氰基修饰的新型寡聚噻吩衍生物的合成
王 静
(商丘师范学院 化学化工学院,河南 商丘 476000)
有机共轭化合物由于在电子学和光电子学领域中的潜在应用而成为人们研究的热点。很多研究工作都集中在p型有机材料的合成和结构与性质关系的研究上[1~3], 关于n型有机半导体材料的报道相对较少,例如氟取代的芳香族化合物[4~7],杂环类芳香化合物[8,9],富勒烯类衍生物[10],萘和二萘嵌苯二酰亚胺[11]等。因此,设计并合成n型有机半导体材料成为研究的热点。得到n型有机半导体材料的方法之一就是在分子的π共轭骨架中引入吸电子取代基[12],这样可以降低分子的LUMO最低轨道能级,有利于电子传输。
本文在文献[13~15]方法的基础上, 以2,3,4,5-四碘代噻吩(2)为原料,设计并合成了3个含有氰基和烷基的新型寡聚噻吩衍生物(1a~1c, Scheme 1),其结构经1H NMR,13C NMR, EI-MS和元素分析表征。将吸电子取代基氰基引入分子中以降低分子的LUMO能级, 增加分子的电子亲和能, 从而有利于电子传输, 提高分子在空气中的稳定性, 得到n型半导体材料。
1 实验部分
1.1 仪器与试剂
Bruker 300 MHz和400 MHz型核磁共振仪(CDCl3为溶剂, TMS为内标);傅立叶变换离子回旋共振质谱仪; UBC型元素分析仪。
(三苯基磷)合钯[Pd(PPh3)4][16],3a~3c[13,14]和2[15]按文献方法合成;2-乙基噻吩, 2-正己

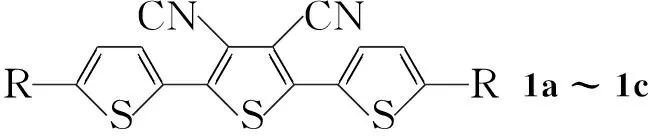
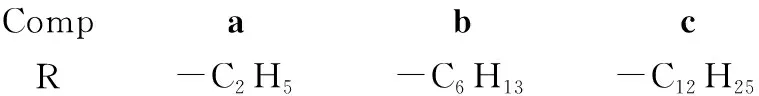
CompabcR-C2H5-C6H13-C12H25
Scheme1
基噻吩,正丁基锂, Alfa Aesar公司;溴代正十二烷,硼酸三甲酯,上海国药集团化学试剂有限公司;其余所用试剂均为分析纯,其中THF经钠和二甲苯酮处理,蒸出立即使用;石油醚沸程为60 ℃~90 ℃;薄层层析(GF254)和柱层析硅胶(200目~300目),山东青岛海洋化工厂。
1.2 合成
(1)4的合成(以4a为例)
在三口圆底烧瓶中加入3a0.98 g(5.00 mmol),2735 mg(1.25 mmol)和NaHCO3饱和溶液10 mL,氩气保护下加入Pd(PPh3)458 mg(0.05 mmol)和无氧THF 15 mL,搅拌下于70 ℃反应至终点(TLC监测)。冷却至室温,倾入饱和NH4Cl溶液中,用乙酸乙酯(3×10 mL)萃取,合并有机相,用无水Na2SO4干燥,浓缩后经硅胶柱层析(洗脱剂:石油醚)纯化得黄色固体3,4-二碘-2,5-二(5-乙基噻吩基-2-)噻吩(4a)264 mg。
用类似方法合成黄色固体4b和4c。
4a: 收率38%;1H NMRδ: 7.25(d,J=3.6 Hz, 2H), 6.80(d,J=3.6 Hz, 2H), 2.87(q,J=7.6 Hz, 4H), 1.34(t,J=7.6 Hz, 6H); EI-MSm/z: 555; Anal.calcd for C16H14S3I2: C 34.55, H 2.54, S 17.29; found C 34.56, H 2.55, S 17.28。
4b: 收率40%;1H NMRδ: 7.24(d,J=3.6 Hz, 2H), 6.78(d,J=3.6 Hz, 2H), 2.82(q,J=7.6 Hz, 4H), 1.70(q,J=7.6 Hz, 4H), 1.28(m, 12H), 0.90(t,J=6.8 Hz, 6H); EI-MSm/z: 667; Anal.calcd for C24H30S3I2: C 43.12, H 4.52, S 14.39; found C 43.15, H 4.50, S 14.39。
4c: 收率35%;1H NMRδ: 7.24(d,J=3.6 Hz, 2H), 6.78(d,J=3.6 Hz, 2H), 2.82(q,J=7.6 Hz, 4H), 1.70(q,J=7.6 Hz, 4H), 1.26(m, 36H), 0.88(t,J=6.6 Hz, 6H); EI-MSm/z: 836; Anal.calcd for C36H54S3I2: C 51.67, H 6.50, S 11.50; found C 51.65, H 6.53, S 11.47。
(2)1的合成(以1a为例)
在两口瓶中加入4a66.6 mg(0.12 mmol)和氰化亚酮32.4 mg(0.24 mmol),氩气保护下加入无水DMF 8 mL,搅拌下回流反应4 h。冷却至室温, 加入六水合三氯化铁121.6 mg的盐酸(4 mL)溶液, 于60 ℃~70 ℃反应30 min。冷却至室温,用二氯甲烷(3×10 mL)萃取, 合并有机相,依次用6 mol·L-1盐酸、水、饱和碳酸钠溶液和饱和食盐水洗涤,无水硫酸钠干燥,浓缩后经硅胶柱层析[洗脱剂:A=V(石油醚) ∶V(二氯甲烷)=2 ∶1]纯化得黄色固体2,5-二(5-乙基噻吩基-2-)噻吩-3,4-二腈(1a)33.9 mg。
用类似方法合成黄色固体1b和1c。
1a: 收率80%;1H NMRδ: 7.48(d,J=3.6 Hz, 2H), 6.84(d,J=3.6 Hz, 2H), 2.90(q,J=7.6 Hz, 4H), 1.34(t,J=7.6 Hz, 6H);13C NMRδ: 151.2, 139.1, 129.4, 129.2, 126.4, 113.2, 105.2, 23.5, 15.8; EI-MSm/z: 354; Anal.calcd for C18H14N2S3: C 60.98, H 3.98, N 7.90, S 27.13; found C 60.96, H 3.95, N 7.92, S 27.16。
1b(洗脱剂:A=2 ∶1): 收率85%;1H NMRδ: 7.47(d,J=3.6 Hz, 2H), 6.82(d,J=3.6 Hz, 2H), 2.85(q,J=7.6 Hz, 4H), 1.70(q,J=7.6 Hz, 4H), 1.28(m, 12H), 0.90(t,J=6.8 Hz, 6H);13C NMRδ: 151.2, 145.0, 128.8, 128.6, 125.8, 113.2, 105.2, 31.6(overlapping signal), 30.2, 28.8, 22.6, 14.0; EI-MSm/z: 466; Anal.calcd for C26H30N2S3: C 66.91, H 6.48, N 6.00, S 20.61; found C 66.96, H 6.45, N 6.02, S 20.57。
1c(洗脱剂:A=3 ∶1): 收率80%;1H NMRδ: 7.47(d,J=3.6 Hz, 2H), 6.82(d,J=3.6 Hz, 2H), 2.85(q,J=7.6 Hz, 4H), 1.70(q,J=7.6 Hz, 4H), 1.26(m, 36 H), 0.88(t,J=6.6 Hz, 6H);13C NMRδ: 151.2, 145.0, 128.8, 128.6, 125.8, 113.2, 105.2, 31.9, 31.5, 30.3, 29.7, 29.6, 29.6, 29.5, 29.4, 29.3, 29.1, 22.7, 14.1; EI-MSm/z: 634; Anal.calcd for C38H54N2S3: C 71.87, H 8.57, N 4.41, S 15.15; found C 71.90, H 8.55, N 4.42, S 15.13。
2 结论
1a~1c均为淡黄色固体,有很好的溶解性,能够溶于常用有机溶剂如氯仿、二氯甲烷、四氢呋喃和甲苯等。因此,可以采用传统的柱层析纯化方法,后处理方便。
[1] Mitschke U, Baüerle P. The electroluminescence of organic materials[J].J Mater Chem,2000,10:1471-1507.
[2] Kraft A, Grimsdale A C, Holmes A B. Electroluminescent conjugated polymers——seeing polymers in a new light[J].Angew Chem Int Ed,1998,37:402-428.
[3] Robinson M R, Wang S, Bazan G C,etal. Electroluminescence from well-defined tetrahedral oligophenylenevinylene tetramers[J].Adv Mater,2000,12:1701-1704.
[4] Fachetti A, MushrushM, Katz H E,etal.n-Type building blocks for organic electronics:A homologous family of fluorocarbon-substituted thiophene oligomers with high carrier mobility[J].Adv Mater,2003,15:33-38.
[5] Facchetti A, Yoon M H, Stern C L,etal. Building blocks forn-type molecular and polymeric electronics. Perfluoroalkyl-versus alkyl-functionalized oligothiophenes(nTs; n=2~6).Systematic synthesis,spectroscopy,electrochemistry,and solid-state organization[J].J Am Chem Soc,2004,126:13480-13501.
[6] Sakamoto Y, Suzuki T, Miura A,etal. Synthesis,characterization,and electron-transport property of perfluorinated phenylene dendrimers[J].J Am Chem Soc,2000,122:1832-1833.
[7] Bao Z, Lovinger A J, Brown J. New air-stablen-channel organic thin film transistors [J].J Am Chem Soc,1998,120:207-208.
[8] Tonzola C J, Alam M M, Kaminsky W,etal. Newn-type organic semiconductors:Synthesis,single crystal structures,cyclic voltammetry,photophysics,electron transport,and electroluminescence of a series of diphenylanthrazolines[J].J Am Chem Soc,2003,125:13548-13558.
[9] Strukelj M, Papadimitrakopoulos F, Miller T M,etal. Design and application of electron-transporting organic materials[J].Science,1995,267:1969-1972.
[10] Thompson B C, Fréchet J M J. Polymer-fullerene composite solar cells[J].Angew Chem Int Ed,2008,47:58-77.
[11] Jones B A, Facchetti A, Wasielewski M R,etal. Tuning orbital energetics in arylene diimide semiconductors.Materials design for ambient stability of n-type charge transport[J].J Am Chem Soc,2007,129:15259-15278.
[12] Zhou Y, Liu W, Ma Y,etal. Single microwire transistors of oligoarenes by direct solution process[J].J Am Chem Soc,2007,129:12386-12387.
[13] Cammidge A N, Goddard V H M, Gopee H,etal. Aryl trihydroxyborates:Easily isolated discrete species convenient for direct application in coupling reactions[J].Org Lett,2006,8:4071-4074.
[14] Wang J, Xu H, Li B,etal. Synthesis and characterization of new planar butterfly-shaped fused oligothiophenes[J].Tetrahedron,2012,68:1192-1197.
[15] Zhang D, Tessier C A, Youngs W J. Synthesis of tris(2,5-dialkynylthieno)cyclotriynes,tris(4,5-dialkoxyphenyl)cyclotriynes,and tetrakis(4,5-dialkoxyphenyl)cyclotetraynes with long-chain alkyl substituents,and the nickel and cobalt complexes of tris[4,5-(didodecyloxy)phenyl]cyclotriyne[J].Chem Mater,1999,11:3050-3057.
[16] Brown H C, Bhat N G, Srebnik M. A simple general synthesis of 1-alkynyldiisop ropoxyboranes[J].Tetrahedron Letters,1988,29: 2631-2634.

