球形、花形和线状钒酸铋的可控合成及光催化性能
林 雪 于丽丽 闫丽娜 关庆丰 闫永胜 赵 晗
(1吉林师范大学化学学院,环境友好材料制备与应用教育部重点实验室,吉林四平136000;2江苏大学材料科学与工程学院,江苏镇江212013;3江苏大学化学化工学院,江苏镇江212013)
1 Introduction
From the viewpoint of the environment issues,the direct use of solar energy such as clean and renewable energy through visible-light-driven photocatalysis for environmental pollution control has attracted great interest.1-8In recent years,there are many papers focused on the preparation of bismuth compounds which can degrade organic pollutants under visible light irradiation.9-13The semiconducting oxides containing bismuth with narrow band gap,such as BiVO4,9,10Bi2WO6,11,12and Bi2MoO6,13have received intensive interest because of their visible-light-driven photocatalytic activities.Among bismuth-containing photocatalysts,BiVO4has received more attention for its high photocatalytic ability for environmental applications and/or water splitting.
Recently,much attention has been paid to the synthesis of BiVO4nanostructures with different sizes and shapes due to their good visible light responsive photocatalytic activites compared with its bulk counterpart.14-17Traditional solid-state reaction usually produces large irregular BiVO4crystals due to its fast crystal growth feature.Moreover,crystallographic facets with high surface energies diminish during the crystal growth process for minimization of surface energy.A variety of solution-based methods including aqueous,hydrothermal,and solvothermal process,have been developed to fabricate BiVO4nanostructures,such as nanoellipsoids,18-20nanowires/nanofibers,21,22nanosheets/nanoplates,23,24and hyperbranched crystals.25However,the ability to control the morphologies of BiVO4nanostructures,such as sizes and well-defined shapes(or crystallographic facets exposed on the surface),remains a great challenge,that can provide a great opportunity to improve their properties.
Herein,we report a simple hydrothermal method without using any surfactant or template for the successful synthesis of BiVO4crystals with well controlled shapes.The photocatalytic activities of the BiVO4samples with different morphologies have been discussed.The aim of the present paper is to study the influence of structure and morphology upon the optical properties and photocatalytic properties of BiVO4photocatalysts.
2 Experimental
2.1 Preparation of BiVO4photocatalysts
All reagents used in our experiment are of analytical purity(purchased from Shanghai Chemical Industrial Company)and used without further purification.In a typical synthesis of BiVO4,0.970 g of Bi(NO3)3·5H2O were dissolved into 35 mL of distilled water.Then an aqueous solution of NH4VO3(0.234 g of NH4VO3,5 mL of distilled water)was added to the above mixture under magnetic stirring to form an orange emulsion.The pH value of the reaction mixture was adjusted from 1 to 3 with ammonia solution(25%-28%(w))under vigorous stirring.After stirring for 0.5 h at room temperature,the reaction mixture was transferred into a stainless steel autoclave,and kept at 180°C for 12 h.The resulting yellow product was collected,washed with ethanol and distilled water several times,and then dried at 100°C for 10 h.The sample prepared for comparison is N-doped TiO2(N-TiO2),which was synthesized according to Hou et al.26
2.2 Characterization of BiVO4photocatalyst
The crystal structures of the samples were characterized by X-ray diffraction(XRD)on a Rigaku(Japan)D/max 2500 X-ray diffractometer(Cu Kαradiation,λ=0.15418 nm).Transmission electron microscopy(TEM)was conducted using a JEM-2100F(Japan JEOL)instrument.The Fourier transform infrared(FTIR)spectra were measured by FTIR spectrometer(America Perkin Elmer,Spectrum One).Raman spectra of BiVO4samples were obtained by a micro laser Raman spectrometer(LabRam inva).Raman spectra were excited with the 514 nm line of an Ar+laser at an incident power of 20 mW.The surface areas of samples were measured by TriStar 3000-BET/BJH Surface Area.The optical property was obtained by the photoluminescence(PL)measurement using HR800 LabRam Infinity Spectro photometer excited by a continuous He-Cd laser with a wavelength of 325 nm at a power of 50 mW.The diffuse reflectance spectra(DRS)were measured by a UV-Vis spectrometer(UV-2550,Shimadzu).BaSO4was used as the reflectance standard material.
2.3 Photocatalytic activities
The photocatalytic activities of BiVO4samples were evaluated using RhB dye as a model compound.In experiments,the RhB dye solution(0.005 mmol·L-1,100 mL)containing 0.02 g of BiVO4photocatalyst was mixed in a pyrex reaction glass.A 500 W Xe lamp(λ>420 nm)was used to provide visible light irradiation.A glass sheet was inserted between the lamp and the sample to filter out UV light(λ<420 nm).Prior to visible light illumination,the suspension was strongly magnetically stirred for 30 min in the dark for adsorption/desorption equilibrium.Then the solution was exposed to visible light irradiation under magnetic stirring.At given time intervals,about 4 mL of the suspension was periodically withdrawn and analyzed after centrifugation.The RhB concentration was analyzed by a UV-2550 spectrometer to record intensity of the maximum band at 552 nm in the UV-Vis absorption spectra.
3 Results and discussion
Fig.1 shows the XRD patterns of the as-prepared BiVO4samples.All diffraction peaks can be assigned to the monoclinic structure of BiVO4(JCPDS No.14-0688).This observation is further confirmed by the splitting of the peaks at 2θ=18.5°,35°,and 46°,which is characteristic of the monoclinic structure of BiVO4.No peaks of impurities were detected from these patterns.The strong and sharp peaks indicate high crystallinity of the samples.However,the intensities of the peaks of BiVO4samples are different which proves that there are some differences among the samples prepared with different pH values.BiVO4sample prepared at pH value of 3 has the strongest peak intensity,which means that the crystallinity of the sample was developing with the increase of pH value.
TEM was used to observe the morphologies and structure details of BiVO4products.Fig.2(a-c)shows the TEM images of the as-prepared BiVO4samples obtained at different conditions.Fig.2(a)gives a typical example of microspheres with average sizes of 200 nm,approximately.It can be observed that there are BiVO4nanorods with average width of about 100 nm(as shown in Fig.2(b)).These nanorods intersect with each other forming microflowers.It can be seen in Fig.2(c)that there are BiVO4microwires with width of approximate 200 nm and lengths up to several micrometres.
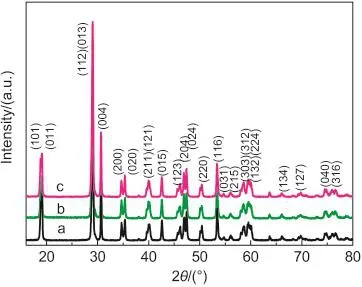
Fig.1 XRD patterns of the as-prepared BiVO4samples obtained at different pH values
These results indicate that pH value seems to play an important role in determining final morphologies of BiVO4.Namely,morphology of BiVO4powders can be controlled through varying the pH value.Although the crystal growth habit is mainly determined by the intrinsic structure,it is also affected by the external conditions such as pH value of the solution,saturation,temperature,etc.27As we all know,the pH value in the precursor solution has been found to be very important for the microstructure.In this work,the condition of the pH value as a factor is considered to play a key part in the formation of BiVO4crystals with different morphologies.At low pH value(pH=1),BiVO4nuclei produced in solution can aggregate to form small mirospheres.With the pH value increase continuing(pH=3),a large amount of BiVO4nuclei produced in the solution lead to form the very high supersaturation solution,which favors the formation of threadlike structure.When pH value is relatively lower(pH=2),only BiVO4nanorods are obtained because of lower driving force,which come from the lower chemical potential.These nanorods intersect with each other forming microflowers.
Diffuse reflectance spectroscopy is a useful tool for characterizing the electron states in optical materials.The UV-Vis diffuse reflectance spectra(UV-Vis DRS)of the as-prepared BiVO4samples are shown in Fig.3.As a comparison,the spectra of P25 TiO2and N-TiO2were also measured.The BiVO4products exhibit strong absorption in the visible range in addition to the UV range.The steep absorption edge in the visible range indicates that the absorption of visible light is not due to the transition from impurity levels but to the band gap transition.28The band gap energies(Eg)of BiVO4samples are calculated to be about 2.33,2.24,and 2.19 eV,based on the formula:Eg=1240/λg,which show marked red shift in the absorbance compared to P25 and N-TiO2.It indicates that BiVO4photocatalysts have a suitable band gap for photocatalytic decomposition of organic contaminants under visible light irradiation.In this system,slight red shift of the absorption band edge of BiVO4samples(from 2.19 to 2.33 eV)can be observed with the pH value increasing from 1 to 3.It is well known that the band gap energy of semiconductor nanoparticles increases with the decrease of grain size.Herein,BiVO4with one-dimensional(1D)microstructure(pH 3)had a red shift in the band-gap tran-sition compared with spherical and flowerlike BiVO4(pH 1,pH 2),attributed to the smaller grain sizes of spherical and flowerlike BiVO4than that of the 1D microwires.

Fig.2 TEM images of the as-prepared BiVO4samples obtained at different pH values
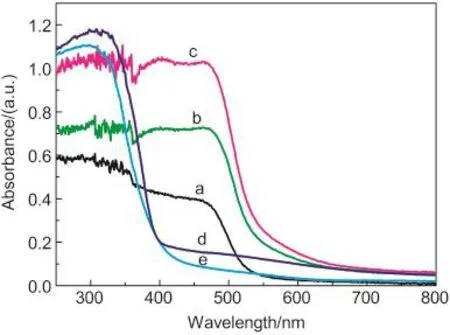
Fig.3 UV-Vis DRS of samples
Notably,it has been established that the photoluminescence(PL)spectra originate from the direct recombination of photoexcited electrons and holes.29In order to further study the efficient separation of photogenerated carriers in BiVO4,the corresponding photoluminescence spectra were obtained to disclose the migration,transfer,and recombination processes of photogenerated electron-hole pairs.Fig.4 shows the PL spectra of Bi-VO4samples measured at room temperature at an excitation wavelength of 325 nm.As shown,a yellow emission near 591 nm was clearly observed in all the three kinds of BiVO4samples,but for a slight blue shift in BiVO4crystals with the particle size decreased,which is in agreement with the previous report.29The fluorescence intensity of BiVO4microspheres was significantly weaker than those of threadlike and flowerlike products,which shows the recombination restraint of the electron-hole(e-/h+)pairs.
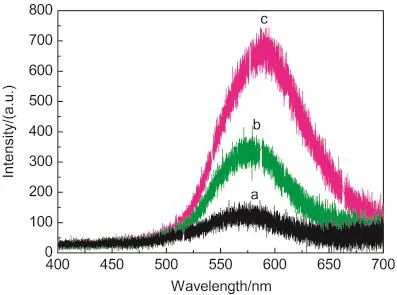
Fig.4 PLspectra of the as-prepared BiVO4samples
During the course of the photocatalysis,the BiVO4catalyst in the RhB resolution destroys not only the RhB conjugated system,but also its molecular structure.When the RhB adsorbed on the surface of BiVO4is excited,it injects an electron(e-)into the conduction band(CB)of BiVO4.The electron can reduce molecular oxygen to superoxide anion and later transform to organic peroxides or hydrogen peroxide.Another reactive intermediate which is responsible for the degradation is hydroxyl radical(·OH).The hydroxyl radical is an extremely strong,non-selective oxidant which leads to the partial or complete mineralization of several organic chemicals.After a series of complicated oxidation reaction,the RhB is decomposed to smaller organics and minerals,like CO2,H2O,and so on.30
Because the direct oxidation of RhB by positive holes(h+)adsorbed on the surface of BiVO4is considered to be the main oxidation pathway,the specific surface area plays an important role in the speed of photocatalytic process.31The BET results show that the specific surface area of BiVO4microspheres is 22.16 m2·g-1,the flowerlike BiVO4nanostructure is 10.30 m2·g-1,and BiVO4microwires is 8.08 m2·g-1(as shown in Table 1).It is clearly revealed that the samples with small size(pH 1)can more efficiently adsorb the RhB due to their higher BET surface area than other samples with larger sizes.Higher specific surface area means that the total active surface area increases at the same catalyst loading,hence availability of more active sites on catalyst surface.32,33In order to prove the effect of the specific surface area,the experiments were performed by comparing in microspheres,microflowers and microwires with 0.02 g catalyst loading for RhB solutions of 0.005 mmol·L-1.The photodegradation for the three kinds of BiVO4samples has been displayed in Fig.5.For comparison,the photodegradation of RhB by P25,N-TiO2,and that without any catalyst were also carried out.From the catalytic studies,BiVO4samples are found to be more photoactive towards RhB solution than P25,and N-TiO2.The photolysis test demonstrates that the degradation rate of RhB by microspheres increases to 100%after 180 min irradiation,much higher than that of microflowers and microwires.The increasing of BET surface areas and the decreaseof crystalline grains were both beneficial to the enhancement of the photocatalytic activity of BiVO4.32In addition,the different photodegradation rates of RhB by different BiVO4samples indicated that the photocatalytic performances of these BiVO4samples are greatly different and strongly dependent on shape,crystal size,and structure.
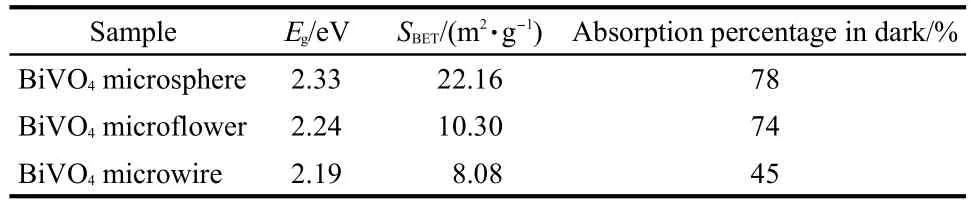
Table 1 Band gap energy(Eg),BET specific surface area(SBET),and absorption percentages of photocatalysts in the dark
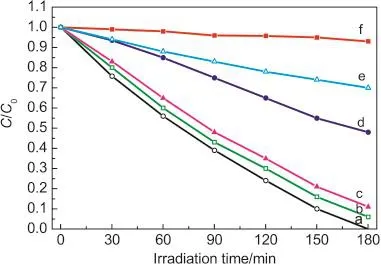
Fig.5 Photodegradation efficiencies of RhB as a function of irradiation time for different photocatalysts
The stability tests were conducted by using recycling reactions four times for the photodegradation of RhB over BiVO4photocatalyst under visible light irradiation(as displayed in Fig.6).No significant decrease in catalytic activity was detected in the recycling reactions.It demonstrates that the BiVO4sample is a stable photocatalyst for degradation of RhB under visible light irradiation.
The photocatalytic property of BiVO4is also related to the distortion of the M-O polyhedron in crystal structure.Raman spectroscopy is effective to probe the local structure of materials because the Raman spectrum reflects the bonding states in the coordination polyhedron of a material.Raman spectra of the as-prepared BiVO4samples are shown in Fig.7.Raman bands at 212,328,369,and 827 cm-1were observed in the spectra of the samples prepared by the hydrothermal method.These Raman bands represented the typical vibration bands of monoclinic scheelite BiVO4and could be assigned to the asymmetric and symmetric deformation modes of VO3-4and the symmetric stretching mode of the V-O bond,respectively.34,35However,an obvious change of the Raman band intensity occurred in this range varying with the increasing of pH values in reaction system.The dependence of the relative intensities of the asymmetric and symmetric deformation vibrations(212 and 328 cm-1)of the VO3-4on the synthesis conditions is observed,demonstrating that different space symmetries are formed and the variation or rearrangement of the crystal structure exists under the influence of reaction conditions(pH value).A similar Raman-band shift of the Mo-O stretching vibration was also observed in the Raman spectrum of Bi2MoO6.32
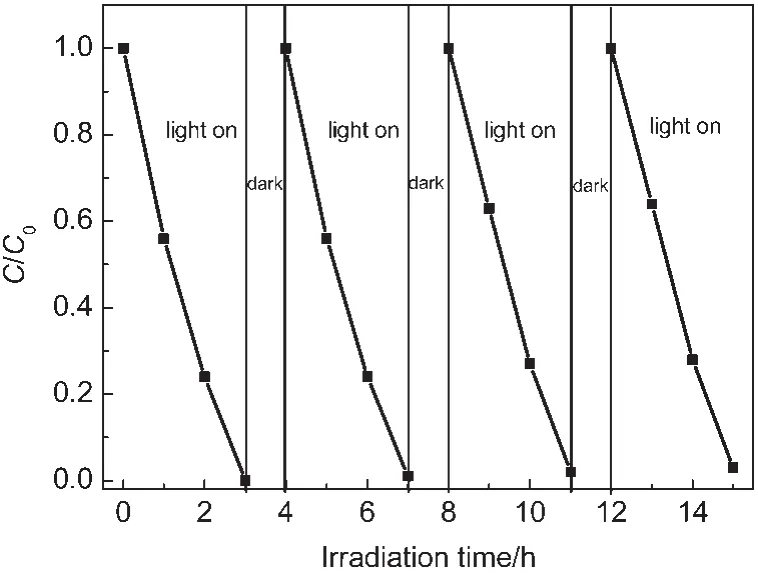
Fig.6 Stability evaluation for the as-prepared BiVO4sample
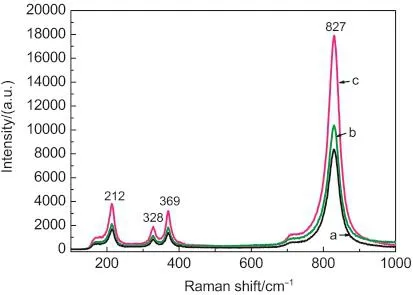
Fig.7 Raman spectra of the as-prepared BiVO4samples obtained at different pH values
FTIR spectra of BiVO4samples are shown in Fig.8.The FTIR spectra of BiVO4samples are similar with each other except for that the absorption bond of BiVO4microspheres at 3446 cm-1displays a slight blue shift compared to BiVO4microflowers and BiVO4microwires.The obvious absorptions at 1651 and 3446 cm-1can be ascribed to bending and stretching vibrations of the adsorbed H2O molecules,respectively.36,37The medium peak at 1419 cm-1indicates adsorbed trace NO-3.38,39The two strong absorption bands at 821 cm-1and 746 cm-1belong to the stretching vibration and deformation bending vibration of VO3-
4,respectively.39Minor down shift of wavenumber at 746 cm-1is observed,indicating that the variation of structure for VO3-4may be occurred with the increasing of pH values in this system.Several differences in half-widths and intensities of the diffraction peaks were observed in the XRD patterns of BiVO4synthesized hydrothermally at various pH values(Fig.1),indicating that slight changes in the structure,even though all the powders exhibited the monoclinic structure.Based on the above results and analysis,it can be concluded that the changing of pH value in reaction system may influence the crystal structure and corresponding photocatalytic performance.
The above experiments have shown the excellent photocatalytic performance of the as-prepared BiVO4samples on the degradation of the widely used dye RhB.It follows that the BiVO4photocatalyst may have highly potential applications in the conservation of the environment.Not only limited to the experimental results,the photodegraded mechanism of RhB in the visible light/BiVO4system was necessary to investigate and guide the further improvement of its photocatalytic performance.
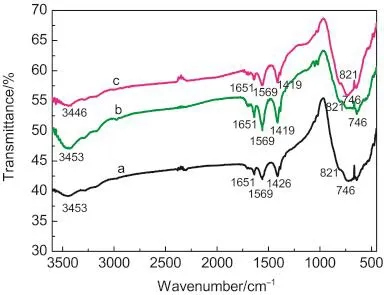
Fig.8 FTIR spectra of the as-prepared BiVO4samples obtained at different pH values
For BiVO4system,photo-oxidations occurring in aqueous media,the mechanism may involve direct reaction of the organic chemical(dye)with surface h+vb(vb:valence band),indirect reaction with·OH radicals or a dual mechanism involving both surfaceand·OH radicals.40,41The pathway of photocatalytic degradation can be described through the following equations:
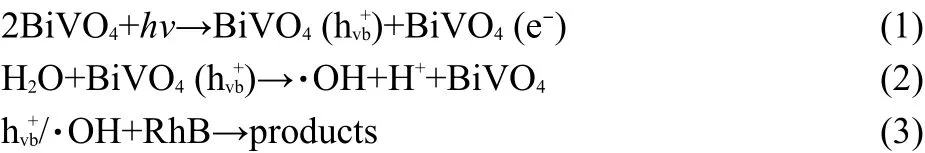
4 Conclusions
BiVO4photocatalysts with different morphologies have been synthesized through a facile,pH-controlled,surfactant-free hydrothermal route.XRD results confirmed that the composition of the as-fabricated samples is of monoclinic structure.The energy band gaps of BiVO4samples are found to be about 2.19-2.33 eV.Extension of the light absorption from the ultraviolet region to the visible-light region was confirmed by UVVis DRS.Due to the structure property relationships,BiVO4photocatalysts show enhanced visible photocatalytic activities over those of P25 and N-TiO2in the decomposition of RhB in water.Our work suggests that the photocatalytic performance of BiVO4is greatly dependent on the local structure and the morphology.Thehydrothermaltechniquepresented here seems an economical and easy way for the morphology and local structure control of such material.
(1)Hu,Y.F.;Li,Y.X.;Peng,S.Q.;Lü,G.X.;Li,S.B.Acta Phys.-Chim.Sin.2008,24(11),2071.[胡元方,李越湘,彭绍琴,吕功煊,李树本.物理化学学报,2008,24(11),2071.]doi:10.3866/PKU.WHXB20081123
(2)Mao,Y.B.;Wong,S.S.J.Am.Chem.Soc.2006,128,8217.doi:10.1021/ja0607483
(3)Li,B.X.;Wang,Y.F.;Liu,T.X.Acta Phys.-Chim.Sin.2011,27(12),2946.[李本侠,王艳芬,刘同宣.物理化学学报,2011,27(12),2946.]doi:10.3866/PKU.WHXB20112946
(4) Zhang,L.S.;Wang,H.L.;Chen,Z.G.;Wong,P.K.;Liu,J.S.Appl.Catal.B:Environ.2011,106,1.
(5) Grasset,F.;Starukh,G.;Spanhel,L.;Ababou-Girard,S.;Su,D.S.;Klein,A.Adv.Mater.2005,17,294.
(6) Grasset,F.;Spanhel,L.;Ababou-Girard,S.Superlattice Microst.2005,38,300.doi:10.1016/j.spmi.2005.08.023
(7)Yao,W.F.;Wang,H.;Xu,X.H.;Zhou,J.T.;Yang,X.N.;Zhang,Y.;Shang,S.X.Appl.Catal.A:Gen.2004,259,29.doi:10.1016/j.apcata.2003.09.004
(8)Yao,W.F.;Xu,X.H.;Wang,H.;Zhou,J.T.;Yang,X.N.;Zhang,Y.;Shang,S.X.;Huang,B.B.Appl.Catal.B:Environ.2004,52,109.doi:10.1016/j.apcatb.2004.04.002
(9) Liu,Y.Y.;Huang,B.B.;Dai,Y.;Zhang,X.Y.;Qin,X.Y.;Jiang,M.H.;Whangbo,M.H.Catal.Commun.2009,11,210.doi:10.1016/j.catcom.2009.10.010
(10)Zhang,Z.J.;Wang,W.Z.;Shang,M.;Yin,W.Z.Catal.Commun.2010,11,982.doi:10.1016/j.catcom.2010.04.013
(11)Zhang,L.W.;Wang,Y.J.;Cheng,H.Y.;Yao,W.Q.;Zhu,Y.F.Adv.Mater.2009,21,1286.doi:10.1002/adma.v21:12
(12) Zhuo,Y.Q.;Huang,J.F.;Cao,L.Y.;Ouyang,H.B.;Wu,J.P.Mater.Lett.2013,90,107.doi:10.1016/j.matlet.2012.09.009
(13)Tian,G.H.;Chen,Y.J.;Meng,X.Y.;Zhou,J.;Zhou,W.;Pan,K.;Tian,C.G.;Ren,Z.Y.;Fu,H.G.ChemPlusChem 2013,78,117.doi:10.1002/cplu.201200198
(14) Kudo,A.;Ueda,K.;Kato,H.;Mikami,I.Catal.Lett.1998,53,229.doi:10.1023/A:1019034728816
(15)Zhou,L.;Wang,W.;Liu,S.;Zhang,L.;Xu,H.;Zhu,W.J.Mol.Catal.A:Chem.2006,252,120.doi:10.1016/j.molcata.2006.01.052
(16) Tokunaga,S.;Kato,H.;Kudo,A.Chem.Mater.2001,13,4624.doi:10.1021/cm0103390
(17) Liu,J.B.;Wang,H.;Wang,S.;Yan,H.Mater.Sci.Eng.B 2003,104,36.doi:10.1016/S0921-5107(03)00264-2
(18)Sun,Y.;Wu,C.;Long,R.;Cui,Y.;Zhang,S.;Xie,Y.Chem.Cummun.2009,4542.
(19) Sun,Y.;Xie,Y.;Wu,C.;Long,R.Cryst.Growth Des.2010,10,602.doi:10.1021/cg900988j
(20) Shang,M.;Wang,W.;Sun,S.;Ren,J.;Zhou,L.;Zhang,L.J.Phys.Chem.C 2009,113,20228.doi:10.1021/jp9067729
(21) Su,J.;Guo,L.;Yoriya,S.;Grimes,C.A.Cryst.Growth Des.2010,10,856.doi:10.1021/cg9012125
(22) Yu,J.;Kudo,A.Chem.Lett.2005,34,850.doi:10.1246/cl.2005.850
(23) Xi,G.;Ye,J.Chem.Commun.2010,46,1893.doi:10.1039/b923435g
(24) Zhang,L.;Chen,D.;Jiao,X.J.Phys.Chem.B 2006,110,2668.doi:10.1021/jp056367d
(25) Zhao,Y.;Xie,Y.;Zhu,X.;Yan,S.;Wang,S.Chem.Eur.J.2008,14,1601.
(26)Hou,Y.D.;Wang,X.C.;Wu,L.;Chen,X.F.;Ding,Z.X.;Wang,X.X.;Fu,X.Z.Chemosphere 2008,72,414.doi:10.1016/j.chemosphere.2008.02.035
(27)Yang,J.H.;Zheng,J.H.;Zhai,H.J.;Yang,L.L.;Lang,J.H.;Gao,M.J.Alloy.Compd.2009,481,628.doi:10.1016/j.jallcom.2009.03.108
(28)Kudo,A.;Tsuji,I.;Kato,H.Chem.Commun.2002,48,1958.
(29)Xu,D.;Gao,A.M.;Deng,W.L.Acta Phys.-Chim.Sin.2008,24(7),1219.[许 迪,高爱梅,邓文礼.物理化学学报,2008,24(7),1219.]doi:10.3866/PKU.WHXB20080717
(30) Wang,D.G.;Li,R.G.;Zhu,J.;Shi,J.Y.;Han,J.F.;Zong,X.;Li,C.J.Phys.Chem.C 2012,116,5082.doi:10.1021/jp210584b
(31) Cao,S.W.;Yin,Z.;Barber,J.;Boey,F.Y.C.;Loo,S.C.J.;Xue,C.ACS Appl.Mater.Interfaces 2012,4,418.doi:10.1021/am201481b
(32)Zhang,L.W.;Xu,T.G.;Zhao,X.;Zhu,Y.F.Appl.Catal.B:Environ.2010,98,138.doi:10.1016/j.apcatb.2010.05.022
(33)Wang,X.;Chen,G.;Zhou,C.;Yu,Y.G.;Wang,G.Eur.J.Inorg.Chem.2012,1742.
(34) Li,G.S.;Zhang,D.Q.;Yu,J.C.Chem.Mater.2008,20,3983.doi:10.1021/cm800236z
(35) Wetchakun,N.;Chaiwichain,S.;Inceesungvorn,B.;Pingmuang,K.;Phanichphant,S.;Minett,A.I.;Chen,J.ACS Appl.Mater.Interfaces 2012,4,3718.doi:10.1021/am300812n
(36) Ke,D.N.;Peng,T.Y.;Ma,L.;Cai,P.;Dai,K.Inorg.Chem.2009,48,4685.doi:10.1021/ic900064m
(37) García,J.;López,T.;Álvarez,M.;Aguilar,H.;Quintana,P.J.NonCryst.Solids 2008,354,729.doi:10.1016/j.jnoncrysol.2007.07.074
(38) Kanagadurai,R.;Sankar,R.;Sivanesan,G.;Srinivasan,S.;Rajasekaran,R.;Jayavel,R.Mater.Chem.Phys.2008,108,170.doi:10.1016/j.matchemphys.2007.09.041
(40)Ge,M.;Liu,L.;Chen,W.;Zhou,Z.CrysEngComm 2012,14,1038.doi:10.1039/c1ce06264f
(41) Fan,H.M.;Jiang,T.F.;Wang,D.J.;Wang,L.L.;Zhai,J.L.;He,D.Q.;Wang,P.;Xie,T.F.J.Phys.Chem.C 2012,116,2425.doi:10.1021/jp206798d