Synthesis and Crystal Structure of the First Complex Derived from a Quinolyl Substituted 1,2,4-Triazole①
SHEN Guo-Ping CAO Dong-Yong JIANG Xue-Yue SHEN Xun LIU Xio-Qin ZHU Dun-Ru② (College of Chemistry nd Chemicl Engineering, Stte Key Lortory of Mterilsoriented Chemicl Engineering, Nnjing University of Technology, Nnjing 210009, Chin) (School of Chemistry & Chemicl Engineering, Fuyng Norml College, Fuyng, Anhui 236041, Chin)
1 INTRODUCTION
Triazoles are an important class of heterocyclic compounds which show various biological activities including antifungal, antimicrobial, antiviral, antiinflammatory, anti-asthmatic, anticonvulsant, antiproliferative, antibacterial and antihelmintic activities[1]. As ligands, 1,2,4-triazole and its derivatives have attracted more and more attention because of their various coordination modes and prospective applications[2,3]. Specially, some iron(II) complexes of substituted 1,2,4-triazoles show intriguing spincrossover properties which can be applied in molecular electronics, such as information storage and switching materials[4,5]. Recently, some substituted 1,2,4-triazoles and their metal complexes have been prepared by us[5-7]and other groups[8,9].As a continuation of our investigation of asymmetrically substituted 1,2,4-triazoles[10-14], we present here the syntheses of L and its iron(II) complex,1, and their crystal structures were determined by single-crystal X-ray analysis. 1 is the first complex with a quinolyl substituted 1,2,4-triazole.
2 EXPERIMENTAL
2.1 Materials and measurements
All chemicals used were of analytical grade.Solvents were purified by conventional methods.Melting points were determined using an X4 digital microscopic melting point apparatus and are uncorrected. Elemental analyses (C, H, N, S) were recorded with a Thermo Finnigan Flash 1112A elemental analyzer. IR spectra were recorded on a Nicolet Avatar 380 FT-IR instrument with KBr pellets in the range of 4,000–400 cm–1.1H NMR spectra were measured on a Bruker AM 500 MHz spectrometer at ambient temperature in CDCl3.Chemical shifts are reported in parts per million(ppm) downfield from tetramethylsilane (TMS).Electrospray ionization mass spectrum (ESI-MS)was recorded with a LCQ ADVANTAGE MAX mass spectrometer, with MeOH on the mobile phase;the flow rate of the mobile phase was 0.2 cm3min–1.The spray voltage was 4 KV and the capillary voltage was 40 V. The capillary temperature was 260 ℃.
2.2 Synthesis of L
The synthetic route of ligand L is outlined in Scheme 1[13]. A mixture of 1,4-dichloro-1-(2-quino-lyl)-4-(p-methylphenyl)-2,3-diaza-1,3-butadiene (0.68 g, 2 mmol) and p-toluidine (10 g) was refluxed at 190 ℃ for 3 h under argon atmosphere,and the excess p-toluidine was removed under reduced pressure. The residue was recrystallized from ethanol to give L as colorless crystals (0.54 g,74.8%), m.p.: 240–242 ℃ . Anal. Calcd. for C24H18N4: C, 79.54; H, 5.01; N, 15.46%. Found: C,79.42; H, 4.83; N, 15.35%. FT-IR (ν, cm-1): 2953,2919, 1596, 1496, 1470, 1448, 1441, 1168, 941, 836,756, 691, 591.1H NMR (CDCl3/500 MHz), δ (ppm):8.37–8.39 (d, 1H, Hd), 8.18–8.20 (d, 1H, Ha),7.76–7.77 (d, 1H, Hc), 7.54–7.56 (t, 1H, He),7.48–7.50 (m, 2H, Hg), 7.40–7.47 (m, 5H, PhH),7.32–7.33 (d, 2H, Hb), 7.10–7.11 (d, 2H, Hf), 2.32 (s,3H, CH3). ESI-MS: m/z 363.4 (L+H)+.
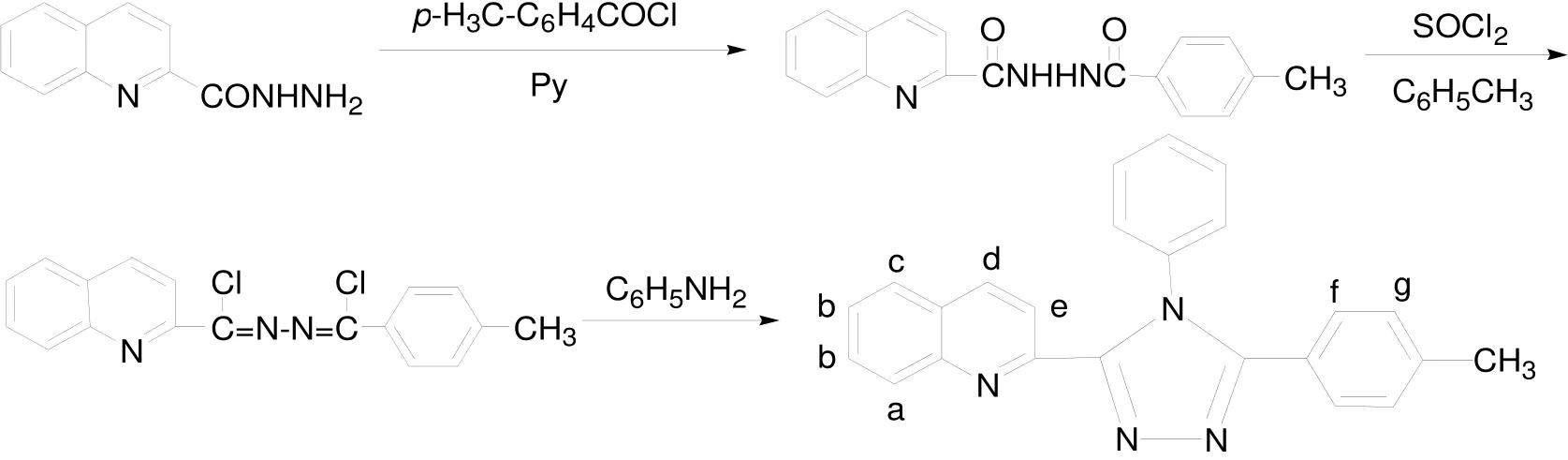
Scheme 1. Synthetic route of ligand L
2.3 Synthesis of cis-[FeL2(NCS)2] (1)
To a solution of KSCN (0.4 mmol) in anhydrous MeOH (2 mL) was added a solution of FeSO4·7H2O(0.2 mmol) in MeOH (2 mL). The mixture was stirred for 30 min, then filtered. The K2SO4precipitate was washed with 2 mL of anhydrous MeOH.The methanolic fractions containing Fe(SCN)2were collected and then poured into a methanol solution of the ligand L (0.4 mmol). The resulting red solution was left to stir for 2 hours during which a purple precipitate formed. The product was filtered and washed with H2O and dried thoroughly in vacuo to give 0.18 g of a purple powder (92.6%). The purple single crystals of 1·Me2CO suitable for X-ray diffraction were obtained by evaporation from the solution of isopropanol/acetone (15:2 v/v) under an argon atmosphere. Anal. Calcd. for C53H42FeN10OS2:C, 66.66; H, 4.43; N, 14.67; S, 6.72%. Found: C,66.53; H, 4.25; N, 14.36; S, 6.51%. FT-IR (cm-1):2948, 2916, 2054, 1597, 1470, 1451, 1441, 1419,1399, 1126, 850, 824, 771, 762, 693, 595 cm–1.
2.4 Structure determination
The well-shaped single crystals of L and 1·Me2CO were selected for X-ray diffraction study.The unit cell parameters and intensity data were collected on a Bruker SMART CCD diffractometer with a detector distance of 5 cm and frame exposure time of 10 s using a graphite-monochromated MoKα (λ = 0.71073 Å) radiation. The structures were both solved by direct methods using the SHELXS software[15]and refined on F2by fullmatrix least-squares procedures with the SHELXL software[16]. All non-hydrogen atoms were anisotropically refined. All H atoms of organic ligand were generated geometrically and allowed to ride on their respective parent atoms, but not refined. Crystallographic data of L and 1·Me2CO are summarized in Table 1. The selected bond lengths and bond angles for L and 1·Me2CO are listed in Tables 2 and 3,respectively.
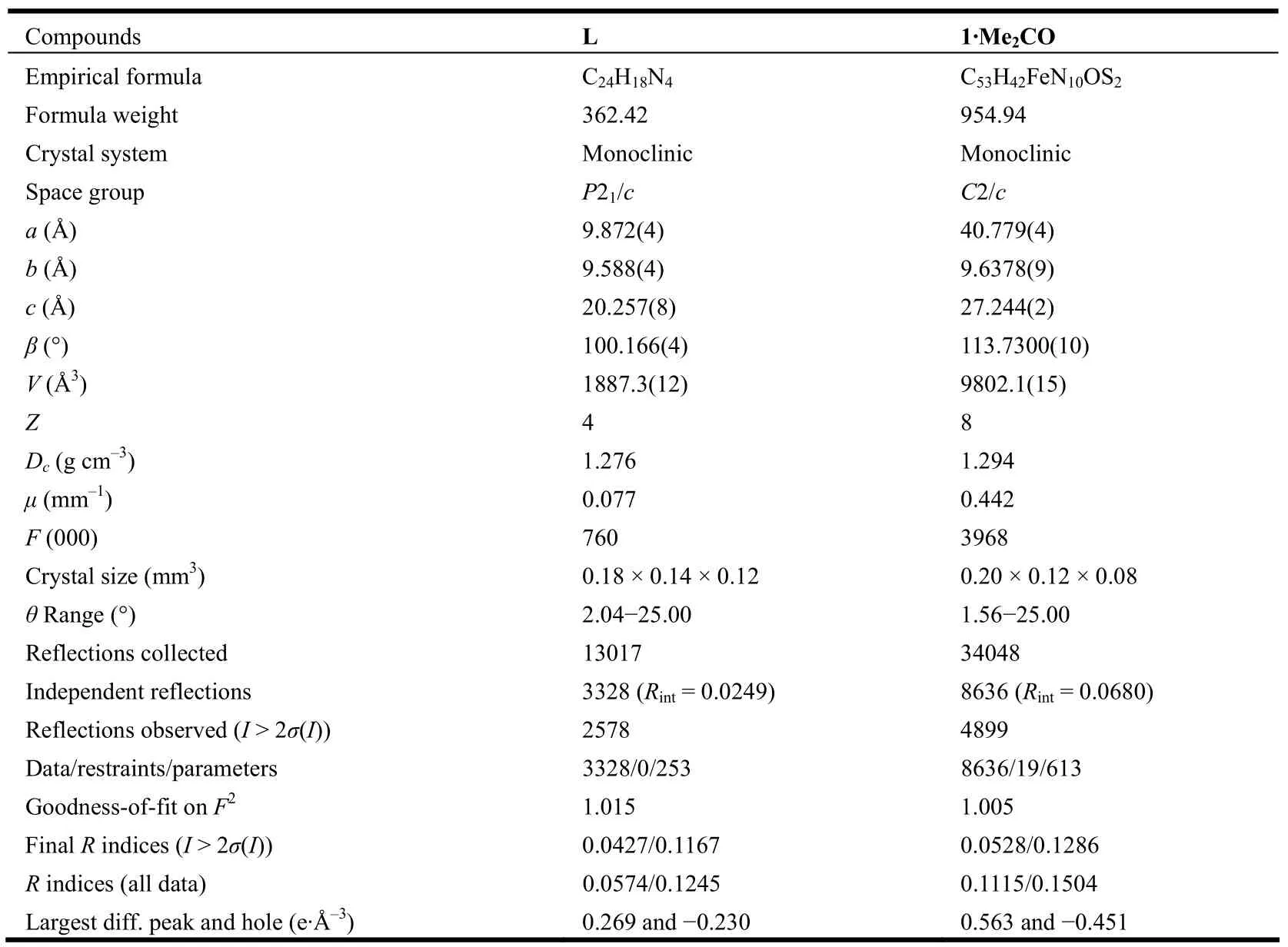
Table 1. Crystal Data and Structure Refinement for L and 1·Me2CO

Table 2. Selected Bond Lengths (Å) and Bond Angles (°) for L
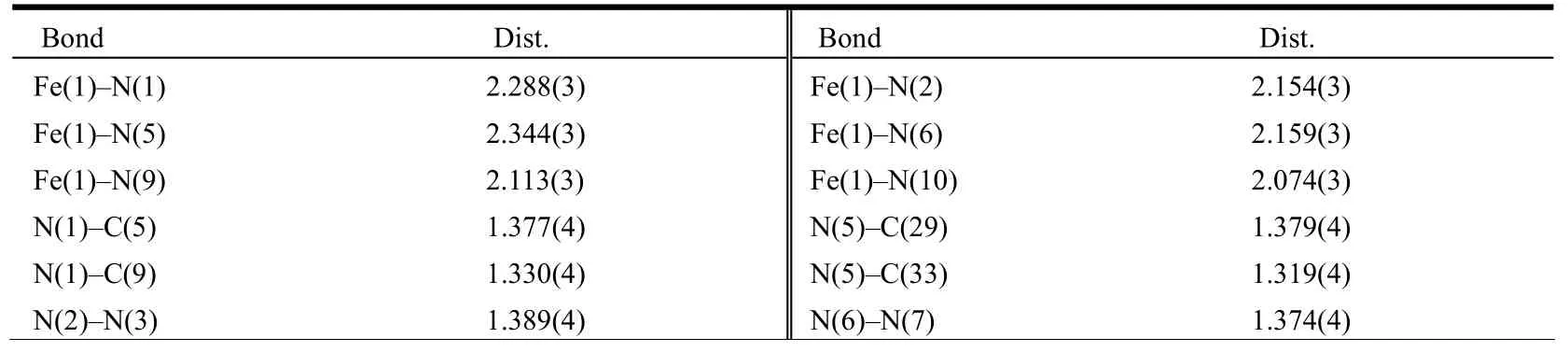
Table 3. Selected Bond Lengths (Å) and Bond Angles (°) for 1·Me2CO
To be continued
3 RESULTS AND DISCUSSION
3.1 Crystal structure of L
A perspective view of L with atomic numbering scheme is shown in Fig. 1a. X-ray structure analysis indicates that L consists of one quinolyl group, one 1,2,4-triazole ring and two phenyl rings and these rings do not share a common plane. The central 1,2,4-triazole ring is oriented at dihedral angles of 33.9(1)°, 31.9(1)° and 69.4(2)° with respect to the quinolyl group, p-methylphenyl ring and phenyl ring, respectively. The bond lengths and bond angles of L are in the normal ranges and similar to those found in literatures[6,13]. The intermolecular C(8)–H(8A)··N(2)ahydrogen bonds link two L molecules to form a dimer, and such dimers are further connected by intermolecular C(1)–H(1A)··πbinteractions to produce 1D chains along the c axis(Fig. 2a). In addition, there is an intramolecular C(13)–H(13A)··π interaction between the p-methylphenyl group and the phenyl ring in each L molecule (Table 4).
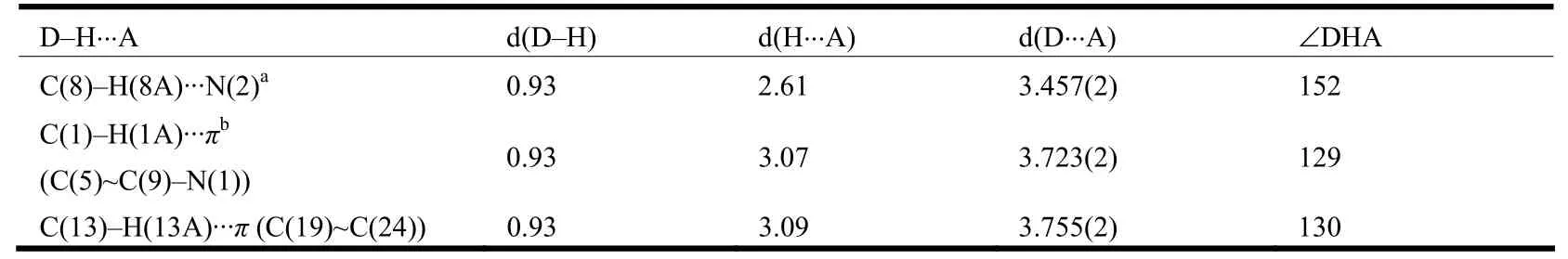
Table 4. Hydrogen Bond Lengths (Å) and Bond Angles (°) for L
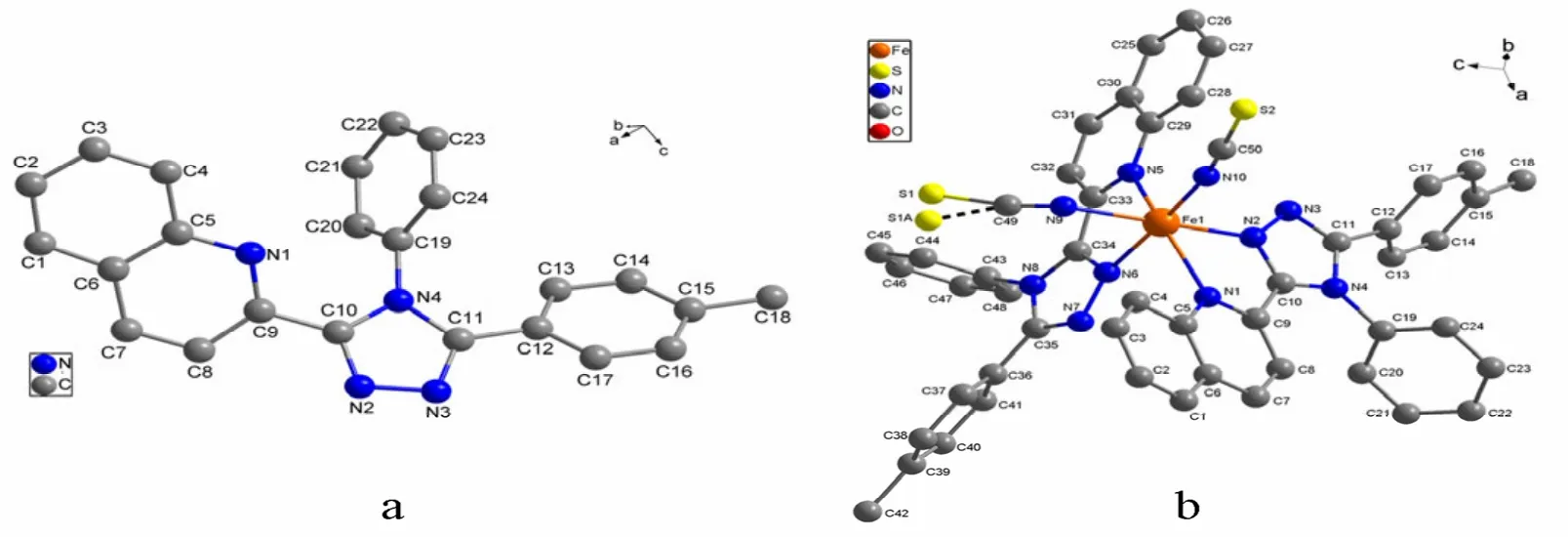
Fig. 1. Structure of L (a) and 1·Me2CO (b) with atomic labeling system.Hydrogen atoms and solvent Me2CO are omitted for clarity
3.2 Crystal structure of 1·Me2CO
The structure of 1·Me2CO with atomic numbering system is shown in Fig. 1b. The asymmetric unit contains an acetone molecule and S(1) atom of one NCS–group is highly disordered (The occupancy factors for S(1) and S(1A) are fixed as 0.6 and 0.4, respectively). The Fe(II) center is octahedrally coordinated by four N atoms from two L ligands and two N atoms from the cis-disposed NCSˉgroups. The octahedron is distorted, as the Fe–N(CS) bond lengths are shorter than Fe–N (L);the N(n)–Fe–N(m) bond angles significantly deviate from the ideal 90° due to the geometry constraints of the L ligand (Table 3). This feature has also been observed in a similar Fe(II) compound[5]. In addition, one NCS–group in the complex is linear(∠N(10)–C(50)–S(2) = 179.5(3)°), whereas the other is bent (∠N(9)–C(49)–S(1) = 169.6(4)°/∠N(9)–C(49)–S(1A) = 163.4(5)°)[14]. One Fe–N–C(S) linkage is almost linear (∠Fe(1)– N(10)–C(50)= 170.1(3)°), whereas the other is obviously bent(∠Fe(1)–N(9)–C(49) = 137.9(3)°). The L ligand coordinates to the iron atom via one N atom (N(1)or N(5)) of the quinolyl group and one N atom (N(2)or N(6)) of the triazole moiety leaving another N atom (N(3) or N(7)) of the triazole uncoordinated.The L ligand in 1·Me2CO is also nonplanar. The triazole ring with N(2) atom makes a dihedral angle of 10.5(3)º with the N(1)- containing quinolyl group,while the triazole ring with N(6) atom makes a dihedral angle of 8.9(3)º with the N(5)-containing quinolyl group. This result reveals that in order to obtain better coordination, the triazole-quinolyl twist angle in 1·Me2CO becomes smaller than that in the free L ligand. It is worthwhile to note that in the crystal structure of 1·Me2CO the phenyl ring of quinolyl group and its symmetry-related partner(symmetric operation: 0.5 – x, 1.5 – y, 1 – z) involve intermolecular offset face-to-face π-π stacking interactions with a separation of 3.546(3) Å and a centroid-centroid distance of 3.731(3) Å (Fig. 2b).Moreover, in 1·Me2CO there are some intramolecular C–H··N hydrogen bonds and intermolecular edge-to-face C–H··π interactions involving: (1)C(4)-containing quinolyl group and SCN–anion with N(9) (C(4)– H(4A)··N(9)); (2) C(28)-containing quinolyl group and SCN–anion with N(10)(C(28)–H(28A)··N(10)); (3) C(8)–H(8A) and the C(19)-containing phenyl ring (C(8)–H(8A)··π); (4)C(51)c–H(51)cand the N(2)- containing triazole ring (C(51)c–H(51)c··π) (Fig. 2b and Table 5). All these interactions are helpful to stabilize the crystal packing.
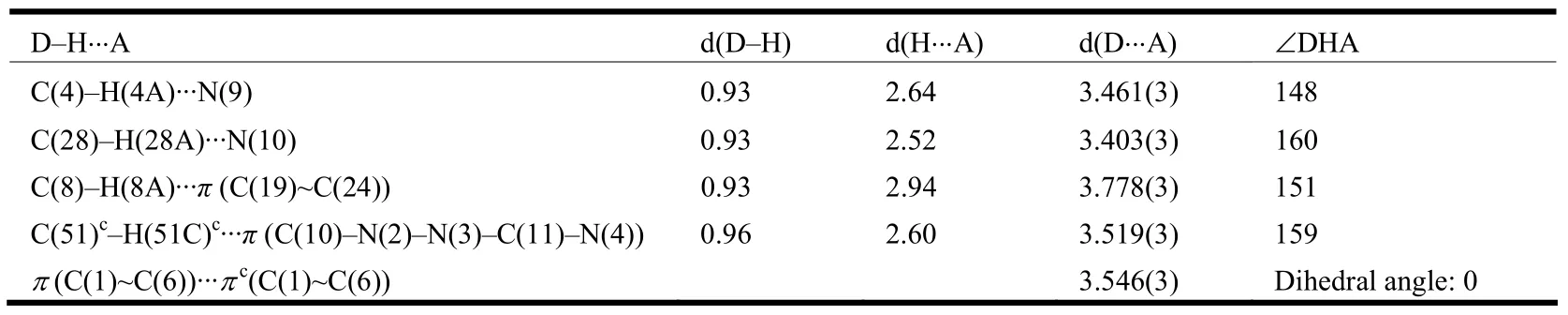
Table 5. Hydrogen Bond Lengths (Å) and Bond Angles (°) for 1·Me2CO
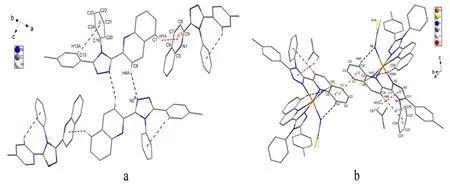
Fig. 2. Crystal packing of L (a) and 1·Me2CO (b) showing the hydrogen bonding and CˉH··π interactions
3.3 IR spectrum
In the IR spectrum of ligand L, two weak bands at 2953 and 2919 cm–1can be assigned to the CˉH stretching vibration of -CH3groups. The bands at 1596, 1496, 1470, 1448 and 1441 cm-1can be attributed to the aromatic ring vibrations[17]. In theIR spectrum of complex 1, two weak bands at 2948 and 2916 cm–1can be assigned to the CˉH stretching vibrations of -CH3groups. A very strong band at 2054 cm-1is due to the C≡N stretching vibration of the cis-coordinated NCS–groups[5]. The bands at 1597, 1470, 1451, 1441 and 1419 cm-1can be assigned to the aromatic ring vibrations.
4 CONCLUSION
A new quinolyl substituted 1,2,4-triazole ligand L and its iron(II) complex 1 have been successfully synthesized and characterized by FT-IR,1H-NMR,ESI-MS, elemental analysis and single-crystal X-ray crystallography. Crystallographic studies reveal that L is a nonplanar molecule and 1·Me2CO is a mononuclear distorted octahedral complex with two cis-coordinated NCS–ions. Each L ligand in the complex adopts a chelating bidentate coordination mode via one N atom of the quinolyl group and one N atom of the triazole.
(1) Potts, K. T. The chemistry of 1,2,4-triazoles. Chem. Rev. 1961, 61, 87–127.
(2) Kahn, O.; Martinez, C. J. Spin-transition polymers: from molecular materials toward memory devices. Science 1998, 279, 44–48.
(3) Haasnoot, J. G. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivatives as ligands. Coord. Chem.Rev. 2000, 200–202, 131–185.
(4) Zhu, D.; Qi, L.; Cheng, H.; Shen, X.; Lu, W. Fe(II) spin crossover molecule-based material. Prog. Chem. 2009, 21, 1187–1198.
(5) Zhu, D.; Xu, Y.; Yu, Z.; Guo, Z.; Sang, H.; Liu, T.; You, X. A novel bis(trans-thiocyanate)iron(II) spin-transition molecular material with bidentate triaryltriazole ligands and its bis(cis-thiocyanate)iron(II) high-spin isomer. Chem. Mater. 2002, 14, 838–843.
(6) Cheng, H. M.; Zhu, D. R.; Lu, W.; Xu, R. H.; Shen, X. Synthesis and crystal structure characterization of 3,5-bis(2-quinolyl)-1,2,4-triazole. J.Heterocycle Chem. 2010, 47, 210–213.
(7) Zhao, J.; Shen, G. P.; Zhang, Y.; Shen, X.; Zhu, D. R. Syntheses, crystal structures and spectral characterization of three new asymmetrical substituted triaryltriazoles. J. Heterocycl. Chem. 2012, 49, 1114–1119.
(8) Shih, C. H.; Sheu, C. F.; Kato, K.; Sugimoto, K.; Kim, J.; Wang, Y,; Takata, M. The photo-induced commensurate modulated structure in site-selective spin crossover complex trans-[Fe(abpt)2(NCS)2]. J. Chem. Soc., Dalton Trans. 2010, 39, 9794–9800.
(9) Cowan, M. G.; Olguín, J.; Narayanaswamy, S.; Tallon, J. L.; Brooker, S. Reversible switching of a cobalt complex by thermal, pressure, and electrochemical stimuli: abrupt, complete, hysteretic spin crossover. J. Am. Chem. Soc. 2012, 134, 2892–2894.
(10) Lu, W.; Zhu, D. R.; Xu, Y.; Cheng, H. M.; Zhao, J.; Shen, X. Syntheses and crystal structures of two novel Cu(II) and Co(II) complexes with 3-methyl-4-(p-bromophenyl)-5-(2-pyridyl)-1,2,4-triazole. Struct. Chem. 2010, 21, 237–244.
(11) Zhao, J.; Cheng, H. M.; Shen, G. P.; Xu, Y.; Zhu, D. R. Syntheses, crystal structures, and spectral properties of Mn(II) and Co(II) complexes with 3-(p-chlorophenyl)-4-(p-methylphenyl)-5-(2-pyridyl)-1,2,4-triazole. J. Coord. Chem. 2011, 64, 942–951.
(12) Shen, G. P.; Zhao, J.; Jiang, J. J.; Liu, Q.; Shen, X.; Xu, Y.; Zhu, D. R.; Liu, X. Q. Abnormal conformation change of an asymmetrical triaryltriazole before and after its coordination. J. Mol. Struct. 2011, 1002, 159–166.
(13) Shen, G. P.; Jiang, J. J.; Sun, F.; Shen, X.; Zhu, D. R.; Liu, X. Q. Syntheses, crystal structures and spectral characterization of two novel quinolyl substituted triazoles. J. Heterocycl. Chem. 2012, DOI 10.1002/jhet.1611.
(14) Wang, L.; Jiang, J. J.; Chen, L.; Shen, X.; Zhu, D. R. Coexistence of two distinct axial ligands and their thermo-induced substitution in trans-[FeL2(NCS)2][FeL2(CH3OH)2](NCS)2(L = 4-amino-3-(p-chlorophenyl)-5-(2-pyridyl)-1,2,4-triazole). Inorg. Chem. Commun. 2013, 28,104–108.
(15) Sheldrick, G. M. SHELXS 97, Program for Crystal Structure Determinations. University of Göttingen, Germany 1997.
(16) Sheldrick, G. M. SHELXL 97, Program for the Refinement of Crystal Structure. University of Göttingen, Germany 1997.
(17) Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds, Wiley, New York 1970.
- 结构化学的其它文章
- Synthesis, Crystal Structure and Photoluminescence of a New Cd-organic meso-Helicate①
- Non-merohedrally Twinned Crystal Structure of the Co-crystal Ethyl 6-(2-[5-(Ethoxycarbonyl)-pyridin-2-yl]-1,2-dihydroxyethyl)pyridine-3-carboxylateethyl 6-(2-[5-(Ethoxycarbonyl)pyridin-2-yl]-2-hydroxyacetyl)pyridine-3-carboxylate (0.69/0.31)
- One New Zinc Coordination Polymer Based on 2-Chloro-benzoate and 4,4΄-Bipyridine:Synthesis, Crystal Structure and Luminescence①
- Synthesis, Crystal Structure and Antibacterial Activity of 4,4΄-Hydrazine-1,2-diylidenebis(methanylylidene)bis(2-ethoxyphenol)①
- A Trinuclear Ni(II) Mixed-ligand Complex Containing Pentadentate N-butylsalicylhydrazide and Monodentate Imidazole:Synthesis, Characterization and Crystal Structure①
- Synthesis, Structure and Photoluminescence of a New Cd(II)Coordination Polymer Based on 4-(Carboxymethoxy)-benzoic Acid and 1,10-Phenanthroline Derivative①

