甲状腺乳头状癌并BRAFV600E突变及原癌基因重排蛋白表达与其侵袭性的关系
孟 超,高 洁,梁 军,梁智勇,林岩松
1青岛大学医学院附属医院肿瘤科,青岛 266003中国医学科学院 北京协和医学院 北京协和医院 2病理科 3核医学科,北京 100730
·论著·
甲状腺乳头状癌并BRAFV600E突变及原癌基因重排蛋白表达与其侵袭性的关系
孟 超1,高 洁2,梁 军1,梁智勇2,林岩松3
1青岛大学医学院附属医院肿瘤科,青岛 266003中国医学科学院 北京协和医学院 北京协和医院2病理科3核医学科,北京 100730
目的探讨BRAFV600E突变及原癌基因重排 (RET) 蛋白表达并存与甲状腺乳头状癌(PTC)侵袭性的关系。方法收集50例PTC患者术后病理,分别用RT-PCR检测 BRAFV600E突变,免疫组织化学SP法检测RET表达情况。分析BRAFV600E突变合并RET表达组(n=24)和BRAFV600E单突变或RET单表达组 (n=19)的临床及病理特征。结果BRAFV600E突变率、 RET表达率分别为76%(38/50)、56%(28/50),存在BRAFV600E突变合并RET表达者占48%(24/50)。与BRAFV600E突变合并RET表达组比较, BRAFV600E单突变或RET单表达组肿瘤组织分化程度差,且甲状腺癌评分系统评分较高(P=0.011,P=0.022)。结论BRAFV600E突变并存RET表达时提示PTC肿瘤组织分化差、更易出现实体亚型等侵袭性较高的病理亚型,有可能增加肿瘤相关死亡风险。
BRAFV600E突变;原癌基因重排蛋白表达;甲状腺乳头状癌;侵袭性
ActaAcadMedSin,2013,35(1):64-68
甲状腺乳头状癌(papillary thyroid cancer, PTC)是甲状腺恶性肿瘤中最常见的一种类型,发病率为70%~80%[1]。Xing等[2]对近30篇PTC相关基因突变文献分析后总结其BRAFV600E突变率为29%~83%,即15外显子第1799位核苷酸T被A取代,导致600密码子编码的缬氨酸被谷氨酸替代(V600E)[3-4],与PTC的局部淋巴结及远处转移相关[5-6]。原癌基因重排(rearranged during transfection proto-oncogene,RET)蛋白,尤其是RET/PTC活化表达不仅是PTC的致病因之一,还与其局部侵袭性[7]、预后及复发[8]有关。目前国内外研究二者突变并存的文献较少,Musholt等[9]对可疑PTC患者的细针穿刺活检组织联合行BRAFV600E突变及RET/PTC检测,有助于PTC术前诊断、恶性程度评估及优化手术方式。Henderson等[10]对PTC复发人群的研究结果提示二者双突变可能与PTC复发有关。在临床工作中PTC患者可同时出现BRAFV600E突变及RET表达,有关BRAFV600E突变和RET表达并存与其侵袭性的关系,目前报道较少。本研究拟对初诊PTC患者手术切除肿瘤组织行BRAF、RET检测,旨在分析BRAFV600E突变和RET表达并存与PTC侵袭性之间的关系。
资料和方法
标本来源选取北京协和医院2011年11月至2012年2月甲状腺全切除术后病理确诊为PTC并均无远处转移的50例患者,男女比为1∶2.1,平均年龄(40.49±11.44)岁。所有患者术后病理组织学切片均由2位高年资病理医师复查确认为PTC,其中PTC亚型包括滤泡亚型9例、实体亚型10例、淋巴上皮瘤样亚型及透明细胞型各1例(因亚型罕见未行分析)。
RT-PCR法检测PTC组织中BRAFV600E突变
组织DNA提取:严格按QIAamp DNA FFPE Tissue Kit(50)(中美艾德AmoyDX公司)说明书进行。紫外光光度计检测DNA纯度及浓度。
PCR扩增:取cDNA 5 μl,上下游引物各35 μl,TaqDNA聚合酶0.4 μl等,加入PCR反应体系中扩增。反应条件:95℃ 5 min 1个循环; 95℃ 25 s,64℃ 20 s,72℃ 20 s 15个循环; 93℃ 25 s,60℃ 35 s,72℃ 20 s 31个循环。
PCR产物分析:(1)确定实验是否成功可信:待测样品的内控内对照信号(VIC信号)应升起。(2)确认未选择校正荧光参照,按管号顺序依次选择单一突变检测反应管进行检测分析。需要同时选择阳性质控品反应孔、无模板对照孔和样品反应孔,然后根据实际情况调节阈值至目的基因FAM扩增曲线升起的拐点处,得到突变组的Ct值,即荧光达到阈值时候的PCR循环次数。若样本的Ct大于或等于28,则样本为阴性;若样本的Ct小于28,则样本为阳性。
免疫组织化学法检测RET在PTC组织中的表达标本经4%甲醛溶液固定,常规石蜡包埋,4μm厚连续切片,分别行HE和免疫组织化学染色。采用免疫组织化学SP法(免疫组织化学试剂购于丹麦DAKO公司,RET鼠抗人单克隆抗体为即用型制剂),具体操作步骤按试剂说明书进行,以1 mmol/L的EDTA pH 9.0 修复液进行高压锅抗原修复,DBS溶液显色,苏木精复染,中性树胶封闭,镜下观察。RET阳性表达位于细胞浆,<5%细胞着色为阴性,≥5%细胞着色为阳性。
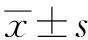
结 果
PTC肿瘤组织BRAFV600E突变、RET表达情况76% (38/50)患者检测到BRAFV600E突变;56% (28/50)患者癌组织中检测出RET表达,2例患者癌旁正常组织中检测出RET蛋白表达(合并桥本氏甲状腺炎和结节性甲状腺肿者各1例);48% (24/50)患者同时存在BRAFV600E突变及RET表达,19例患者为BRAFV600E单突变或RET单表达,7例患者既不存在BRAFV600E突变也无RET表达(统计数据时已将其剔除)。
临床病理特征BRAFV600E突变和RET表达并存组(G1组)患者肿瘤组织呈中分化者明显高于二者单表达组(G2组)(P=0.011);G1组患者实体亚型者明显高于G2组(P=0.020);甲状腺乳头状癌评分系统(MACIS评分)高于G2组(P=0.022)。G1组中患者肿瘤侵出甲状腺包膜与周围组织黏连例数明显高于G2组(P=0.036)(表1、图1)。
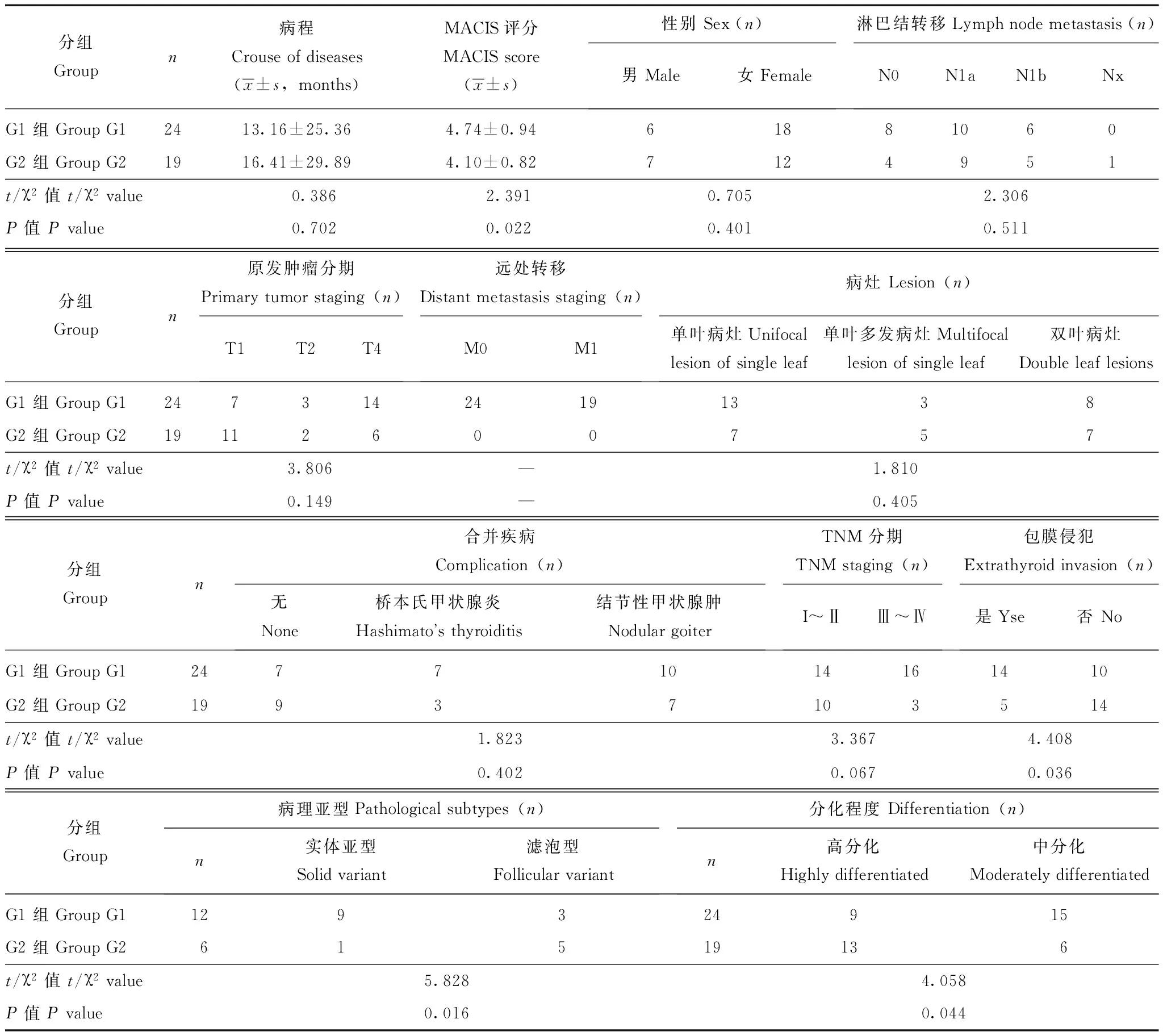
表 1 两组患者临床病理资料比较
G1组:BRAFV600E突变合并RET表达组;G2组:BRAFV600E单突变或RET单表达组;RET:原癌基因重排;Nx:未被评估的局部结节
GroupG1: concurrent BRAFV600Eand RET protein expression; GroupG2: only one of BRAFV600Emutation and RET protein expression; RET: rearranged during transfection proto-oncogene; Nx:regional nodes cannot be assessed
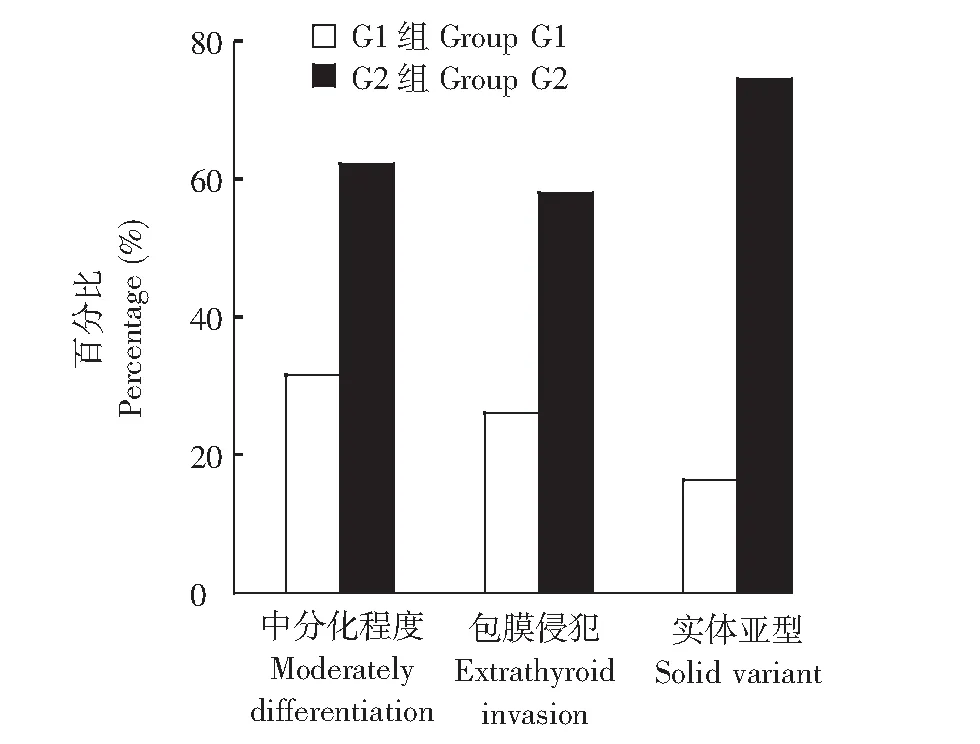
G1组:BRAFV600E突变合并RET表达组;G2组:BRAFV600E单突变或RET单表达组GroupG1: concurrent BRAFV600E and RET protein expression; GroupG2: only one of BRAFV600E mutation and RET protein expression图 1 两组患者肿瘤组织实体亚型、中分化程度及包膜侵犯发生率Fig 1 Percentages of solid variant, moderately differentiation and extrathyroid invasion in two groups
讨 论
PTC是甲状腺恶性肿瘤中最常见的类型[1],经甲状腺全切或次全切除术辅以选择性131I治疗及甲状腺激素抑制治疗后,大多数患者预后较好,但仍约30%复发率[11]。包膜侵犯、颈部淋巴结转移可使PTC肿瘤组织侵袭性增强,导致患者10年生存率下降[12]。研究显示PTC患者BRAFV600E突变率为29%~83%[2],RET/PTC重排发生率为17%~44%[13]。二者均与PTC患者局部侵袭性、预后及复发相关[5-8,14]。有关BRAFV600E突变合并RET表达对PTC侵袭性影响的报道较少,Musholt等[9]证实联合检测BRAFV600E突变及RET/PTC异常表达有助于PTC术前细针穿刺活检组织诊断、恶性程度评估及优化手术方式;针对复发性PTC的研究显示BRAFV600E突变和RET/PTC重排并存可能与PTC复发及恶性程度有关[10]。本研究首次观察了PTC患者初次手术病理BRAFV600E突变并存RET表达与PTC侵袭性的关系,结果显示BRAFV600E突变和RET表达并存组(G1组)患者包膜外侵犯率明显高于单一突变或表达组(G2组),提示两者并存时肿瘤组织的侵袭性更高。
PTC患者肿瘤组织的侵袭性与其分化程度及病理亚型类型有关。RET原癌基因高表达,RAS/BRAF/MAPK信号通路持续激活被认为是PTC发病的主要分子机制之一,影响PTC肿瘤组织的分化程度及亚型[15]。Kakudo等[16]研究提示中分化甲状腺癌患者10年生存率较经典型甲状腺癌者下降约20%,提示其预后较差。实体亚型是PTC病理特殊组织学亚型,属于侵袭性强、预后差的类型,其患者颈部淋巴结及远处转移率、复发率高[16-18]。本研究G1组PTC患者肿瘤组织呈中分化者明显高于G2组,同时实体亚型者明显高于G2组,提示BRAFV600E突变和RET表达并存时使PTC肿瘤组织分化程度降低、更易出现实体亚型等侵袭性较高的病理亚型。
MACIS评分主要依据患者年龄、原发肿瘤直径、局部侵犯情况、是否完全切除及远处转移进行计算。有报道MACIS评分>6时可作为PTC患者死亡率的预测指标之一,其分值高,提示PTC患者肿瘤组织的侵袭性增强,生存率降低[19]。本研究G1组PTC患者MACIS评分较G2组高,分析其原因主要与本组患者包膜侵犯率较高有关,提示BRAFV600E突变和RET表达并存时,患者肿瘤组织侵袭性增高,增加了肿瘤相关死亡风险。
将G2组进一步分为两个亚组,即BRAFV600E突变或RET单表达组,两组患者病理资料差异无统计学意义,可能与患者病例数较少有关,在今后研究中将增加病例数继续对其进一步观察。
综上,PTC患者初次术后病理BRAFV600E突变和RET表达并存时与肿瘤组织的侵袭性明显相关,使其具有更高包膜侵犯率,导致分化程度变差且更易出现实体亚型等侵袭性较高的病理亚型,有可能增加肿瘤相关死亡风险。
[1] Ain KB. Papillary thyroid carcinoma. Etiology, assessment, and therapy[J]. Endocrinol Metab Clin North Am, 1995, 24(4):711-760.
[2] Xing M. BRAF mutation in thyroid cancer[J]. Endocr Relat Cancer, 2005, 12(2):245-262.
[3] Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage[J]. Nat Rev Mol Cell Biol, 2004, 5(11):875-885.
[4] Wojciechowska K, Lewinski A. BRAF mutations in papillary thyroid carcinoma[J]. Endocr Regul, 2006, 40(4):129-138.
[5] Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis [J].Cancer, 2012, 118(7):1764-1773.
[6] Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer[J]. Ann Surg, 2007, 246(3):466-470.
[7] Wang YL,Zhang RW,Luo ZW,et al. High frequency of level Ⅱ-Ⅴ lymph node involvement in RET/PTC positive papillary thyroid carcinoma[J]. Eur J Surg Oncol, 2008,34(1):77-781.
[8] Kogan EA, Rozhkova EB, Seredin VP, et al. Prognostic value of the expression of thyreoglobulin and oncomarkers (p53, EGFR, ret-oncogene) in different types of papillary carcinoma of the thyroid: clinicomorphological and immunohistochemical studies[J]. Arkh Patol, 2006, 68(4):8-11.
[9] Musholt TJ, Fottner C, Weber MM, et al. Detection of papillary thyroid carcinoma by analysis of BRAF and RET/PTC1 mutations in fine-needle aspiration biopsies of thyroid nodules[J]. World J Surg, 2010, 34(11):2595-2603.
[10] Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutation associated with recurrent papillary thyroid carcinoma[J]. Clin Cancer Res, 2009, 15(2):485-491.
[11] Tuttle RM, Leboeuf R. Follow up approaches in thyroid cancer: a risk adapted paradigm[J]. Endocrinol Metab Clin N Am, 2008, 37(2):419-435.
[12] Hamzany Y, Soudry E, Strenov Y. et al. Early death from papillary thyroid carcinoma[J]. Am J Otolaryngol, 2012, 33(1):104-108.
[13] Zhu Z, Ciampi R, Nikiforova MN, et al. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity[J]. J Clin Endocrinol Metab, 2006, 91(9):3603-3610.
[14] Zafón C, Castellví J, Obiols G. Usefulness of the immunohistochemical analysis of several molecular markers in the characterization of papillary thyroid carcinoma with initial lymph node metastasis[J]. Endocrinol Nutr, 2010, 57(4):165-169.
[15] Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications[J]. J Chin Med Assoc,2010, 73(3):113-128.
[16] Kakudo K, Bai YH, Katayama S. et al. Classification of follicular cell tumors of the thyroid gland: analysis invoving Japanese patients form one institute[J]. Pathol Int, 2009, 59(6):359-367.
[17] Nikiforov YE, Erickson LA, Nikiforova MN,et al. Solid variant of papillary thyroid carcinoma: incidence, clinical-pathologic characteristics, molecular analysis, and biologic behavior[J]. Am J Surg Pathol, 2001, 25(12):1478-1484.
[18] Silver CE, Owen RP, Rodrigo JP, et al. Aggressive variants of papillary thyroid carcinoma[J]. Head Neck, 2011, 33(7):1052-1059.
[19] Holler T, Theriault J, Payne RJ, et al. Prognostic factors in patients with multiple recurrences of well-differentiated thyroid carcinoma[J]. J Oncol, 2009, doi:10.1155/2009/650340.
Invasive Properties of Papillary Thyroid Cancer with Concurrent BRAFV600EMutationand Rearranged during Transfection Proto-oncogene Protein Expression
MENG Chao1, GAO Jie2, LIANG Jun1, LIANG Zhi-yong2, LIN Yan-song3
1Department of Oncology, the Affiliated Hospital of Qingdao University Medical College, Qingdao 266003, China2Department of Pathology,3Department of Nuclear Medicine, PUMC Hospital,CAMS and PUMC, Beijing 100730, China
LIN Yan-song,Tel:010-69155610,E-mail:linyansong68@yahoo.com.cn
Objective To investigate the aggressive properties of papillary thyroid cancer (PTC) with concurrent BRAFV600Emutation and rearranged during transfection (RET) proto-oncogene protein expression.MethodsFifty pathologically confirmed PTC patients who had
thyroidectomy were enrolled in this study. BRAFV600Emutation was detected by real time polymerase chain reaction (RT-PCR), while RET protein expression was measured by immunohistochemical SP method. Clinical and pathological features were compared between the concurrent BRAFV600Emutation and RET protein expression group (n=24) and BRAFV600Emutation or RET protein expression alone group (n=19). Seven patients were ruled out from the final analysis due to the absence of either BRAFV600Emutation or RET protein expression.ResultsOf these 50 patients, BRAFV600Emutation and RET protein expression were detected in 38 patients (76%) and 28 patients (56%), respectively. Concurrent BRAFV600Emutation and RET expression was detected in 24 patients (48%). Compared with the concurrent BRAFV600Emutation and RET protein expression group, the BRAFV600Emutation or RET protein expression alone group had relatively poorer tissue differentiation and higher prognostic score (P=0.011,P=0.022).ConclusionPTC patients with concurrent BRAFV600Emutation and RET expression present poorer differentiation, more highly aggressive variant in carcinoma tissues, and higher cancer-related mortality risk.
BRAFV600Emutation; tyrosine kinase receptor protein expression; papillary thyroid cancer; aggressiveness
国家自然科学基金(30970850)和卫生行业科研专项项目(201202012)Supported by the National Natural Sciences Foundation of China (30970850)and the Research Special Fund of the Health Industry(201202012);第一、二位作者对本文贡献一致The first two authors contributed equally to this article
林岩松 电话:010-69155610,电子邮件:linyansong68@yahoo.com.cn
R736.1
A
1000-503X(2013)01-0064-05
10.3881/j.issn.1000-503X.2013.01.012
2012-04-25)

