Synthesis, Crystal Structure and Photoluminescence of a New Cd-organic meso-Helicate①
YANG Shn-Shn WANG Xin-Fng ZHANG Ming-Xing CHEN Xin XIAO Feng ZHU Yi LIU Yu-Jun HUANG Kun-Lin② (College of Chemistry, Chongqing Norml University,Chongqing 400047, Chin) (Deprtment of Chemistry, Dezhou University, Dezhou 253023, Chin)
1 INTRODUCTION
The self-assembly of metal-organic supramolecular architectures from transition metal ions and organic moieties has attracted great interest because of their fascinating structure and promising application as functional materials[1-3]. Among a plenty of supramolecular structures published to date, helicates are considered the most simple and fundmental architectures[4-5]. Due to biomimetic relevance of helicates[6]and extensive use of helicates as templates for the synthesis of topologically interesting derivatives such as molecular knots and related compounds, they have been highly concerned by crystal experts. However, directed synthesis is still a great challenge because the assembly progress is seriously influenced by several factors (metal or ligand properties, solvents, templates, counterions,and reaction conditions). Fortunately, these uncertainties can be reduced by using flexible ligands because flexible ligands can be optionally distorted and wrapped around the metal ions to form a helical structure.
In this context, we choose (2E,2΄E)-3,3΄-((propane-1,3-diylbis(oxy))bis(2,1-phenylene)) diacrylic acid (H2ppa) as the flexible ligand. The ligand not only inherits the advantages of fluorescent analogues of cinnamic acid, such as the rigidity arising from the extended conjugated structure, but also has much longer spacer between the carboxylate groups, which could lead to the interesting torsion of ligand. To our knowledge, so far H2ppa has not been reported in building coordination polymers or supramolecular architectures. Herein we report a new meso-helicate from the flexible H2ppa, cadmium salt and phen, and the title complex possesses expected luminescent property.
2 EXPERIMENTAL
Infrared (IR) spectrum was recorded from KBr pellets in the range of 400–4000 cm-1on a NicoletImpact 410 FTIR spectrometer. Elemental analysis was performed on a Perkin-Elmer 2400 elemental analyzer. Photoluminescence spectra were measured using a RF-5301PC spectrofluorometer equipped with a 450W xenon lamp as the excitation source and the solid-state samples were measured at room temperature.
2.1 Synthesis of Cd(ppa)(phen)(H2O)
A mixture of Cd(NO3)2·4H2O (30.8 mg, 0.1 mmol), H2ppa (36.8 mg, 0.1 mmol), phen (29.8 mg,0.1 mmol), DMF (2 mL) and H2O (8 mL) was sealed in a 25 mL Teflon-lined stainless steel reactor and directly heated to 75 ℃ for 3 days, and then cooled to room temperature spontaneously. The crystal samples were washed with methanol to give compound 1 in about 65% yield (based on H2ppa ligand). Elemental analysis calcd. (%) for C66H56N4O14Cd2: C, 58.55; H, 4.17; N, 4.14; O,16.54. Found (%): C, 58.48; H, 4.25; N, 4.18; O,16.52. FT-IR (KBr, cm-1): 1635 (m), 1599(m),1555(s), 1406(s), 846(m), 723(s).
2.2 X-ray crystallography study of complex 1
The single-crystal X-ray diffraction data of 1 were collected on a CCD area detector equipped with a graphite-monochromatized MoKα radiation (λ =0.71073 Å). A total of 16538 reflections were collected in the range of 1.53≤θ≤25.02º, of which 5232 were unique with Rint= 0.0174, and 4673 observed reflections (I > 2σ(I)) were used in the succeeding refinements. Data processing was performed using the SAINT program. The structure was solved by direct methods with SHELXS-97[7]program and refined on F2using SHELXL-97[7]program by full-matrix least-squares techniques with equivalent isotropic displacement parameters for the non-hydrogen atoms. The hydrogen atoms of water molecules were found by Difference Fourier maps and all hydrogen atoms attached to carbon atoms were placed according to theoretical models. The final R = 0.0248 and wR = 0.0667 (w = 1/[σ2(Fo)2+(0.0491P)2+ 0.0000P], where P = (Fo2+ 2Fc2)/3). S= 1.065, (Δ/σ)max= 0.001, (Δρ)max= 0.767 and(Δρ)min= –0.418 e/Å3. The selected bond distances and bond angles are listed in Table 1. The hydrogen bonds are shown in Table 2.
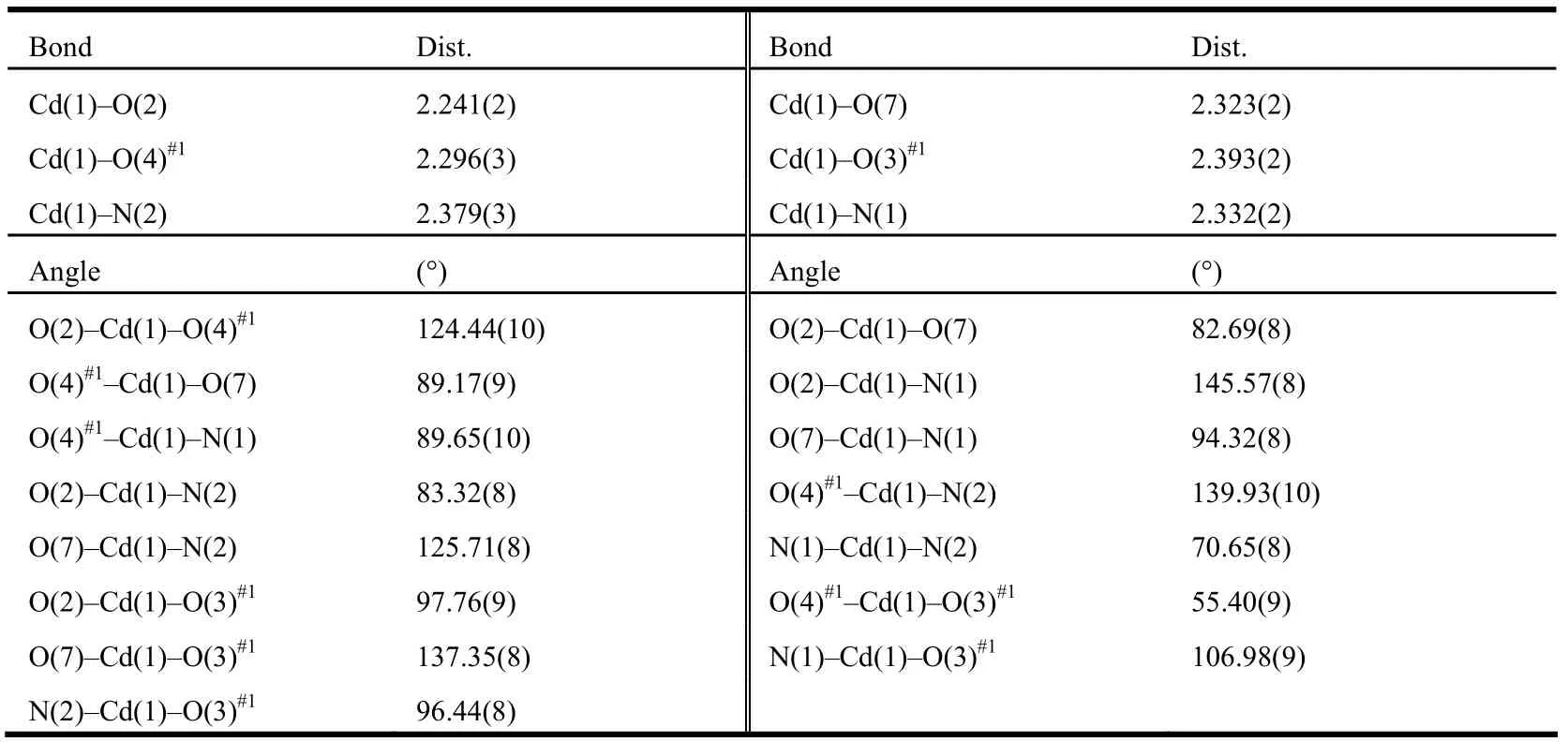
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
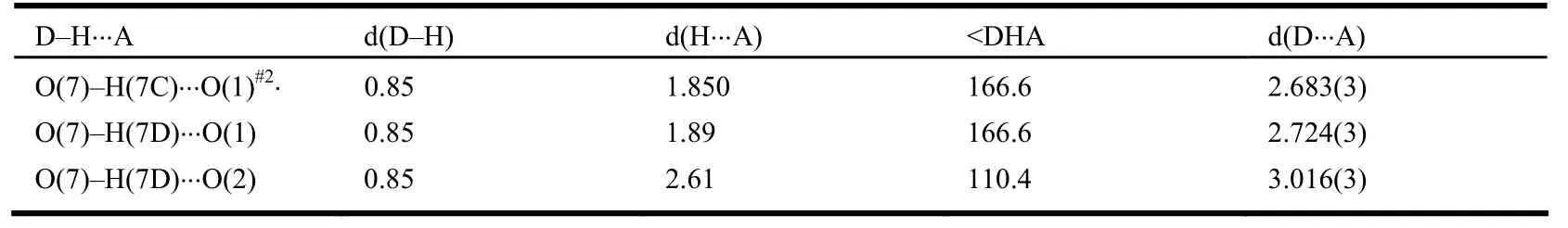
Table 2. Hydrogen Bonds with Distances (Å) and Angles (°)
3 RESULTS AND DISCUSSION
3.1 Structure description
X-ray single-crystal diffraction study reveals that compound 1 crystallizes in the P21/c space group and is composed of [Cd(ppa)(phen)(H2O)]2. Asymmetric unit of 1 contains one crystallographically independent Cd center (Cd(1)), one deprotonated ppa2-ligand, one phen, and one coordinated water molecule (Fig. 1). The Cd2+center adopts a six-coordinated mode with two N atoms (N(1), N(2)) from a chelating phen and four O atoms: three (O(2#1), O(3),O(4)) from two ppa2-ligands and one (O(7)) from the water molecule (Fig. 1). The Cd–O and Cd–N bond lengths are in the normal ranges of 2.241(2)–2.393(2) Å and 2.332(2)–2.379(3) Å respectively(Table 1), similar to those observed in the reported Cd-complexes[8-11].
As shown in Fig. 1, the completely deprotonated ppa2-ligand adopts only one coordinated mode with one acrylate group connected with one Cd2+via a bridging monodentate mode, whereas the other is linked to one crystallographically equivalent Cd2+via a chelating coordinated fashion. Interestingly,two crystallographically equivalent Cd atoms (Cd(1),Cd(1#1)) are bridged by two ppa2-ligands in a chelating/bridging monodentate mode. Two ppa2-ligands coordinated to equivalent Cd atoms were distorted in different M (left-handed) and P (righthanded) methods. Therefore, this complex can be termed as a meso-helicate (Fig. 2a). Moreover, there exists a 36-membered large ring with the dimensions of 9.58 and 12.43 Å (nucleus-to-nucleus separation,Fig. 2b).
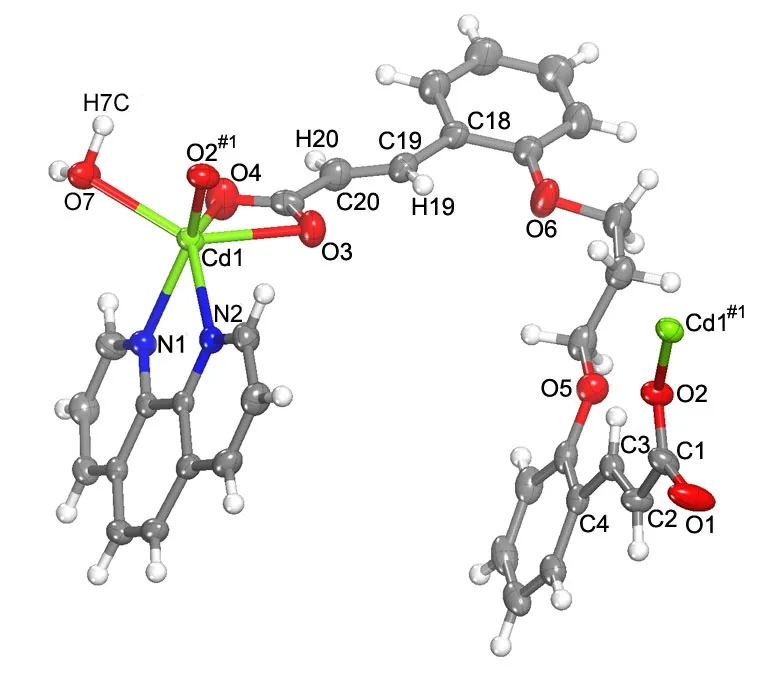
Fig. 1. Coordination environments and the sectional crystal structure of [Cd(ppa)(phen)(H2O)]2

Fig. 2. (a) A space-filling representation of the meso-helical structure in 1, and(b) View of the large-ring structure of complex 1. Phen groups have been omitted for clarity
Another interesting structure feature of complex 1 is non-covalent interactions. As illustrated in Fig. 3,owing to the π-π stacking interactions with the separation of 3.5 Å between phen groups from adjacent rings and the π-π interactions of 3.4 Å between phen and phenyal (C6H5) groups of ppa2-ligands, complex 1 presents a one-dimensional (1-D)chain structure. To move forward a single step,inter-chain O–H··O hydrogen bonds (O(1)··O(7)2.683(3) Å, Table 2) extend the 1-D chains into a 2-D supramolecular architecture (Fig. 4).
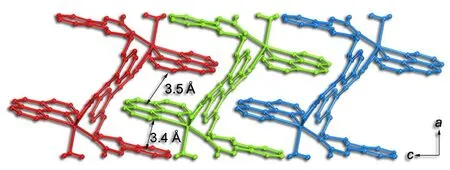
Fig. 3. View of the one-dimensional [Cd(ppa)(phen)(H2O)]n chain along the b axis with abundant π-π interactions
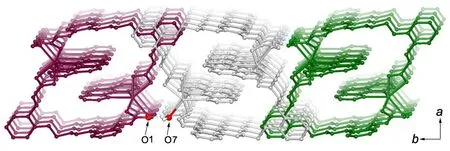
Fig. 4. Two-dimensional layer built up from 1-D chains through inter-chain O-H··O Hydrogen bonds. See along the c direction
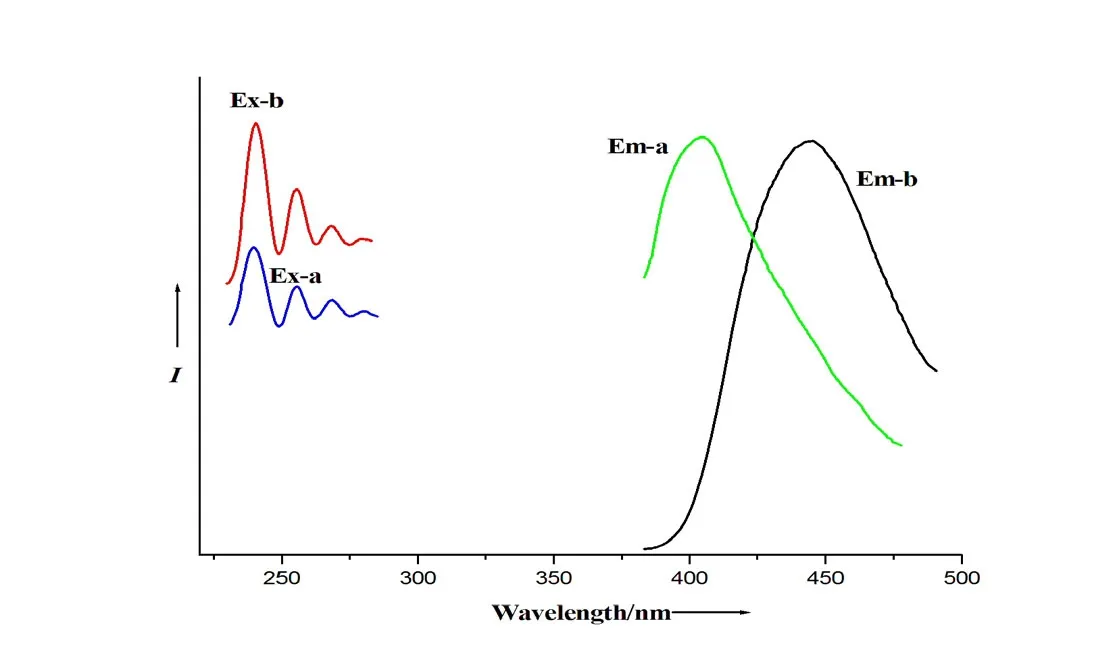
Fig. 5. Photoluminescent spectra of compounds: free H2ppa (λEm-a, max at 404 nm upon λEx-a, max at 240 nm) and the title complex (λEm-b, max at 445 nm, λEx-b, max at 240 nm) in the solid state at room temperature (Em = emission, Ex = excitation, and max = maximum)
3.2 Luminescent properties
Fig. 5 presents the photoluminescent spectra of compound 1 in the solid state at room temperature.Free H2ppa has an emission (Em) maximum at 404 nm upon excitation (Ex) maximum at 240 nm, while the emission maximum of phen under 409 nm upon excitation is at 356 nm. Compound 1 shows strong photoluminescence with emission maximum at λ =445 nm (upon λEx,max= 240 nm). The emission peak of complex 1 is red-shifted by about 40 nm compared to that of the pure H2ppa ligand, which can be assigned to the ligand-metal charge transfer(LMCT) and π-π interactions[12].
4 CONCLUSION
A new metal-organic meso-helicate is successfully synthesized by using the new flexible ligand, a fluorescent analogue of cinnamic acid, (2E,2΄E)-3,3΄-((propane-1,3-diylbis(oxy))bis(2,1-phenylene))diacrylic acid. And the 2-D layer structure is formed from the 1-D chains through interchain hy- drogen bonds. The strong photoluminescent emis- sion makes 1 a potentially useful photoactive solid- state material.
(1) Yaghi, O. M.; O΄Keeffe, M.; Ockwig, N. W.; Chae, H. K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003,423, 705–714.
(2) Huang, K. L.; Chen, X.; Liu, Y.; Liu, X.; Yang, Y. A photoluminescent bimetallic zinc-pyridinedicarboxylate: diverse supramolecular motifs from distinct hydrogen bonds. Chin. J. Struct. Chem. 2008, 27, 570–574.
(3) Chen, X.; Huang, K. L.; Liu, X. Alternate array of zigzag (PbO5)nand (PbO6)npolynuclear chains in a 2-D coordination polymer with diverse coordinate modes of nitrate (NO3-). Chin. J. struct. Chem. 2010, 29, 199–204.
(4) Piguet, C.; Bernardinelli, G.; Hopfgartner, G. Helicates as versatile supramolecular complexes. Chem. Rev. 1997, 97, 2005–2062.
(5) Albrecht, M. “Let΄s twist again”-double-stranded, triple-stranded, and circular helicates. Chem. Rev. 2001, 101, 3457–3497.
(6) Hannon, M. J.; Childs, L. J. Helices and helicates: beautiful supramolecular motifs with emerging applications. Supramol. Chem. 2004, 16, 7–12.
(7) Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr Sect. A 2008, 64, 112–122.
(8) Wang, G. H.; Li, Z. G.; Jia, H. Q.; Hu, N. H.; Xu, J. W. Topological diversity of coordination polymers containing the rigid terephthalate and a flexible N,N΄-type ligand: interpenetration, polyrotaxane, and polythreading. Cryst. Growth Des. 2008, 8, 1932–1939.
(9) Fang, S. M.; Zhang, Q.; Hu, M.; Yang, X. G.; Zhou, L. M.; Du, M.; Liu, C. S. Regulation and properties of diversiform Cd(II) supramolecular complexes with a bulky naphthalene-based dicarboxyl tecton and different N-donor Co-ligands. Cryst. Growth Des. 2010, 10, 4773–4785.
(10) Tzeng, B. C.; Yeh, H. T.; Chang, T. Y.; Lee, G. H. Novel coordinated-solvent induced assembly of Cd(II) coordination polymers containing 4,4΄-dipyridylsulfide. Cryst. Growth Des. 2009, 9, 2552–2555.
(11) Sun, X. L.; Wang, Z. J.; Zang, S. Q.; Song, W. C.; Du, C. X. A series of Cd(II) and Zn(II) coordination polymers with helical subunits assembled from a versatile 3-(4-hydroxypyridinium-1-yl)phthalic acid and N-donor ancillary coligands. Cryst. Growth Des. 2012, 12, 4431–4440.
(12) Huang, K. L.; Liu, X.; Chen, X.; Wang, D. Q. Spontaneous assembly of 63topological metal-organic nanotubes with distinct asymmetric subunits for the construction of hydrophilic intertube channels encapsulating rare helical water-chains. Cryst. Growth Des. 2009, 9, 1646–1650.
- 结构化学的其它文章
- Synthesis and Crystal Structure of the First Complex Derived from a Quinolyl Substituted 1,2,4-Triazole①
- Non-merohedrally Twinned Crystal Structure of the Co-crystal Ethyl 6-(2-[5-(Ethoxycarbonyl)-pyridin-2-yl]-1,2-dihydroxyethyl)pyridine-3-carboxylateethyl 6-(2-[5-(Ethoxycarbonyl)pyridin-2-yl]-2-hydroxyacetyl)pyridine-3-carboxylate (0.69/0.31)
- One New Zinc Coordination Polymer Based on 2-Chloro-benzoate and 4,4΄-Bipyridine:Synthesis, Crystal Structure and Luminescence①
- Synthesis, Crystal Structure and Antibacterial Activity of 4,4΄-Hydrazine-1,2-diylidenebis(methanylylidene)bis(2-ethoxyphenol)①
- A Trinuclear Ni(II) Mixed-ligand Complex Containing Pentadentate N-butylsalicylhydrazide and Monodentate Imidazole:Synthesis, Characterization and Crystal Structure①
- Synthesis, Structure and Photoluminescence of a New Cd(II)Coordination Polymer Based on 4-(Carboxymethoxy)-benzoic Acid and 1,10-Phenanthroline Derivative①

