One New Zinc Coordination Polymer Based on 2-Chloro-benzoate and 4,4΄-Bipyridine:Synthesis, Crystal Structure and Luminescence①
MA De-Yun GUO Hi-Fu QIN Ling (School of Chemistry nd Chemicl Engineering, Zhoqing University, Zhoqing 526061, Chin) (Key Lortory of Fuel Cell Technology of Gungdong Province,South Chin University of Technology, Gungzhou 510641, Chin)
1 INTRODUCTION
Many researches in recent years have focused on the design and synthesis of new potentially multifunctional coordination polymers with useful structure-derived properties, such as porosity, gas storage and ion exchange, or other physical properties like magnetism and photoluminescence[1-8]. Much of the reported work is based on the use of mixed functional organic ligands containing N- and/or O-donor atoms to bind to the d-block transition metal ions[9-11]. The selection of organic ligands is of great importance in the construction of specific supramolecular networks with useful physicochemical properties and intriguing structural topologies[12-14]. As is well known, the 4,4΄-bpy ligand may act in bidentate bridging or monodentate terminal modes,leading to a variety of one-dimensional chain, twodimensional layer and three-dimensional network motifs[15]. The 2-chlorobenzoate ligand has versatile binding and coordination modes, and can also be used to construct multinuclear structures[16-17].Polynuclear d-block metal complexes have been found to exhibit interesting structural motifs and photoluminescence[18-19]. On the basis of these considerations, we chose 2-chlorobenzoic acid, 4,4΄-bipyridine and ZnIIas our building blocks. A new one-dimensional zinc coordination framework, 1,resulting from the hydrothermal treatment of Zn(NO3)2·6(H2O) with 2-chlorobenzoic acid and 4,4΄-bipyridine in alkaline aqueous solution, has been structurally characterized. Furthermore, the photoluminescent properties of 1 are also investigated in this paper.
2 EXPERIMENTAL
2.1 Materials and physical measurements
All chemicals purchased were of reagent grade and used without further purification. All syntheses were carried out in 23 mL Teflon-lined autoclaves under autogenous pressure. Elemental analyses (C,H and N) were performed on a Perkin-Elmer 240 CHN elemental analyzer. Infrared spectra were recorded (4000–400 cm-1) as KBr disks on a Bruker 1600 FTIR spectrometer. Thermogravimetry analyses (TGA) were executed on an automatic simultaneous thermal analyzer (DTG-60, Shimadzu) under a flow of N2at a heating rate of 10 ℃/min between ambient temperature and 800 ℃. Luminescence spectra and lifetime for the crystalline samples were recorded at room temperature on an Edinburgh FLS920 phosphorimeter.
2.2 Synthesis of 1
A mixture of Zn(NO3)2·6(H2O) (0.15 g, 0.5 mmol),2-chlorobenzoic acid (0.156 g, 1 mmol) 4,4΄-bipyridine (0.08 g, 0.5 mmol), NaOH (0.04 g, 1 mmol) and H2O (15 mL) was placed in a 23 mL Teflon reactor and kept under autogenous pressure at 150 ℃ for 72 h. The mixture was cooled to room temperature at a rate of 5 ℃/h, and colorless block crystals were obtained in a yield of 52% based on 2-cb. Calcd. (%) for C19H12Cl2NO4Zn: Anal. found(%): C, 50.16; H, 2.64; N, 3.08. Calcd. (%): C, 50.22;H, 2.60; N, 3.06. IR (KBr pellet) (cm-1): 1626(vs),1566(m), 1509(w), 1488(w), 1401(s), 1350(s),1256(w), 1218(m), 1066(m), 1043(w), 1013(m),810(m), 798(vs), 753(w), 724(w), 634(s), 574(w),482(m).
2.3 Structure determination
A single crystal with dimensions of 0.30mm ×0.27mm × 0.21mm was mounted on a glass fiber for data collection which was performed on a Bruker SMART APEXII CCD diffractometer operating at 50 kV and 30 mA using a MoKa radiation (λ =0.71073 Å) at 296(2) K by using an ω scan mode. In the range 1.74≤θ≤27.55°, a total of 14962 reflections were collected, of which 4076 were unique (Rint= 0.0365) and 3167 observed ones (I >2σ(I)) were used in the succeeding structure calculations. Data collection and reduction were performed using the APEX II software[20]. Multiscan absorption corrections were applied using the SADABS[20]. The structure was solved by direct methods and refined on F2by full-matrix leastsquares technique using the SHELX-97 program package[21]. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms attached to carbon were placed in the geometrically idealized positions and refined using a riding model. The final R =0.0359 and wR = 0.0936 (w = 1/[σ2(Fo2) +(0.0600P)2+ 0.3000P], where P = (Fo2+ 2Fc2)/3) for 3167 observed reflections with I > 2σ(I). S = 1.033,(Δ/σ)max= 0.000, (Δρ)max= 0.463 and (Δρ)min=–0.303 e/Å3. The selected bond lengths and bond angles are given in Table 1. H-bonding parameters for 1 are given in Table 2.
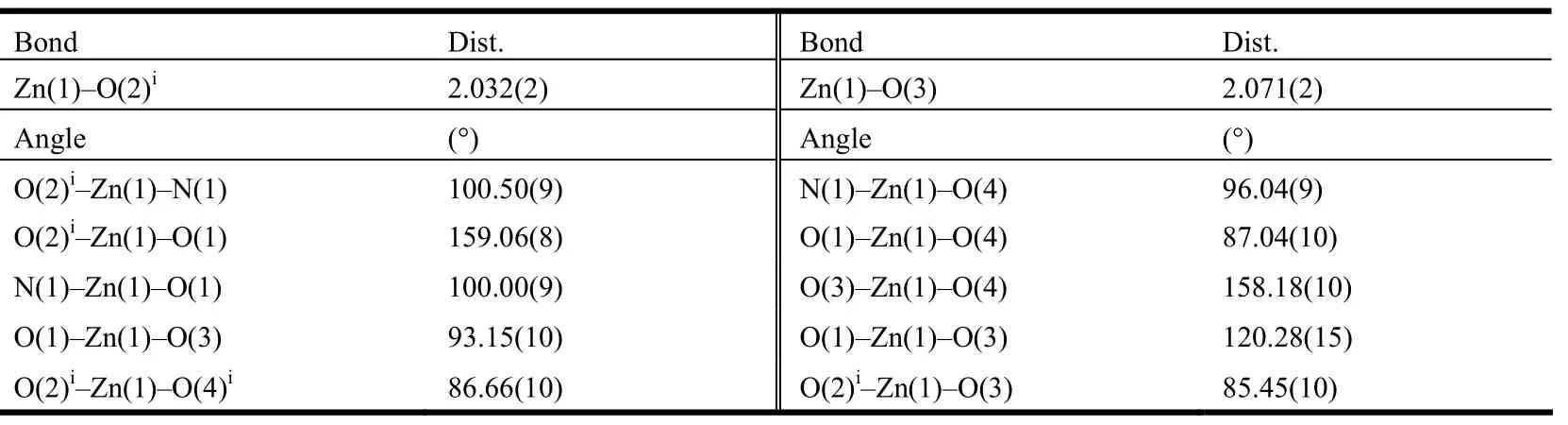
Table 1. Selected Bond Lengths (Å) and Bond angles (°)

Table 2. Hydrogen Bonds of 1
3 RESULTS AND DISCUSSION
3.1 Description of the structure
Single-crystal X-ray diffraction analysis reveals that compound 1 crystallizes in the triclinic space group Pand exhibits a 1D infinite chain structure constructed by 4,4΄-bpy and a dicaryon secondarybuilding-unit of [Zn2(2-cb)4]. As shown in Fig. 1, the asymmetric unit of 1 contains one ZnIIion, two 2-cb ligands and half of a 4,4΄-bpy ligand. Each Zn(II)center is five-coordinated by four carboxylate oxygen atoms from four different 2-cb ligands and one nitrogen atom from one 4,4΄-bpy ligand,adopting a distorted trigonal biyramidal geometry.The Zn–O, Zn–N bond lengths and O–Zn–O,O–Zn–N bond angles range from 2.032(2) to 2.074(2) Å and from 70.93(7) to 166.47(7)°, respectively, which are within the reasonable ranges for other five-coordinated Zn(II) complexes with oxygen and nitrogen donating ligands[19,22]. The four 2-cb ligands link two ZnIIions to construct a dicaryon zinc building block, with a Zn··Zn separation of 2.95(1) Å, which can be regarded as a supramolecular secondary building unit (SBU) or knot.The construction of the knot is similar to the structures[23-25], but unlike to that of the Zn2(acetate)4[26]and Zn2(4-methylbenzoate)4[19]units in the infinite chain. Each 4,4΄-bpy ligand bridges two neighboring SBUs to form a one-dimensional infinite chain along the a axis of the unit cell (Fig. 2a). The distance between two knots is 14.027(2) Å. Adjacent chains are further connected into a layer (Fig. 2b) in the ab plane through π-π stacking interactions among benzene rings (Cg1 and Cg3) and pyridine rings(Cg2), with the centroid-to- centroid distances of 3.642(8) and 3.680(4) Å, respectively, thus indicating weak π-π stacking contacts[27](Cg1, Cg2 and Cg3 are the centroids of N(1)/C(15)–C(19),C(1)–C(6) and C(8)–C(13) rings, respectively).Moreover, intramolecular C–H··O hydrogen bons are also observed (Table 2).
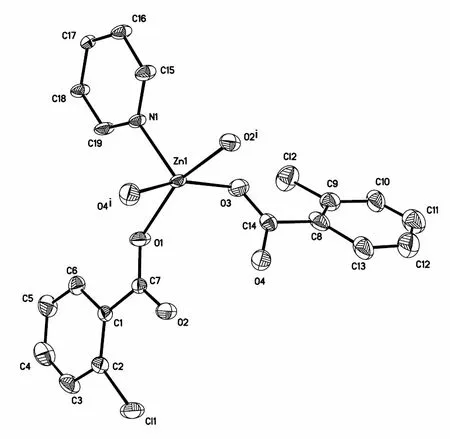
Fig. 1. View of the asymmetric unit of 1 (30% probability ellipsoids).Symmetry codes: i = –0.5+x, –0.5+y, z

Fig. 2. (a) View of the 1D infinite chain of 1; (b) View of the 2D layer network formed by π··π stacking interactions (dashed lines)
3.2 IR and TGA
IR spectra of compound 1 show strong acuate bands at 1566 and 1401 cm-1are associated with the asymmetric (COO) and symmetric (COO) stretching vibrations. The Δν(νas(COO-) – νs(COO-)) value is 166 cm-1, indicating the coordination of 2-cb with Zn(II) in the bidentate-bridging modes[28], which is well consistent with the X-ray diffraction structural analysis.
Thermal gravimetric analyses (TGA) were carried out to examine the thermal stability of 1. The samples were heated up in flowing N2with a heating rate of 10 ℃/min. The TG and DTA curves are depicted in Fig. 3, which shows that 1 has thermal stability as no strictly clean weight loss step occurs below 230 ℃. The weight-loss step appears above 230 ℃, corresponding to the decomposition of the framework structure. Finally, 1 was completely degraded into ZnO with the total loss of 82.23 wt.%(calcd. 82.10 wt.%).

Fig. 3. Thermogravimetric curves (DTA and TG) for compound 1
3.3 Photoluminescent properties
Photoluminescence experiments for ZnII-containing polymers, as a typical d10transition-metal configuration which exhibits photoluminescent pro-perty[29], were performed at room temperature in the solid state. The 2-cb and 4,4΄-bpy ligands with their extended aromaticity are thus regarded to be excellent candidates for enhanced emissive properties. As illustrated in Fig. 4, complex 1 exhibits indigotin photoluminescence with an emission maximum of 430 nm upon excitation at 360 nm.Complex 1 represents an obvious qualitative change of luminescence property resulting from the interaction between metal ion and the ligand. The emission of 1 probably origins from the ligandto-metal charge transfer (LMCT)[30]excited state,because both organic ligands have relatively large π-conjugated system of benzene-pyridyl rings and use carboxylate oxygen and nitrogen donors to coordinate to the Zn(II) ions, which benefits the charge transfer between the Zn(II) ion and organic ligands. The luminescent lifetime of solid complex 1 using an Edinburgh FLS920 phosphorimeter with 450 W xenon lamp as the excitation source shows lifetime for complex 1 of 55.03 ns at 430 nm (Fig. 5).

Fig. 4. Solid-state emission spectra of 1 at room temperature
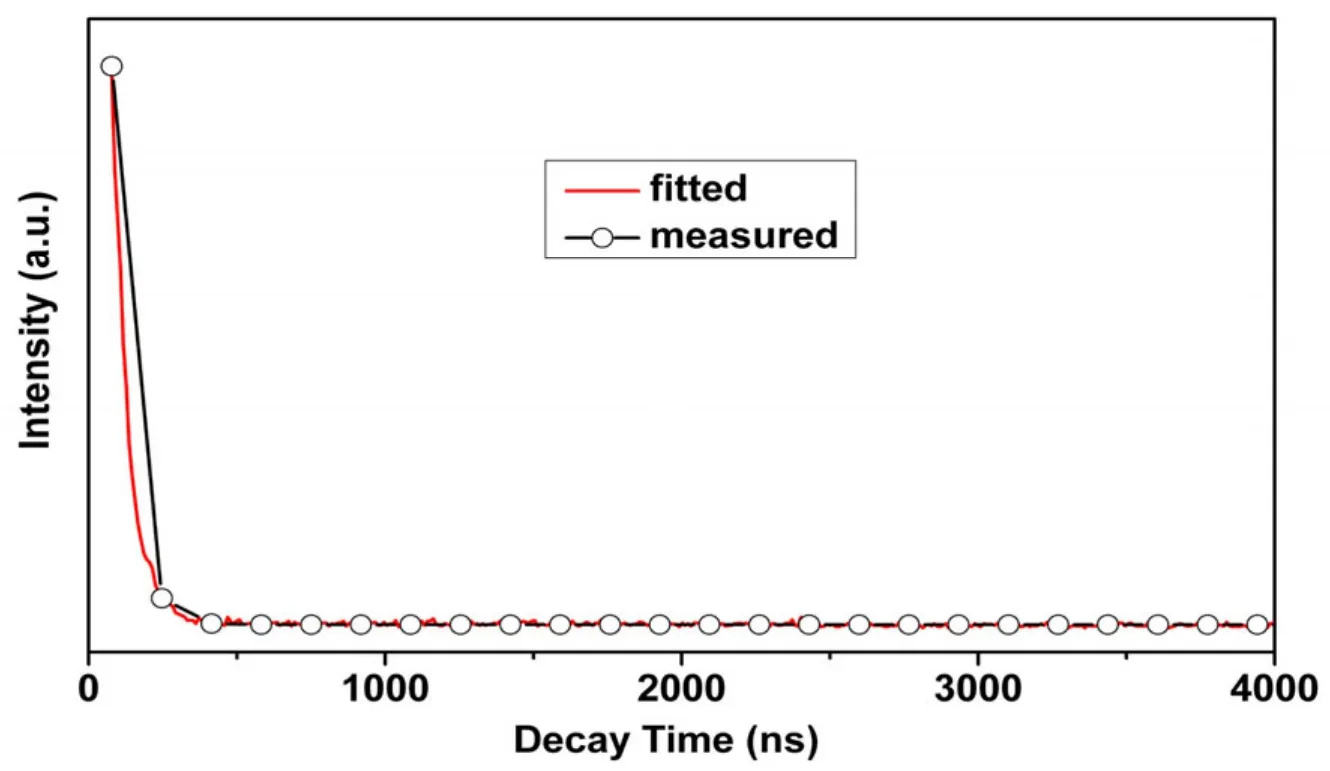
Fig. 5. Luminescent lifetime for complex 1 in the solid state at room temperature
In conclusion, a new one-dimensional zinc(II)coordination polymer [Zn(2-cb)2(4,4΄-bpy)0.5]ninvolving 2-cb and 4,4΄-bpy ligands has been hydrothermally synthesized. Complex 1 emits intense indigotin luminescence of Zn2+ion with the fluorescence lifetime of 55.03 ns (430 nm) in the solid state at room temperature, which may have potential applications as luminescent materials in indigotin light-emitting devices.
(1) Shi, X.; Zhu, G.; Fang, Q.; Wu, G.; Tian, G.; Wang, R.; Zhang, D.; Xue, M.; Qiu, S. Novel supramolecular frameworks self-assembled from one-dimensional polymeric coordination chains. Eur. J. Inorg. Chem. 2004, 1, 185–191.
(2) Wu, P.; He, C.; Wang, J.; Peng, X.; Li, X.; An, Y.; Duan, C. Photoactive chiral metal-organic frameworks for light-driven asymmetric α-alkylation of aldehydes. J. Am. Chem. Soc. 2012, 134, 14991–14999.
(3) Gong, Y.; Wu, T.; Jiang, P. G.; Lin, J. H.; Yang, Y. X. Octamolybdate-based metal-organic framework with unsaturated coordinated metal center as electrocatalyst for generating hydrogen from water. Inorg. Chem. 2012, ASAP.
(4) Ma, D. Y.; Qin, L.; Lu, K.; Guo, H. F.; Liu, J. Q. A new 3D photoluminescent Cu(I) coordination polymer built from 5-[4-(imidazol-1-yl)phenyl]tetrazolide with 4-connected SrAl2(sra) topology. Inorg. Chem. Commun. 2012, 24, 87–90.
(5) Ma, D.; Li, Y.; Li, Z. Tuning the moisture stability of metal-organic frameworks by incorporating hydrophobic functional groups at different positions of ligands. Chem. Commun. 2011, 47, 7377–7379.
(6) Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162.
(7) Zhang, J. P.; Zhang, Y. B.; Lin, J. B.; Chen, X. M. Metal azolate frameworks: from crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033.
(8) Rowsell, J. L. C.; Millward, A. R.; Park, K. S.; Yaghi, O. M. Hydrogen sorption in functionalized metal-organic frameworks. J. Am. Chem. Soc. 2004, 126, 5666–5667.
(9) Kleij, A. W.; Kuil, M.; Tooke, D. M.; Lutz, M.; Spek, A. L.; Reek, J. N. H. ZnII-salphen complexes as versatile building blocks for the construction of supramolecular box assemblies. Chem. Eur. J. 2005, 11, 4743–4750.
(10) Ma, D. Y.; Guo, H. F.; Lu, K.; Pan, Y.; Qin, L.; Blatov, V. A. A 1D→3D two-fold interpenetration array formed by hydrogen bonding interactions. Z.Anorg. Allg. Chem. 2012, 638, 992–995.
(11) Eddaoudi, M.; Li, H.; Yaghi, O. M. Highly porous and stable metal-organic frameworks: structure design and sorption properties. J. Am. Chem. Soc. 2000, 122, 1391–1397.
(12) Zhang, M. B.; Zhang, J.; Zheng, S. T.; Yang, G. Y. A 3D coordination framework based on linkages of nanosized hydroxo lanthanide clusters and copper centers by isonicotinate ligands. Angew. Chem. Int. Ed. 2005, 44, 1385–1388.
(13) Gu, X.; Xue, D. Selected controlled synthesis of three-dimensional 4d-4f heterometallic coordination frameworks by lanthanide carboxylate subunits and silver centers. Cryst. Growth Des. 2006, 6, 2551–2557.
(14) Blatov, V. A.; O΄Keeffe, M.; Proserpio, D. M. Vertex-, face-, point-, Schläfli-, and Delaney-symbols in nets, polyhedra and tilings: recommended terminology. CrystEngComm. 2010, 12, 44–48.
(15) Biradha, K.; Sarkar, M.; Rajput, L. Crystal engineering of coordination polymers using 4,4΄-bipyridine as a bond between transition metal atoms.Chem. Commun. 2006, 4169–4179.
(16) Albeia, B.; EI Fallah, M. S.; Ribas, J.; Folting, K.; Christou, G.; Hendrickson, D. N. Two new mixed-valence manganese complexes of formula[Mn4O2(Xbenzoato)7(bpy)2] (X = 2-Cl, 2-Br) and the crystal structure of the 2-Cl complex: ground-state spin variability in the [Mn4O2]7+complexes.Inorg. Chem. 2001, 40, 1037–1044.
(17) Clegg, W.; Harbron, D. R.; Hunt, P. A.; Little, I. R.; Straughan, B. P. Structures of polymeric zinc 3,3-dimethylacrylate and zinc 2-chlorobenzoate.Acta. Cryst. 1990, C46, 750–753.
(18) Fu, Z. Y.; Wu, X. T.; Dai, J. C.; Hu, S. M.; Du, W. X.; Zhang, H. H.; Sun, R. Q. The structure and fluorescence properties of two novel mixed-ligand supramolecular frameworks with different structural motifs. Eur. J. Inorg. Chem. 2002, 7, 2730–2735.
(19) Ma, D. Y.; Deng, G. H. A novel one-dimensional zinc coordination polymer containing 4-methylbenzoate and 4,4΄-bipyridine. Acta Cryst. 2008, C64, m271–m273.
(20) Bruker. APEXII software, Version 6.3.1, Bruker AXS Inc, Madison, Wisconsin, USA (2004).
(21) Sheldrick, G. M. SHELXS-97 and SHELXL-97. University of Göttingen, Germany 1997.
(22) Zhang, J.; Gao, S.; Che, Z. M. A blue photoluminescent 2D coordination polymer constructed by dinuclear zinc(II) subunits [Zn2(oz)2] (Hoz =2-(2΄-hydroxyphenyl)-2-oxazoline) and dicyanamide. Eur. J. Inorg. Chem. 2004, 956–959.
(23) Zhang, E.; Hou, H.; Meng, X.; Liu, Y.; Fan, Y. Ferrocenyl functional coordination polymers based on mono-, bi-, and heterotrinuclear organometallic building blocks: syntheses, structures, and properties. Cryst. Growth Des. 2009, 9, 903–913.
(24) Chen, X. Y.; Li, G. M.; Hu, H. B.; Chen, Y. P.; Li, C. X.; Yang, Q. Y.; Sun, Y. Q.; Zhang, H. H.; Luo, R. G. The crystal structure and properties research on compounds Co(II) and Zn(II) with 3-methylbenzoic acid. Chin. J. Struct. Chem. 2011, 30, 839–847.
(25) Feng, G. D.; Jiang, L.; Zhao, W. X.; Wang, Y.; Li, Z. X. Chin. J. Inorg. Chem. 2011, 27, 1664–1668.
(26) Lee, T. W.; Lau, J. P. K.; Wong, W. T. Synthesis and characterization of coordination polymers of Zn(II) with 1,3,-bis(4-pyridyl)propane and 4,4΄-pyridine ligands. Polyhedron 2004, 23, 999–1002.
(27) Li, H.; Yin, K. L.; Xu, D. J. Catena-poly[[bis(1H-benzimidazole)(salicylate)copper(II)]μ-salicylate]. Acta Cryst. 2005, C61, m19–m21.
(28) Zheng, Y.; Xu, D. M.; Liu, S. X. Three mononuclear copper complexes of 9-hydroxy-9H-fluorene-9-carboxylic acid. Inorg. Chim. Acta 1999, 294, 163–169.
(29) Tian, Z.; Lin, J.; Su, Y.; Wen, L.; Liu, Y.; Zhu, H.; Meng, Q. J. Flexbile ligand, structural, and topological diversity: isomerism in Zn(NO3)2coordination polymers. Cryst. Growth Des. 2007, 7, 1863–1867.
(30) Liu, B.; Qiu, Q. C.; Peng, G.; Ma, L.; Jin, L. M.; Cai, J. B.; Deng, H. Construction and photoluminescence properties of two novel zinc(II) and cadmium(II) benzyl-tetrazole coordination polymers. Inorg. Chem. Commun. 2009, 12, 1200–1203.
- 结构化学的其它文章
- Synthesis and Crystal Structure of the First Complex Derived from a Quinolyl Substituted 1,2,4-Triazole①
- Synthesis, Crystal Structure and Photoluminescence of a New Cd-organic meso-Helicate①
- Non-merohedrally Twinned Crystal Structure of the Co-crystal Ethyl 6-(2-[5-(Ethoxycarbonyl)-pyridin-2-yl]-1,2-dihydroxyethyl)pyridine-3-carboxylateethyl 6-(2-[5-(Ethoxycarbonyl)pyridin-2-yl]-2-hydroxyacetyl)pyridine-3-carboxylate (0.69/0.31)
- Synthesis, Crystal Structure and Antibacterial Activity of 4,4΄-Hydrazine-1,2-diylidenebis(methanylylidene)bis(2-ethoxyphenol)①
- A Trinuclear Ni(II) Mixed-ligand Complex Containing Pentadentate N-butylsalicylhydrazide and Monodentate Imidazole:Synthesis, Characterization and Crystal Structure①
- Synthesis, Structure and Photoluminescence of a New Cd(II)Coordination Polymer Based on 4-(Carboxymethoxy)-benzoic Acid and 1,10-Phenanthroline Derivative①

