月间,在复旦大学附属肿瘤医院乳腺外科接受手术治疗的绝经后激素受体阳性乳腺癌患者为主要研究对象,共293例。所有患者均接受5年的AI治疗。
1.2 随访
手术治疗时间为随访起始时间,采用电子病历(住院病历、门诊复查病历)跟踪,结合电话方式[随访截止日期为2006年12月,中位随访时间为72个月(6~140个月)],有具体完整的临床及病理资料,包括年龄、是否糖尿病、是否高血压、肿瘤大小、分期、淋巴结状态、病理分级、ER及孕激素受体(progesterone receptor,PR)、HER-2蛋白表达、P53蛋白表达、化疗与否、放疗与否等。
1.3 免疫组化检测P53蛋白表达
石蜡切片经常规脱蜡至水,用3%甲醇过氧化氢溶液封闭内源性过氧化氢酶,0.01 mol/L柠
檬酸溶液(pH=6.0)抗原修复,煮沸10 min,保温10 min,取出后置于柠檬酸缓冲液中在室温下自然冷却。封闭20 min。依次加入鼠抗人P53单克隆抗体(Clone DO-7)(丹麦Dako公司),工作液稀释比例为1∶100和即用型辣根过氧化物酶标记的二抗,DAB显色,苏木精对比染色细胞核,常规脱水,透明,中性树胶封固。PBS取代一抗作为阴性对照,其余步骤同上。用已知阳性切片作为各蛋白的阳性对照。随机选取5个高倍视野(×400),计数肿瘤细胞和阳性细胞数,得出阳性细胞百分率。阳性细胞率≤25%记为0分,>25%~50%为1分,>50%~75%为2分,>75%为3分。再按多数阳性细胞呈现的染色强度予以计分,无显色为0分,浅棕黄色为1分,棕黄色为2分,棕褐色为3分。将上述2项得分相加,0分判为“-”,1~2分判为“+”,3~4分判为“++”,5~6分判为“+++”。由3位有经验的临床病理医师阅片,采用双盲评估的方法,根据其评分的平均值来确定判定结果。本研究进行结果分析时,以“+”,“++”和“+++”定义该蛋白为阳性表达,“-”定义为阴性表达。
1.4 复发转移的诊断
①局部区域复发:乳腺癌的局部复发包括术区的胸壁复发和同侧区域淋巴结的复发,以临床和组织学认定,对保乳患者包括同侧乳房的复发。②远处转移:常见转移部位分别是骨、肺、胸膜、软组织和肝,以临床生化、影像学检查及病理诊断(对可触及的转移灶)认定(75%的乳腺癌转移发生在原发性乳腺癌诊断后的5年之内)。
1.5 统计学处理
应用SPSS 12.0统计学软件,组间差异采用χ2检验,Kaplan-Meier法进行生存分析,Logrank法进行显著性检验,Cox比例风险模型用于单因素和多因素分析,P<0.05为差异有统计学意义。
2 结 果
2.1 患者一般情况与基线特征
本研究回顾性分析了293例绝经后激素受体阳性乳腺癌患者。表1归纳了这293例患者的人口学及临床病理特征见表1。
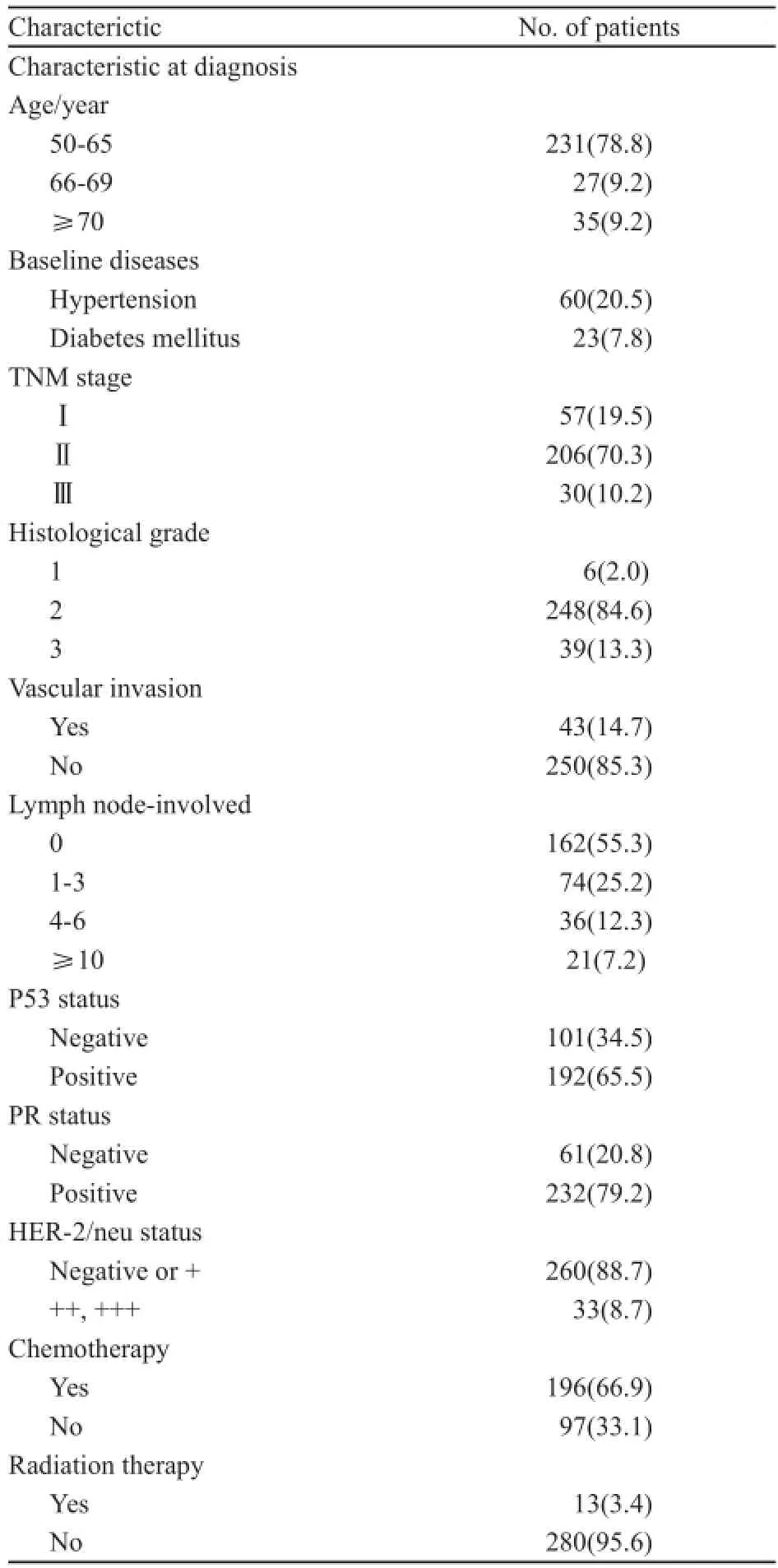
表1 293例ER阳性早期绝经后乳腺癌患者的一般情况和临床病理特征。Tab. 1 Patient characteristics at diagnosis in 293 ER-positive early stage postmenopausal breast cancer patients [n(%)]
2.2 患者的随访结果和预后分析
在293例患者中,有191(65.4%)例患者免疫组化有P53蛋白表达。P53免疫组化阳性结果见图1。两组的无进展生存期(progress free survival,PFS)差异有统计学意义(P=0.035)。Kaplan-Meier曲线分析发现P53阳性组和阴性组的无病生存率(disease free survival,DFS)差异有统计学意义(P<0.05,图2)。所有患者均接受5年AI治疗,具体用药种类见表2。
2.3 与预后相关的单因素分析结果
使用Cox比例风险模型来进行单因素分析。发现高龄、伴有糖尿病、高组织学分级、高病理分期、淋巴结转移、P53表达与复发转移呈正相关,术后放疗与复发转移呈负相关(表3)。
2.4 与预后相关的多因素分析结果
为排除多因素间的相互干扰,将表3中临床病理因素放入Cox回归模型做多因素分析,结果显示P53表达状态、组织学分级、临床分期以及年龄是乳腺癌预后的独立危险因素(表4)。
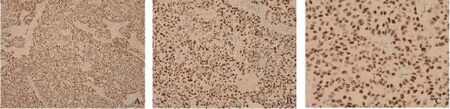
图1 免疫组化检测结果显示P53染色阳性Fig. 1 Immunohistochemistry positive results for P53
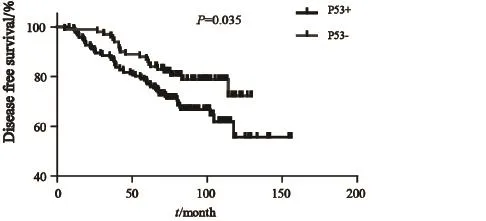
图2 P53阳性组与阴性组的无复发生存曲线Fig. 2 Kaplan-Meier estimated the effect of P53 overexpression on DFS for 293 postmenopausal ER-positive breast cancer patients
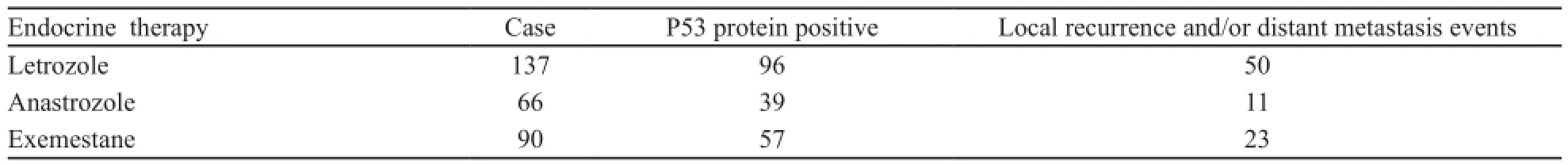
表2 293例ER阳性早期绝经后乳腺癌患者AI治疗及耐药情况Tab. 2 Use of AI and resistance conditions in 293 ER-positive early stage postmenopausal breast cancer patients(n)
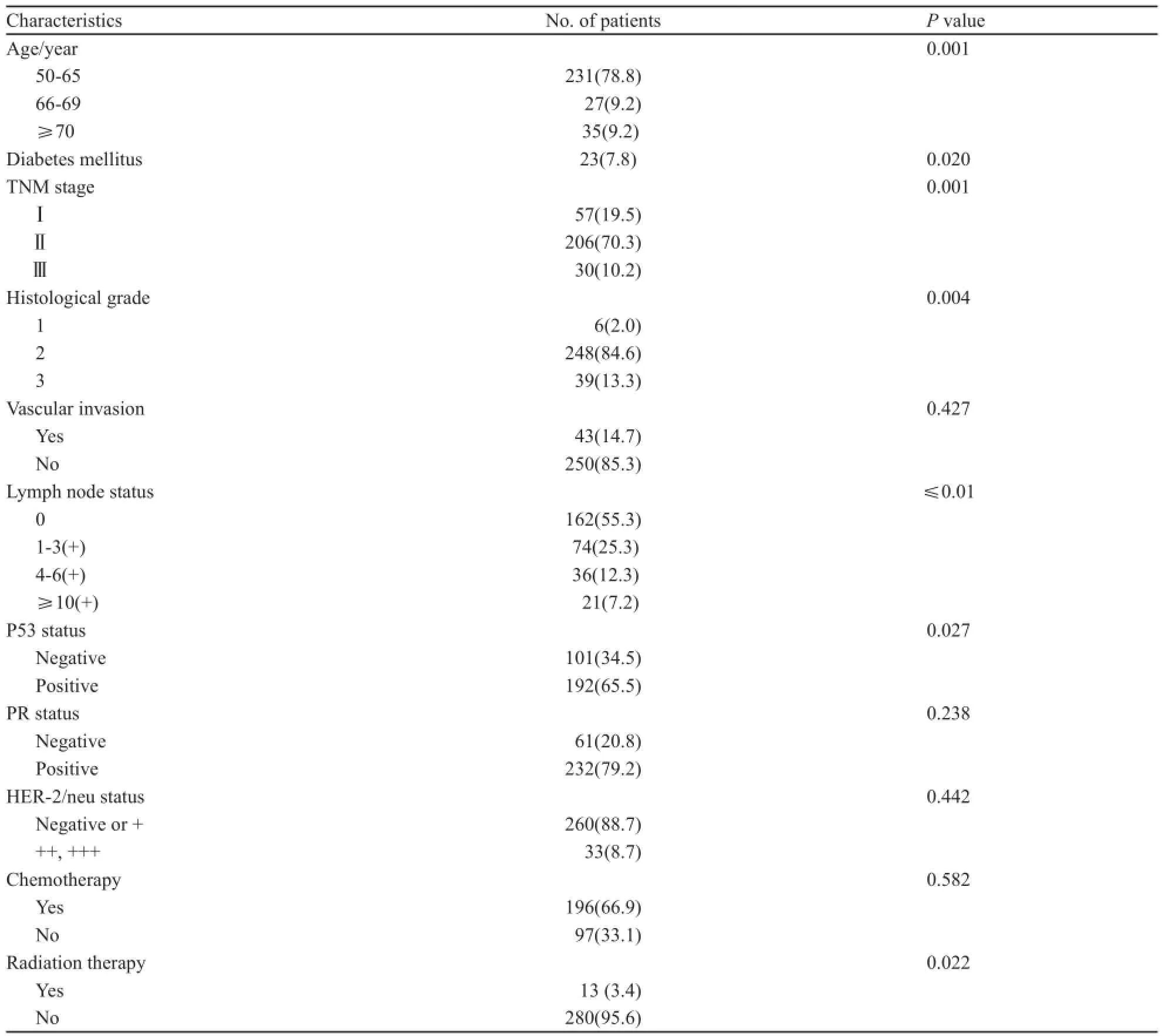
表3 293例患者基线特征与无病生存之间的关系Tab. 3 Summary of the association between the patients’ baseline characteristics with disease free survival for all patients[n(%)]
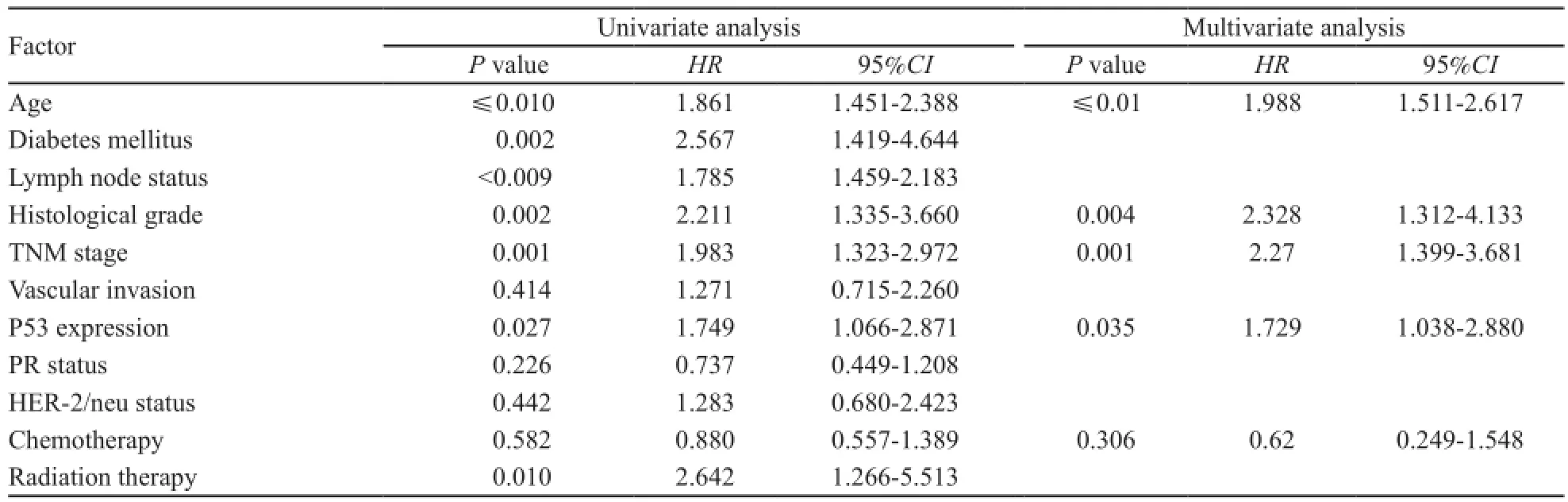
表4 单因素和多因素生存分析的参数和结果Tab. 4 Summary of the association between patients baseline characteristics with diseases free survival: Cox univariate and multivariate regression analysis
3 讨 论
本研究从回顾性分析的角度,分析了P53表达与绝经后乳腺癌对AI治疗敏感性。AI治疗是绝经后ER阳性乳腺癌患者的标准治疗方式[1],然而仍有一部分患者在初始AI治疗后出现耐药。本次研究发现P53过表达是接受AI治疗的ER阳性早期绝经后乳腺癌的独立预后因素,由此我们推测P53可能预测ER阳性早期绝经后患者对AI治疗的敏感性。
P53基因编码一种核磷酸化蛋白,此蛋白在正常人类细胞中表达量较低,可调节细胞生长和分化。P53蛋白作为肿瘤潜在的分子标志物,很早就得到了认可。在2005年的ASCO年会上,Kai等[21]报道了P53蛋白作为预测激素敏感性复发转移性乳腺癌对第3代AI耐药的关键因子。全基因组分析发现P53突变与AI耐药呈显著正相关[22]。所有这些研究都表明P53基因及蛋白可能在AI治疗的敏感性中具有一定预测作用。
近1/3的乳腺癌出现P53基因突变,导致高组织学分级及快速进展[7]。有研究表明尽管P53表达预后较差,但P53与他莫昔芬的反应性无相关性[18]。相反,Berns等[20]将乳腺癌细胞的细胞质提取物做酶联免疫吸附实验,发现P53表达与他莫昔芬耐药相关。进一步研究每一种内分泌治疗药物与P53表达状态之间的关系非常必要,有助于了解P53在内分泌耐药中所扮演的角色。此外,由于评估P53状态的方法不同,可能会得出相矛盾的结果。Geisler等[11]研究结果表明,突变的P53有助于评估P53状态与内分泌耐药之间的关系。
需要指出的是,本研究存在一些局限性。本研究的P53表达状态是通过免疫组化的方法确定的,而免疫组化主观性较强,多少存在一些偏倚。如果将来P53用于乳腺癌患者的预后指标,那么最好各中心之间统一检测方法和标准。另外,此研究的样本量较小,可能会使结果的可信度下降。本研究所有患者均为ER阳性患者,故并未对ER状态进行分析。对于ER与 P53的研究较多,有研究报道,P53是ERα的一个靶点,ER可激活P53的转录从而调节P53的表达[23]。Choi等[24]发现P53失活可导致芳香化酶的表达增加,这可能作为本研究的理论基础。我们会继续深入此方面的研究,尤其侧重基础方面的研究。
基于本研究的结果,P53结合其他预后因素可能作为ER阳性绝经后早期乳腺癌对AI治疗反应性的预测指标,这需要进一步的前瞻性研究加以证实。P53也可能作为未来随机临床试验的重要分层因子。
[1]BUAM M, BUDZAR A U, CUZICK J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial [J]. Lancet, 2002, 359(9324): 2131-2139.
[2]JOHNSTON S R, HEAD J, PANCHOLI S, et al. Integration of signal transduction inhibitors with endocrine therapy: an approach to overcoming hormone resistance in breast cancer[J]. Clin Cancer Res, 2003, 9(Suppl 1): 524-532.
[3]SCHIFF R, MASSARWEH S, SHOU J, et al. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response [J]. Clin Cancer Res, 2003, 9 (Suppl 1): 447-454.
[4]ELLEDGE RM, GREEN S, CIOCCA D, et al. HER-2 expression and response to tamoxifen in estrogen receptorpositive breast cancer: a Southwest Oncology Group Study[J]. Clin Cancer Res, 1998, 4(1): 7-12.
[5]YAMAUCHI H, STEARNS V, HAYES D F. When is a tumor marker ready for prime time? A case study of c-erbB-2 as a predictive factor in breast cancer [J]. J Clin Oncol, 2001, 19(8): 2334-2356.
[6]STAL O, BORG A, FERNO M, et al. ErbB2 status and the benefit from two or five years of adjuvant tamoxifen in postmenopausal early stage breast cancer[J]. Ann Oncol, 2000, 11(12): 1545-1550.
[7]FITZGIBBONS P L, PAGE D L, WEAVER D, et al. Prognostic factors in breast cancer[J]. Arch Pathol Lab Med, 2000, 124(7): 966-978.
[8]YAMASHITA H, NISHIO M, TOYAMA T, et al. Coexistence of HER2 over-expression and p53 protein accumulation is a strong prognostic molecular marker in breast cancer[J]. Breast Cancer Res, 2004, 6(1): 24-30.
[9]KANDIOLER-ECKERSBERGER D, LUDWIG C, RUDAS M, et al. TP53 mutation and p53 overexpression for prediction of response to neoadjuvant treatment in breast cancer patients
[J]. Clin Cancer Res, 2000, 6(1): 50-56.
[10]THOR A D, BERRY D A, BUDMAN D R, et al. erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer[J]. J Natl Cancer Inst, 1998, 90(18): 1346-1360.
[11]GEISLER S, LONNING P E, AAS T, et al. Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer[J]. Cancer Res, 2001, 61(6): 2505-2512.
[12]AAS T, BORRESEN A L, GEISLER S, et al. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients[J]. Nat Med, 1996, 2(7): 811-814.
[13]RAHKO E, BLANCO G, SOINI Y, et al. A mutant TP53 gene status is associated with a poor prognosis and anthracyclineresistance in breast cancer patients[J]. Eur J Cancer, 2003, 39(4): 447-453.
[14]CLAHSEN P C, VANDE VELDE C J, DUVA L, et al. p53 protein accumulation and response to adjuvant chemotherapy in premenopausal women with node-negative early breast cancer[J]. J Clin Oncol, 1998, 16(2): 470-479.
[15]BERTHEAU P, PLASSA F, ESPIE M, et al. Effect of mutated TP53 on response of advanced breast cancers to high-dose chemotherapy[J]. Lancet, 2002, 360(9336): 852-854.
[16]KNOOP A S, BENTZEN S M, NIELSEN M M, et al. Value of epidermal growth factor receptor, HER2, p53, and steroid receptors in predicting the efficacy of tamoxifen in high-risk postmenopausal breast cancer patients[J]. J Clin Oncol, 2001, 19(14): 3376-3384.
[17]BERRYD A, MUSS H B, THOR A D, et al. HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer[J]. J Clin Oncol, 2000, 18(20): 3471-3479.
[18]ELLEDGE R M, GREEN S, HOWES L, et al. Bcl-2, p53, and response to tamoxifen in estrogen receptor-positive metastatic breast cancer: a Southwest Oncology Group study[J]. J Clin Oncol, 1997, 15(5): 1916-1922.
[19]ARCHER S G, ELIOPOULOS A, SPANDIDOS D, et al. Expression of ras, p21, p53 and c-erbB-2 in advanced breast cancer and response to first line hormonal therapy[J]. Br J Cancer, 1995, 72(5): 1259-1266.
[20]BERNS E M, KLIJN J G, VAN PUTTEN W L, et al. p53 protein accumulation predicts poor response to tamoxifen therapy of patients with recurrent breast cancer[J]. J Clin Oncol, 1998, 16(1): 121-127.
[21]KAI K, NISHIMURA R, MATSUDA M, et al. P53 overexpression is a significant factor in predicting resistance to 3rd generation aromatase inhibitors (AIs) in hormonesensitive recurrent or advanced breast cancer[J]. J Clin Oncol (Meeting Abstracts), 2005, 23(Suppl 16): 715.
[22]ELLIS M J, DING L, SHEN D, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition[J]. Nature, 2012, 486(7403): 353-360.
[23]BERGER C E, QIAN Y, LIU G, et al. p53, a target of estrogen receptor (ER) α, modulates DNA damage-induced growth suppression in ER-positive breast cancer cells[J]. J Biol Chem, 2012, 287(36): 30117-301127.
[24]CHOI H K, ROH S H, KIM H G, et al, Enhanced expression of aromatase in p53-inactivated mammary epithelial cells[J]. Endocr Relat Cancer, 2008, 15(1): 139-147.
Overexpression of P53 is prognostic for aromatase inhibitor resistance in early stage postmenopausal patients with ER-positive breast cancer
JIA Xiao-qing1, HONG Qi1, CHENG Jing-yi2, LI Jian-wei1, WANG Yu-jie1, MO Miao3, SHAO Zhi-min1, SHEN Zhen-zhou1, LIU Guang-yu1(1. Department of Breast Surgery, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; 2. Department of Nuclear Medicine, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; 3. Department of Epidemiology, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China)
Background and purpose:Tumor suppressor gene P53 has long been studied in tumors, including breast cancer. More studies focused on the relationship between P53 and prognosis of breast cancer and found that P53 overexpression suggested a bad prognosis. However, the effect of P53 on early stage postmenopausal patients with ER-positive breast cancer has not been clari fi ed yet. This study was to investigate the role of P53 plays in aromatase
P53; Breast cancer; Prognosis; Endocrine resistance
10.3969/j.issn.1007-3969.2014.05.006
R737.9
A
1007-3639(2014)05-0354-07
2013-11-20
2014-03-01)
柳光宇 E-mail:liugy123@yahoo.com
inhibitor (AI) resistance among early stage postmenopausal patients with ER-positive breast cancer patients.Methods:A total number of 293 operable breast cancer patients who received surgical treatment during Jul. 2000 to Jul. 2006 in Fudan University Shanghai Cancer Center were enrolled into this study. All patients received AI treatment. The SPSS 12.0 software was used to estimate the survival rate. Univariate and multivariate analysis were also performed via above software.Results:The median follow-up time is 72 months (6-140 months). The 5 year disease free survival (DFS) of P53 positive and negative were 78% and 89%. The results showed that P53 overexpression (HR=1.729, 95%CI:1.038-2.880, P=0.035), pathological stage (HR=2.270, 95%CI: 1.399-3.681, P=0.001); histological grade (HR=2.328, 95%CI: 1.312-4.133, P=0.004); age (HR=1.988, 95%CI:1.511-2.617, P<0.005) were still the independent risk factors of recurrence and metastasis in breast cancer patients treated with AI.Conclusion:P53 overexpression correlated strongly with AI resistance in early stage postmenopausal patients with ER-positive breast cancer patients who were treated with AI and con fi rmed the relevance of previously described prognostic factors. It is reasonable to take P53 expression into account when we evaluate the risk of breast cancer patients and decide the anti-cancer treatment strategy.

