A Trinuclear Ni(II) Mixed-ligand Complex Containing Pentadentate N-butylsalicylhydrazide and Monodentate Imidazole:Synthesis, Characterization and Crystal Structure①
CHEN Xiao-Hua WU Qiong-Jie LV Wei YANG Ming-Xing CHEN Li-Juana (College of Materials Siene and Engineering,Fujian Normal University, Fuzhou 350007, China) (College of Life Siene, Fujian Agriulture and Forestry University, Fuzhou 350002, China) (College of Chemistry and Chemial Engineering,Fujian Normal University, Fuzhou 350007, China)
1 INTRODUCTION
In recent years, N-acylsalicylhydrazides (H3xshz)have attached much interest as effective pentadentate constructing ligands due to their ease formation of metallacrowns with different ring-sizes and nuclearities based on trivalent 3d metal ions such as Fe3+,Co3+, Ga3+and Mn3+[1-7]. Some of these complexes have potential applications in chemically modified electrodes, anion-selective separation agents, magnetic materials and biological activities[1-7]. Meanwhile, H3xshz have also been utilized to construct trinuclear complexes based on bivalent 3d metals like Ni2+, Cu2+and Zn2+[8-17]. Of further importance is the fact that these kinds of trinuclear complexes had potential applications for their antibacterial[11,12,15],antitumor[10]and magnetic[13]activities. As for the reaction of H3xshz and metal salts, the conditions such as the selection of solvent and adjusting the pH value are very important[14]. Among those trinuclear nickel(II) complexes, pyridine molecules have always been used as the ancillary ligands[9-17]because they can not only adjust the pH of the reaction system, but also coordinate to the nickel ions and complete the coordination geometries[14].However, neutral N-donor heterocyclic imidazole has rare been used as the ancillary ligand in those reported trinuclear nickel(II) complexes[8]. As a contribution to this field, we report the synthesis, characterization and crystal structure of the trinuclear nickel(II) compound [Ni3(bushz)2(Himdz)2(H2O)2]·2DMF with N-butylsalicylhydrazide (H3busha) and monodentate N-heterocycle Himdz. The self-assembly of the complex molecules via intermolecular hydrogen bonds involving the heterocyclic imidazole NH group has been demonstrated. The complex is connected to form a supramolecule with an infinite three-dimensional network through intermolecular hydrogen bonds.
2 EXPERIMENTAL
2.1 Materials and methods
The ligand (H3bushz) was synthesized according to the reported method[15]. All other chemicals were of analytical reagent grade and used without further purification.
Elemental analyses for C, H, and N were carried out with an Elementar Vario EL III microanalyser.IR spectra were recorded on a Perkin-Elmer spectrum 2000 spectrophotometer with KBr pellets in the range of 4000~400 cm-1. Electronic spectra in solid state were recorded on a Perkin Elmer lamda 900 spectrometer. Thermogravimetric curves were measured on a Perkin-Elmer Diamond TG/DTA at a heating rate of 5 ℃·min-1from room temperature to 800 ℃ under air. Cyclic voltammetry was performed by using a CHI-660 electrochemical analysis system with a glass-carbon working electrode, a Pt wire auxiliary electrode and a Ag–AgCl reference electrode.
2.2 Synthesis of complex 1
To a mixture solution of MetOH (15 mL), DMF (6 mL) of H3bushz (22.2 mg, 0.1 mmol) and Himdz(13.6 mg, 0.2 mmol), a methanol solution (10 mL)of Ni(CH3COO)2·4H2O (24.8 mg, 0.1 mmol) was added gradually with stirring. The resulting red solution was further stirred for 1 h and filtered. The red crystals separated after several days were collected in 35% yield based on Ni. Anal. Calcd. for C34H48N10Ni3O10(%): C, 43.77; H, 5.19; N, 15.01.Found (%): C, 43.98; H, 5.35; N, 14.72. IR (KBr pellet, cm-1): 3253 (m); 2980 (m); 1603 (vs); 1565(s); 14689s), 1379 (m); 1265 (m); 573 (w); 540 (w),420(w).
2.3 Crystal structure determination
A red single crystal with dimensions of 0.38mm ×0.24mm × 0.08mm was mounted on a Rigaku RAXIS RAPID Weisserberg IP diffractometer equipped with a graphite-monochromatic MoKα radiation(λ = 0.7103 Å) for data collection at 293(2) K using an ω scan mode. A total of 17770 reflections were collected in the range of 2.99≤θ≤27.46º, of which 4590 (Rint= 0.0427) were independent and 3660 were observed with I > 2σ(I). The structure was solved by direct methods with SHELXS-97[18]and refined by full-matrix least-squares calculations with SHELXL-97[19]. All of the non-hydrogen atoms were refined anisotropically. H atoms bound to O(4)and N(4) atoms were located in a difference Fourier map and refined isotropically with O–H and N–H distance restraints of 0.85 and 0.86 Å, respectively.H atoms bound to the C atoms were placed in their calculated positions. The final R = 0.0359 and wR =0.0771 (w = 1/[σ2(Fo2) + (0.0347P)2+ 0.5596P],where P = (Fo2+ 2Fc2)/3), S = 1.086, (Δ/σ)max=0.001, (Δρ)max= 0.300 and (Δρ)min= –0.272 e/Å3.
3 RESULTS AND DISCUSSION
3.1 Crystal structure of complex 1
The selected bond lengths and bond angles for complex 1 are listed in Table 1. Single-crystal X-ray diffraction analysis of 1 reveals that the crystal is of monoclinic, space group P21/c with Z = 2. As shown in Fig. 1, the symmetric unit of 1 consists of three Ni(II) ions, two bushz3-ligands, two imidazole molecules and two water molecules. The ligand serves as both bidentate for the central Ni(II) cation and, at the same time, tridentate for the two terminal Ni(II) ions. Thus, three Ni(II) centers (Ni(1), Ni(2),Ni(1a)) are bridged by two μ2-bridging bushz3-ligands, furnishing a centrosymmetric trimetallic unit with the Ni(1)··Ni(2) separation in 4.607(2) Å and the Ni(1)··Ni(2)··Ni(1a) angle of 180.0º. The coordination geometry of the three Ni(II) ions follows a square-planar/octahedral/square-planar mode. The central Ni(2) atom possesses an elongated octahedral geometry, in which the basal plane is defined by two carbonyl oxygen atoms and two hydrazine nitrogen atoms from the two bushz3-ligands (Ni(2)–O(2) = Ni(2)–O(2a) = 2.029(2) Å,Ni(2)–N(2) = Ni(2)–N(2a) = 2.060(2) Å). The apical positions are occupied by two coordinated water oxygen atoms (Ni(2)–O(4) = Ni(2)–O(4a) = 2.153(2)Å). The terminal Ni(1) atom is coordinated in a square-planar configuration composed of the other hydrazine nitrogen (Ni(1)–N(1) = 1.823(2) Å),carbonyl oxygen (Ni(1)–O(3) = 1.844(2) Å) and phenolic oxygen (Ni(1)–O(1) = 1.824(2) Å) of one bushz3-ligand, as well as one coordinated Himdz nitrogen (Ni(1)–N(3) = 1.907(2) Å). There is nearly no deviation of the Ni(1) center from the N2O2square-plane. The Ni(1) atom deviates from the basal plane by 0.0098(7) Å. The bond angles around Ni(1) range from 83.92(8) to 178.19(8)º, and those around Ni(2) vary from 78.99(7) to 180.00(7)º. The Ni–N and Ni–O distances are in the ranges of 1.823(2)~2.060(2) Å and 1.824(2)~2.153(2) Å,respectively, which drop in the normal scope of values reported for nickel complexes based on N-acylsalicylhydrazide ligands[8-17].
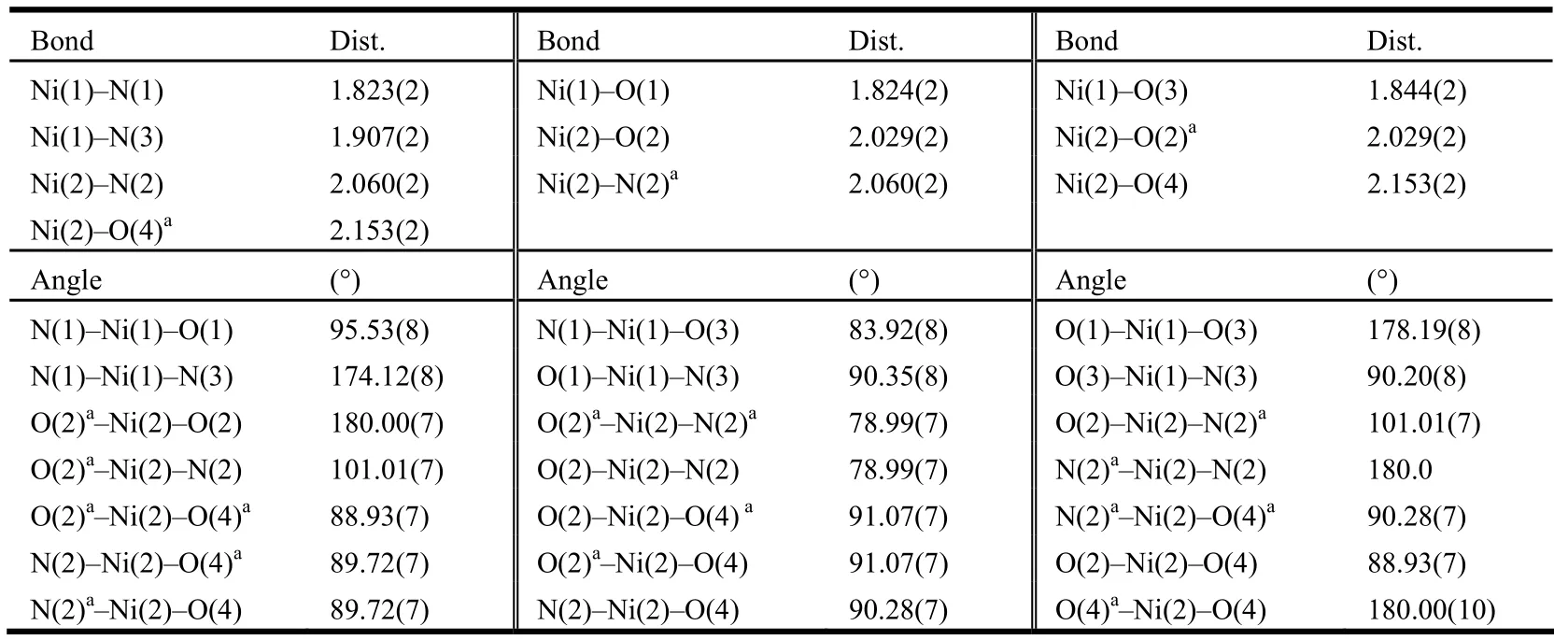
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for Complex 1
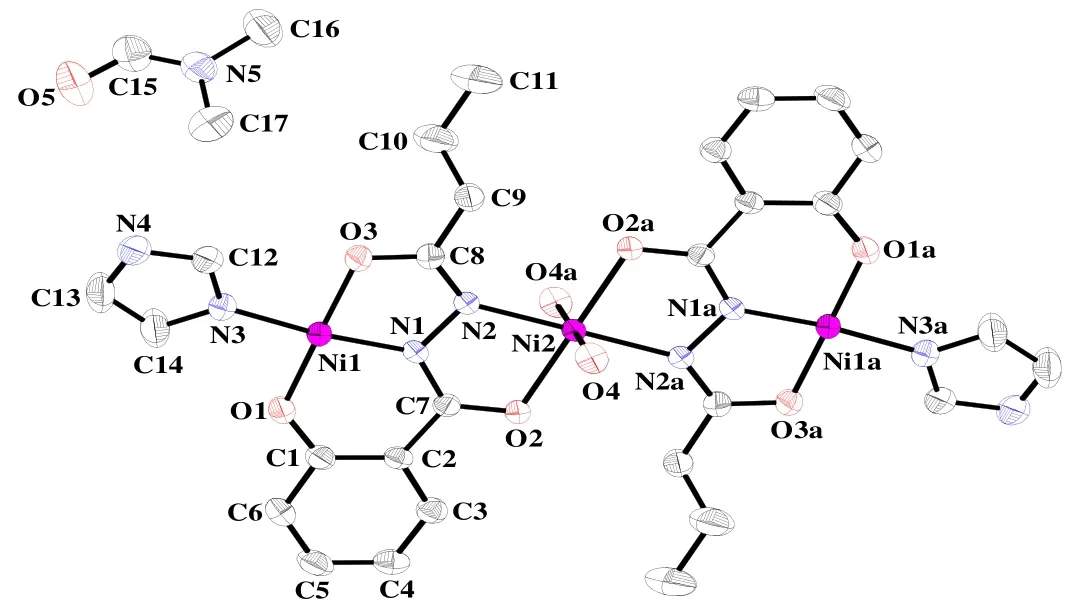
Fig. 1. Molecular structure of 1 at 50% probability thermal ellipsoids.H-atoms were omitted. Symmetry code: (a) –x+1, –y+1, –z+1
There are many different hydrogen bonds in complex 1 (Table 2), playing an important role in the supramolecular assembly process. Firstly, there are intermolecular hydrogen bonding interactions between dissociative DMF molecules and coordinated water molecules (O(4)–H(4B)··O(5b), 2.848(3) Å,166(3)º; O(4)–H(4C)··O(5c) 2.802(2) Å, 164(3)º,symmetry codes: b: –x+1, y+1/2, –z+1/2; c: x+1,–y+1/2, z+1/2), resulting in an extended onedimensional chain along the a axis (Fig. 2). In this uniform chain-like arrangement, two Ni(2) atoms are bridged by an 8-membered ring, with the Ni(2)··Ni(2) distance being 7.706(2) Å. Meanwhile,there are intermolecular hydrogen bonding interactions between carbonyl oxygen O(2) and nitrogen N(4) of Himdz (N(4)–H(4D)··O(2d) , 2.955(2) Å,171(3)º, symmetry code: d: –x+1, y–1/2, –z+1/2),leading to an extended two-dimensional layer parallel to the bc plane. These layers and chains are linked to construct a novel three-dimensional hydrogen bonding framework (Fig. 3).
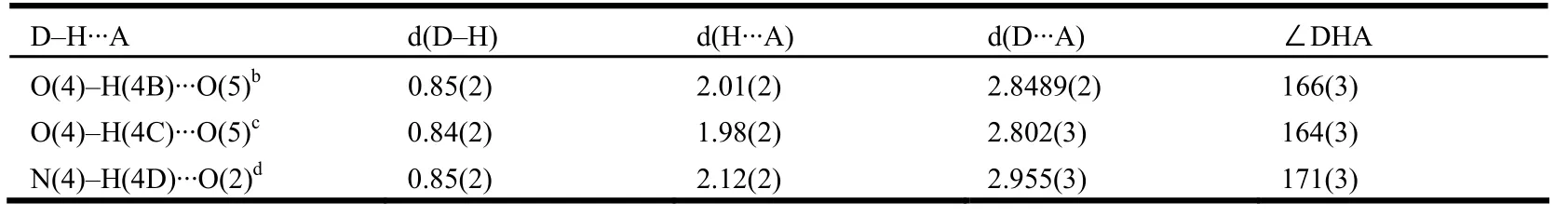
Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°) of 1

Fig. 2. One-dimensional chain of 1 formed through O–H··O hydrogen bonding interactions
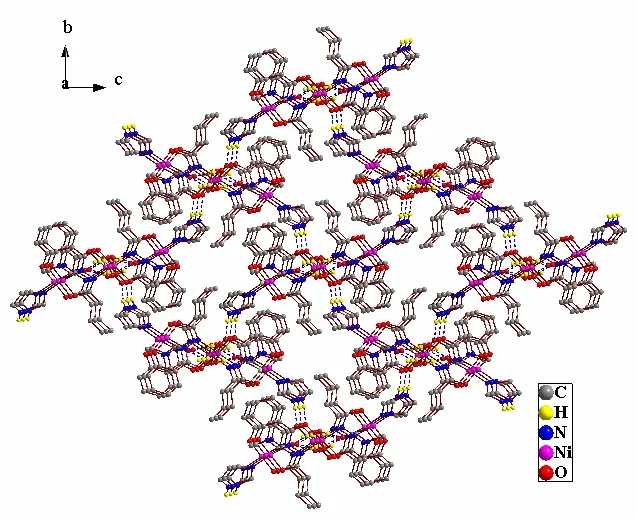
Fig. 3. Three-dimensional supramolecular network structure of 1 along the bc plane
3.2 UV spectra
The electronic spectra were recorded in solid state for complex 1. As shown in Fig. 4, the electronic spectra exhibit one very weak d-d transition band at 583 nm[20]. The absorption in the 200~320 nm range should be assigned to the π-π* and n-π* transitions of the ligand[20-21]. In addition, the absorption at 363 nm may be due to the ligand-to-metal charge transfer transition (MLCT)[20].
3.3 Cyclic voltammogram
The cyclic voltammogram of the title complex was measured in DMF with 0.1 mol·dm-3(n-Bu)4NClO4(TBAP) as the supporting electrolyte in the potential range of 0~1.0 V with 0.08 V/s scan rate (Fig. 5). It shows only one pair of redox peaks corresponding to the redox couple of Ni(III)/Ni(II),Epa= 0.498 V and Epc= 0.372 V, which are consistent with those reported in the mononuclear nickel complexes [Ni(en)2(dpas)2][22]and [Ni(L)(Mf)]n[23].The average formal potential (E1/2= (Epa+ Epc)/2) is 0.435 V. The peak-to-peak separation is 0.126 V,exhibiting a quasi-reversible electrode process[24].
3.4 Thermogravimetric analyses
To study the stability of the title complex, thermogramimetric analysis (TGA) was performed. The TG curve (Fig. 6) shows two clear and well separated weight loss steps. The first weight loss of 34.52%(calcd. 34.13%) from 147 to 271 ℃ results from the departure of DMF, water and imidazole molecules, while the second step between 327 and 542 ℃is attributed to the decomposition of organic ligands(obsd. 49.55%, calcd. 49.86%). The final residuals may be NiO.
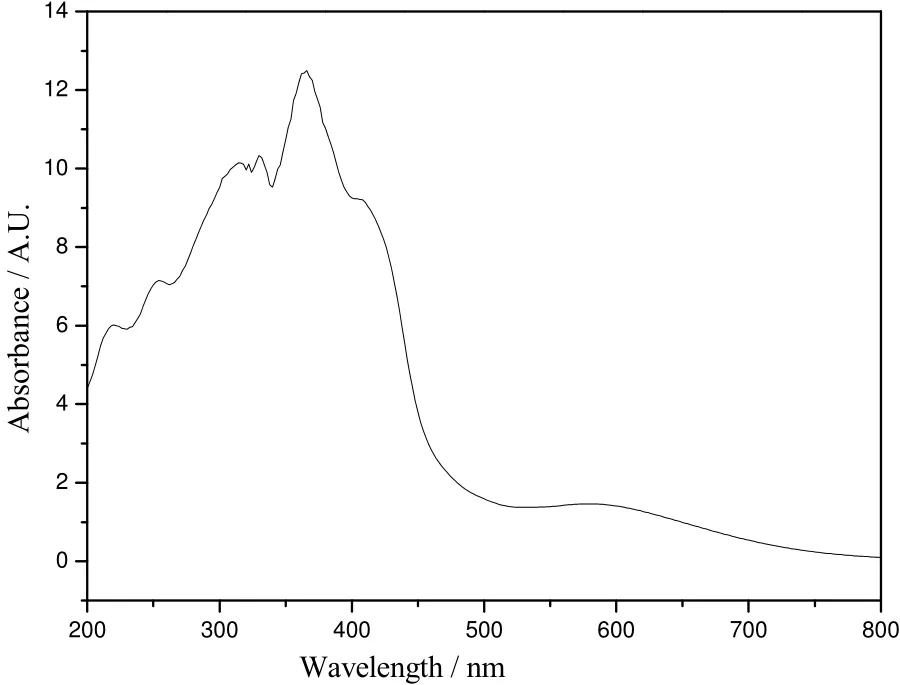
Fig. 4. UV spectra of compound 1
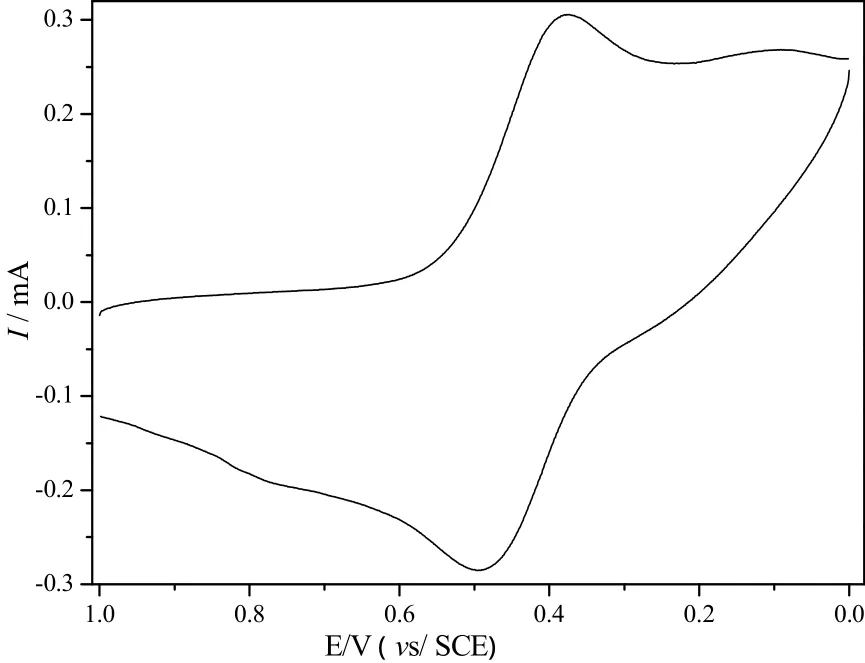
Fig. 5. Cyclic voltammogram of compound 1
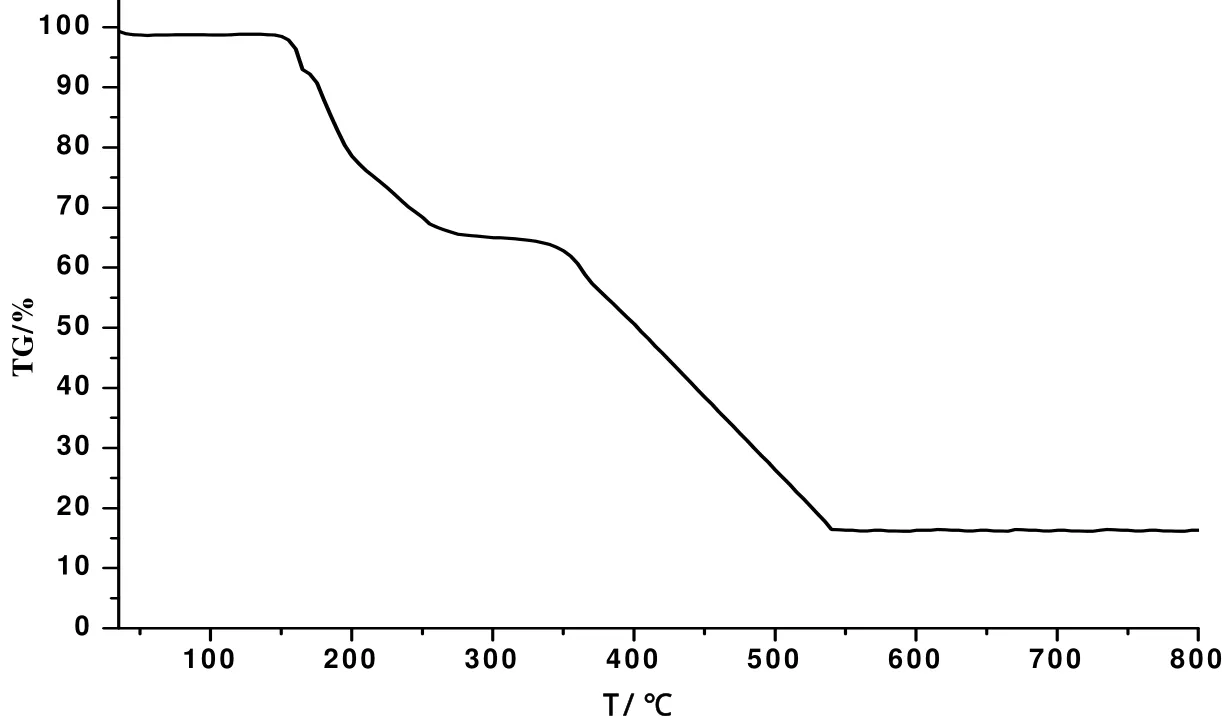
Fig. 6. TG curve of compound 1
(1) Dou, J. M.; Liu, M. L.; Li, D. C.; Wang, D. Q. Synthesis, characterization, and crystal structure of two manganese metallacrowns:30-metallacrown-10 and 18-metallacrown-6 with ligands derived from 3-hydroxy-2-naphthalenecarbohydrazide. Eur. J. Inorg. Chem. 2006,4866–4871.
(2) Xiao, F. P.; Jin, L. F.; Cheng, G. Z.; Ji, Z. P. Two novel 18-metallacrown-6 complexes: synthesis, structural characterization and bioactivity.Polyhedron 2007, 26, 2695–2702.
(3) Liu, S. X.; Lin, S.; Lin, B. Z.; Lin, C. C.; Huang, J. Q. Metallacrown-10 compounds: [Mn(C14H9N2O3)(CH3OH)10·5CH2Cl2·10CH3OH·H2O] and[Fe(C14H9N2O3)(CH3OH)10·3CH2Cl2·12.5CH3OH·5H2O]. Angew. Chem. Int. Ed. 2001, 49, 1084–1087.
(4) Lin, S.; Liu, S. X.; Chen, Z. Synthesis, structure, and magnetism of a ferric 24-azametallacrown-8 complex. Inorg. Chem. 2004, 43, 2222–2224.
(5) Lin, S.; Liu, S. X.; Lin, B. Z. Synthesis, crystal structure and magnetic properties of a novel iron(III) 18-azametallacrown-6 compound. Inorg. Chim.Acta 2002, 328, 69–73.
(6) Kim, I.; Kwak, B.; Lah, M. S. A series of nanometer-sized hexanuclear Co-, Fe-, and Ga-metallamacrocycles. Inorg. Chim. Acta 2001, 317, 12–20.
(7) Jin, L. F.; Xiao, F. P.; Cheng, G. Z.; Ji, Z. P. Synthesis, crystal structure and bioactivity of a novel 18-metallacrown-6 [Mn6(H2O)6(abshz)6]·36H2O.Inorg. Chem. Commun. 2006, 9, 758–760.
(8) Chen, X. H.; Wu, Q. J.; Lu, W.; Yang, M. X.; Chen, L. J. A 3D hydrogen bonding framework of trinuclear Ni(II) complex in mixed ligands system:structural and magnetic studies. Inorg. Chem. Commun. 2011, 14, 694–696.
(9) Chen, X. H.; Xie, C. L.; Yang, M. X.; Chen, L. J. Bis[μ-N΄-(2-methyl-1-oxidopropanylidene)-2-oxidobenzohydrazidato]tetrapyridinetrinickel(II).Acta Cryst. 2011, E67, m1308–m1309.
(10) Qiu, X. Y.; Xu, M. T.; Zhu, H. L.; Liu, W. S.; Zhao, W. X. Synthesis, crystal structure and antitumor activity of a new trinuclear Ni(II) complex Ni3(C14H8N3O5)2(C5H5N)4. Chin. J. Inorg. Chem. 2010, 26, 355–359.
(11) Luo, W.; Meng, X. G.; Xiang, J. F.; Duan, Y.; Cheng, G. Z.; Ji, Z. P. Synthesis, characterization and bioactivity of four novel trinuclear copper(II)and nickel(II) complexes with pentadentate ligands derived from N-acylsalicylhydrazide. Inorg. Chim. Acta 2008, 361, 2667–2676.
(12) Luo, W.; Meng, X. G.; Sun, X. Z.; Xiao, F. P.; Shen, J. F.; Zhou, Y.; Cheng, G. Z.; Ji, Z. P. Synthesis, crystal structure and bioactivity of a novel linear trinuclear nickel(II) complex. Inorg. Chem. Commun. 2007, 10, 1351–1354.
(13) Luo, W.; Wang, X. T.; Meng, X. G.; Cheng, G. Z.; Ji, Z. P. Synthesis, crystal structure, and magnetic properties of two bent trinuclear Cu(II)/Ni(II)complexes of ligands derived from N-acyl-salicylhydrazides. Inorg. Chem. Commun. 2008, 11, 1044–1047.
(14) Xiao, F. P.; Jin, L. F. Synthesis and characterization of two trinuclear nickel(II) complexes. Z. Anorg. Allog. Chem. 2008, 634, 397–400.
(15) Yang, M. X.; Lin, S.; Yu, P.; Chen, L. J.; Liu, S. X. Syntheses, structures and antibacterial activities of two nickel(II) complexes with N-hexanoylsalicylhydrazide ligand. Chin. J. Chem. 2005, 23, 1407–1411.
(16) Lin, S.; Yang, M. X.; Liu, S. X. Three novel trinuclear zinc(II)/nickel(II) complexes with pentadentate ligands N-nitrobenzoylsalicylhydrazidate.Polyhedron 2007, 26, 4793–4798.
(17) Yang, M. X.; Lin, S.; Chen, L. J.; Liu, S. X. Synthesis and crystal structure of the trinuclear nickel(II) complex with Schiff base ligand N-butylsalicylhydrazide. Chin. J. Inorg. Chem. 2003, 19, 433–436.
(18) Sheldrick, G. M. SHELXS-97: Program for X-ray Crystal Structure Solution. University of Göttingen, Göttingen, Germany 1997.
(19) Sheldrick, G. M. SHELXL-97: Program for X-ray Crystal Structure Refinement. University of Göttingen, Göttingen, Germany 1997.
(20) Bu, X. H.; Du, M.; Zhang, L.; Liao, D. Z.; Tang, J. K.; Zhang, R. H.; Shionoya, M. Novel nickel(II) complexes with diazamesocyclic ligands functionalized by additional phenol donor pendant(s): synthesis, characterization, crystal structures and magnetic properties. J. Chem. Soc., Dalton Trans. 2001, 593–598.
(21) Liu, H. Y.; Wang, H. Y.; Niu, D. Z.; Lu, Z. S. Synthesis, structure and fluorescence of copper(II) complex with substituted aroylhydrazone and monodentate N-heterocycle. Chin. J. Inorg. Chem. 2007, 23, 611–614.
(22) Wang, X.; Luo, F.; Gao, W. T. Synthesis, crystal structure and electrochemical properties of 2D hydrogen-bonded network complex [Ni(en)2(dpas)2].Chin. J. Inorg. Chem. 2009, 25, 154–157.
(23) Chen, X. H.; Wu, Q. J.; Chen, S. Y.; Huang, S. M.; Jiang, F. F. Synthesis, crystal structure and electrochemic of 1D chain complex [Ni(L)(Mf)]n.Chin. J. Inorg. Chem. 2010, 26, 1573–1576.
(24) Chen, S. L.; Liu, Z.; Han, G. C. Synthesis, crystal structure and properties of a novel tetranuclear nickel(II) complex with azo-enolic-2-hydroxybenzamide. Chin. J. Struct. Chem. 2012, 31, 1641–1649.
- 结构化学的其它文章
- Synthesis, Structure and Photoluminescence of a New Cd(II)Coordination Polymer Based on 4-(Carboxymethoxy)-benzoic Acid and 1,10-Phenanthroline Derivative①
- Syntheses, Crystal Structures and Properties of Two Cd(II) Coordination Polymers with Mixed Ligands of 2,5-Di(pyridin-4-yl)benzaldehyde and Carboxylates①

