Non-merohedrally Twinned Crystal Structure of the Co-crystal Ethyl 6-(2-[5-(Ethoxycarbonyl)-pyridin-2-yl]-1,2-dihydroxyethyl)pyridine-3-carboxylateethyl 6-(2-[5-(Ethoxycarbonyl)pyridin-2-yl]-2-hydroxyacetyl)pyridine-3-carboxylate (0.69/0.31)
ZHANG Wen-Hu NG Seik Weng (Institute of Mterils Reserh & Engineering, 3 Reserh Link, Singpore 117602, Singpore) (Deprtment of Chemistry, University of Mly, 50603 Kul Lumpur, Mlysi) (Chemistry Deprtment, King Adulziz University, Jeddh, Sudi Ari)
A ‘co-crystal’ is roughly defined as a crystalline compound having two distinct species[1]; this definition serves to define multi-component crystals in which the individual components are linked by, for example, hydrogen bonds. Not included in the broad definition, however, are the crystals in which the two components are superimposed over each other;with such superimposition, the crystal structure may,and often does, manifest disorder. 5-Ethoxycarbonyl-2-hydroxypyridine dissolved in N,N-dimethylformide undergoes dimerization in the presence of indium trichloride to yield C2H5O2CC5H3N—CH(OH)—CH(OH)—C5H3NCO2C2H5; a portion of the product is oxidized to C2H5O2CC5H3N—CH(OH)—C(O)—C5H3NCO2C2H5. The refinement of this co-crystal[2]and the determination of the ratio of the two overlapping components is presented here.
The ethylene link connecting the two aromatic rings consists of a hydroxymethylene HO–C portion that is disordered with respect to a carbonyl O=C portion. The carbon atom was refined as two atoms(C1, C1') as was the oxygen atom (O1, O1'). The temperature factors of the primed atoms were set to those of the unprimed ones. Carbon-carbon distances were restrained to 1.50 ± 0.01 Å and the C–Ocarbonyldistance to 1.25 ± 0.01 Å; the C–Ohydroxydistance was not restrained. Additionally, the 1,3-related C··C distance for the carbonyl unit was restrained to 2.35 ± 0.02 Å. The four-atom Caliphatic–C(=O)–Caromaticwas restrained to within 0.01 Å of a plane.As the disorder refined to a 0.845(5):0.155 ratio, the formula unit then consists of 0.690 of the 1,2-dihydroxylethyl component and 0.310 of the 2-hydroxyacetyl component. Carbon-bound H-atoms were placed in the calculated positions (C–H 0.95 to 0.98 Å, Uiso(H) 1.2 to 1.5Ueq(C)) and were included in the refinement in the riding model approximation; the hydroxy H-atom was located in a difference Fourier map, and was refined with a distance restraint O–H 0.84 ± 0.01 Å and its temperature factor was refined.The two component formula units are illustrated in Figs. 1 and 2.

Fig. 1. Thermal ellipsoid plot of the major C18H20N2O6 component at the 70% probability level
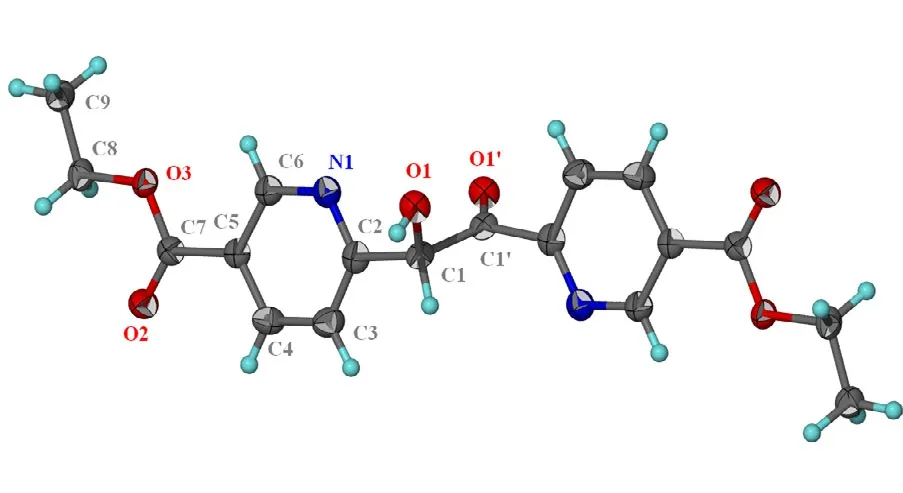
Fig. 2. Thermal ellipsoid plot of the minor C18H18N2O6 component at the 70% probability level
The crystal is a non-merohedral twin, with a minor component of 37.7(2)% (Fig. 3). The Rintfor the major component was 0.021 and that of the minor one was 0.034; the overall Rintwas 0.025. The two reciprocal lattices do not coincide exactly but are related by the approximate twin law (–1 0 0 0 –1 0 1/3 0 1).
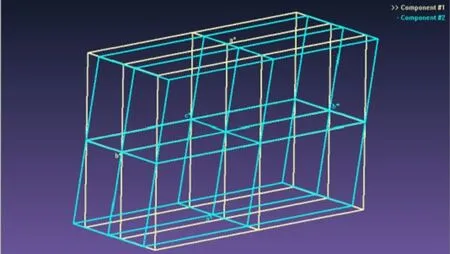
Fig. 3. Unit cells of the two twin components
If only one set of the reflections is measured,PLATON[3]and ROTAX[4]can separate the twin domains based on the fact that a significant number of reflections will have Iobssignificantly larger than Icalc. Both programs treat twinning in an ‘after-thefact’ treatment only. In this study, twinning was already detected in a pre-experiment and ‘twinned’data were collected; owing to the extra data, the ratio of unique:expected reflections of 1.678 exceeded the usual value of unity for untwined crystals. 2-Amino-4-phenyl-5,6-dihydrobenzo[h]quinoline-3-carbonitrile[5]forms a co-crystal with 3-amino-1-phenyl-9,10 dihydrophenanthrene-2,4-dicarbonitrile in a 5:3 molar ratio; the co-crystal also features two molecules occupying the same positions. The first component is a dihydrobenzoquinoline and has only one cyano substituent whereas the second component is a dihydrophenanthrene with two cyano substituents, and the two-coordinate N atom of the first molecule occupies the same site as the three-coordinate C atom of the second molecule[7]. Two other related compounds are also similarly overlapped, one with the components in a 1:19[7]ratio and the second in a 1:4[8]ratio. These ratios are worked out manually on the basis of the refined occupancies of the disordered portions of the molecule. In the present study,the ratio gives rise to a non-integral number of hydrogen atoms in the C18H19.38N2O6formula unit.
ACKNOWLEDGEMENTS
We thank the University of Malaya (grant No. UM.C/HIR/MOHE/SC/12) for supporting this study.
(1) Zukerman-Schpector, J.; Tiekink, E. R. T. Z. Kristallogr. 2008, 223, 233–234.
(2) The sphere of 6567 reflections was measured on a SuperNova diffractometer equipped with Cu-Ka radiation (l 1.54184 Å) at –173 ℃ to a 2qmaxof 150°. The major component had 2332 isolated reflections and the second 2320 isolated reflections; 3135 were overlapping ones. The reflections averaged (Rint0.025) to 3055 reflections, with 2909 satisfying the I > 2σ(I) criterion. The structure refined on 130 parameters and 7 restraints to a final R index of 0.057 (CCDC 918609).
(3) Spek, A. L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155.
(4) Cooper, R. I.; Gould, R. O.; Parson, S.; Watkins, D. J. The derivation of non-merohedral twin laws during refinement by analysis of poorly fitting intensity data and the refinement of non-merohedrally twinned crystal structures in the program CRYSTALS. J. Appl. Cryst. 2002, 35, 168–174.
(5) Indium trichloride (0.022 g, 0.1 mmol) and 5-ethoxycarbonyl-2-hydroxymethylpyridine (0.036 g, 0.2 mmol) were mixed in a glass bottle and a mixture of DMF/H2O (3/3 mL) was added. The bottle was then sealed and heated at 95 ℃ for 72 h and then cooled to room temperature. A while powder was removed by filtration; the faint yellow filtrate was allowed to evaporate to yield faint yellow crystals; these were colorless when viewed under a microscope.1H NMR (400 MHz, CDCl3) δ 9.21 (d, J = 1.6 Hz, 1H), 8.97 (d, J = 1.7 Hz, 1H), 8.39 (dd, J = 8.2, 2.1 Hz, 1H), 8.23 (dd, J = 8.5,2.1 Hz, 1H), 8.06 (d, J = 8.2 Hz, 1H), 7.24 (d, J = 8.5 Hz, 1H), 6.99 (s, 1H), 4.38–4.46 (m, 4H), 1.49–1.35 (m, 6H) ppm.13C {1H} NMR (101 MHz,CDCl3): δ 164.24, 161.27, 156.97, 150.62, 147.01, 138.42, 138.15, 126.71, 122.50, 120.32, 97.67, 61.84, 61.72, 14.68 ppm. MS (ESI) (CH2Cl2, 150℃): 747.17 m/z: [M(diketone form) + M(acyloin form) + CH3OH + H]+. IR (KBr pellet): 2996 (w), 2957 (w), 2923 (w), 2897 (w), 2849 (w), 1714(s), 1590 (s), 1555 (s), 1473 (s), 1448 (s), 1394 (s), 1368 (s), 1298 (s), 1267 (s), 1131 (s), 1106 (s), 1067 (s), 1020 (s), 970 (s), 944 (s), 886 (m), 864(s), 828 (m), 792 (m), 729 (s), 695 (w), 652 (w), 637 (w), 525 (w), 513 (w), 497 (w) cm–1.
(6) Asiri, M. A.; Al-Youbi, A. O.; Faidallah, M. H.; Ng, S. W. 2-Amino-4-phenyl-5,6-dihydrobenzo[h]quinolone-3-carbonitrile 3-amino-1-phenyl-9,10-dihydrophenanthrene-2,4-dicarbonitrile (5/3). Acta Cryst. 2011, E67, o2872.
(7) Asiri, M. A.; Al-Youbi, A. O.; Faidallah, M. H.; Ng, S. W. 2-Amino-4-(3,4-dimethoxy)-5,6-dihydrobenzo[h]quinolin-3-carbonitrile 3-amino-1-(3,4-dimethoxylphenyl)-9,10-dihydrophenanthrene-2,4-dicarbonitrile (1/19). Acta Cryst. 2011, E67, o2873.
(8) Asiri, M. A.; Al-Youbi, A. O.; Faidallah, H. M.; Ng, S. W. 2-Amino-4-(4-chlorophenyl)-5,6-dihydrobvenzo[h]quinolone-3-carbonitrile 3-amino-1-(4-chlorophenyl)-9,10-dihydrophenanthrene-2,4-dicarbonitrile (1/4). Acta Cryst. 2011, E67, o2874.
- 结构化学的其它文章
- Synthesis and Crystal Structure of the First Complex Derived from a Quinolyl Substituted 1,2,4-Triazole①
- Synthesis, Crystal Structure and Photoluminescence of a New Cd-organic meso-Helicate①
- One New Zinc Coordination Polymer Based on 2-Chloro-benzoate and 4,4΄-Bipyridine:Synthesis, Crystal Structure and Luminescence①
- Synthesis, Crystal Structure and Antibacterial Activity of 4,4΄-Hydrazine-1,2-diylidenebis(methanylylidene)bis(2-ethoxyphenol)①
- A Trinuclear Ni(II) Mixed-ligand Complex Containing Pentadentate N-butylsalicylhydrazide and Monodentate Imidazole:Synthesis, Characterization and Crystal Structure①
- Synthesis, Structure and Photoluminescence of a New Cd(II)Coordination Polymer Based on 4-(Carboxymethoxy)-benzoic Acid and 1,10-Phenanthroline Derivative①

