Reproductive Cycle and Seasonal Variations in Lipid Content and Fatty Acid Composition in Gonad of the Cockle Fulviamutica in Relation to Temperature and Food
LIU Wenguang1), LI Qi2), *, and KONG Lingfeng2)
Reproductive Cycle and Seasonal Variations in Lipid Content and Fatty Acid Composition in Gonad of the Cocklein Relation to Temperature and Food
LIU Wenguang, LI Qi, and KONG Lingfeng
1),,,510301,2),,,266003,
From March 2004 to February 2005, seasonal variations in lipid content andfatty acid composition of gonad of the cockle(Reeve) were studied on the eastern coast of China in relation to the reproductive cycle and environment conditions (., temperature and food availability). Histological analysis as well as lipid and fatty acid analyses were performed on neutral and polar lipids of the gonad. Results showed that gametogenesis occurred in winter and spring at the expense of lipids previously accumulated in summer and autumn, whereas spawning occurred in summer (20.4–24.6℃). The seasonal variation in lipid content was similar to that of the mean oocyte diameter. In both neutral and polar lipids, the 20:5n-3 and 22:6n-3 levels were relatively higher than saturated fatty acids, and polyunsaturated fatty acids were abundant, with series n-3 as the predominant component. Seasonal variations in the 20:5n-3 and 22:6n-3 levels and the principal n-3 fatty acids were clearly related to the reproductive cycle. The ∑(n-3) and ∑(n-6) values were relatively high during January–May, and the associated unsaturation index was significantly higher than that in other months. The results suggest that fatty acids play an important role in the gametogenesis of.
; lipids; fatty acids; reproductive cycle; food; temperature
1 Introduction
The cockle(Reeve) in the family Cardiidae is widely distributed in coastal waters of the northern Yellow Sea in China, southern Mutsu Bay to Kyushu in Japan, and the Korean Peninsula. It inhabits from intertidal to muddy-sand bottoms at a depth of 50m, and is a functional hermaphrodite bivalve species with rapid growth rates (Xu., 1995; Liu., 2008).is a food source of commercial importance, but current exploitation of this species is mainly based on natural populations (Li., 1994; 1999). For better management ofpopulations and successful development of relevant artificial breeding techniques, it is essential to understand its reproductive physiology.
The reproductive activity of marine bivalves is affected by complex interactions between endogenous factors (., energy storage-utilization cycle) and exogenous factors (., food availability and water temperature) (Barber and Blake, 1981; Bayne, 1976). Gametogenesis requires a continuous supply of nutrients for the biosynthesis of reproductive materials (Brokordt and Guderley, 2004).
However, there are interspecific and intraspecific differences in the energy sources of gametogenesis (Bayne, 1976). In some species of bivalves, gametogenesis takes place at the expense of the recently ingested food, whereas in other species, gametogenesis occurs using the previously stored nutrient reserves (Taylor and Venn, 1979; Barber and Blake, 1981). Reproduction, on the other hand, is influenced by the environmental conditions such as temperature and food availability (Gabbott, 1983).
In the reproductive processes of marine bivalves, lipids are important components as a major source of metabolic energy and essential materials for the formation of cell and tissue membranes (Holland, 1978). When food is abundant, the lipids are stored in the gonad prior to gametogenesis. They are then utilized for production of gametes when the metabolic demand is high (Mann, 1979; Li., 2000). In addition, the lipids have been shown to provide energy for growth in winter when carbohydrate sources are depleted (Li., 2006). The seasonal variations in lipid content and fatty acid composition of adult bivalves are thought to be closely linked to the reproductive cycle and affected by the quality and quantity of natural diet and water temperature (Beninger and Lucas 1984; Galap., 1997). The importance of fatty acids for maturation of bivalves has also been widely reported (Napolitano and Ackman, 1993; Soudant., 1996; Soudant., 1997; Soudant., 1999). Polyunsaturated fatty acids (PUFA) are considered to be the structural components during embryogenesis and the precursors of physiologically active molecules such as prostaglandins and other eicosanoids (Soudant., 1996; Delaunay., 1993; Langdon and Waldock, 1981). In case bivalves have a limited capacity to synthesize and modify fatty acids, the fatty acid composition of their lipids is markedly influenced by the food consumed. Therefore, the fatty acid composition may reflect useful information regarding trophic sources (Allan., 2010; Dalsgaard., 2003). Some fatty acids can be used as fatty acid trophic markers (FATMs) to trace the food sources of consumers (Kharlamenko., 2001; Pernet., 2012). Detailed knowledge of variations in the fatty acid composition ofin relation to gametogenesis and environment conditions is important. However, data is lacking regarding annual fatty acid variation induring reproductive cycle in relation to environment variables.
The aim of this study is to provide evidence for seasonal variations in the lipid content and fatty acid composition in gonad of, with particular interest in their correlation with the reproductive cycle and environment parameters. Results can be used forpopulation management and mariculture.
2 Materials and Methods
2.1 Sample Preparation
From March 2004 to February 2005, 30 cockles (shell height, 5.1cm±0.4cm; shell length, 4.6cm±0.3cm; wet flesh weight, 13.4g±2.2g) were collected monthly on the eastern coast of China (Weihai, Shandong) (36˚41΄N– 37˚36΄N and 121˚11΄E–121˚42΄E). The cockles were immediately transported to the laboratory for measurements of the fresh weight and linear dimension (length, height). Then, the cockles were dissected and the gonad was collected and stored at −80℃ before use.
During sampling, sea water temperature was measuredusing centigrade thermometer, and the chlorophyllcontent (0–1m depth) was determined according to Parsons. (1984). Phytoplankton were sampled with a standard Shallow III Microplankton Net (diameter 37 cm, mesh fiber JF62, mesh size 0.077mm) that was hauled vertically, strictly following the CSBTS standard method (CSBTS, 1991). The phytoplankton samples were fixed with Lugol’s solution, with cell number (counting units) counted under a light microscope.
2.2 Histology
Fifteen specimens from each gonad sample were selected for histological examination. A 5-mm-thick cube of the gonad was fixed in Bouin’s solution, dehydrated using graded series of ethanol, cleared in xylol and embedded in paraffin. The 6-μm thick sections were cut on a rotary microtome, stained with haematoxylin and eosin (Humason, 1979), and then examined by microscopy to develop a profile of gametogenesis. The diameter of 100 oocytes from five animals was measured monthly to determine the degree of maturity. There was no dioecism in the animals examined.
According to the scale of maturity (Gallucci and Gallucci, 1982), five gametogenic stages were classified, including 1) Undifferentiated stage: this stage was characterized by a total absence of gametes; the connective tissue occupied almost all of the space (Fig.1A). 2) Developing stage: in the female, there were rounded oocytes along with oocytes attached to the follicle wall; some detached oocytes occurred. In the male, varying quantities of spermatogenic cells were present (Fig.1B). 3) Ripe stage: in the female, most oocytes were free-living within the follicles, with some oocytes attached to the follicle wall. In the male, follicles were filled by spermatozoa arranged in characteristic bands (Fig.1C). 4) Partially spawned: in the female, large spaces inside the follicles and between free oocytes were present. Some follicles were completely devoid of oocytes. In the male, a substantial decrease in the quantity of spermatozoa was observed. Large spaces inside the follicles occurred. In some follicles, only a few residual spermatozoa were present (Fig.1D). 5) Spent: at this stage, some unspawned oocytes and spermatozoa were observed within follicles (Fig.1E).
2.3 Lipid and Fatty Acid Analyses
Five specimens from each gonad sample were used for lipid and fatty acid analyses. Total lipids were extracted from the gonad with chloroform/methanol 2:1 containing 0.1% butylated hydroxytoluene (BHT) as antioxidant following the Folch’s method (Folch., 1957). The lipid extracts were fractionated into neutral lipids (including triglycerides, free fatty acids, and sterols) and polar lipids (mainly including phospholipids with a small amount of glycolipids) using column chromatography on a silica gel hydrated with 6% water (Pernet., 2006). Briefly, the 100-mg columns were preconditioned with 1mL of methanol and 1mL of chloroform. The lipid aliquots (200μL) corresponding to about 1mg of lipid were loaded to the solid-phase extraction column. Samples were gently drawn into the solid phase under light vacuum, and the columns were washed with 1mL of chloroform-methanol (98:2) to elute neutral lipids followed by 5mL of methanol to elute polar lipids. The eluted fractions were collected in 7mL tubes positioned in a vacuum manifold apparatus. The vacuum was adjusted to generate a flow rate of about 1mLmin.
The fractions of polar lipids and neutral lipids were saponified with 1molLKOH/methanol solution and esterified with 2molLHCl/methanol solutions. Methyl esters were extracted with n-hexane. The fatty acid profile was determined by gas chromatography (HP 5890) using a Carbowax 20m column (25mm×0.35mm). The oven temperature was kept at 150℃ for 1min, and then increased to 200℃ for 15℃ per min, followed by an increase to 250℃ for 2℃ per min. Injector and detector temperatures were 270℃. Helium was used as the carrier gas. The identification of fatty acids was performed by comparing retention times with a standard mixture of fatty acids. The quantification was based on the relative peak areas. Results are expressed as a percentage of the total fatty acid area (Xue., 1995).
2.4 Statistical Analysis
The concentration of 16:1 represents the sum of two fatty acids: 16:1n-7 and 16:1n-9. All data are reported as mean ± standard error (=5). The relationships between the chlorophyllconcentration and the phytoplankton biomass and cell number were examined using a regression analysis. Pearson correlation was used to explore relationships between lipid content and the two environmental factors (sea water temperature and chlorophyllconcentration). One-way ANOVA followed by Tukey’s multiple comparison tests was used to assess the monthly differences in oocyte diameter, lipid content and fatty acids. Fatty acids from the neutral and polar fractions were analyzed separately.
3 Results
3.1 Environment Parameters
The seasonal variation in phytoplankton biomass in Rushan Bay was typical double-peak type (Fig.1). The highest peaks appeared in August and September, and the second highest peaks appeared in May and April. The cell number of phytoplankton ranged from 248×10to 3024×10cellsm, with an average of 1092×10cellsm. The phytoplankton biomass ranged from 2.0–40.7mgmannually, with an average of 13.3mgm.
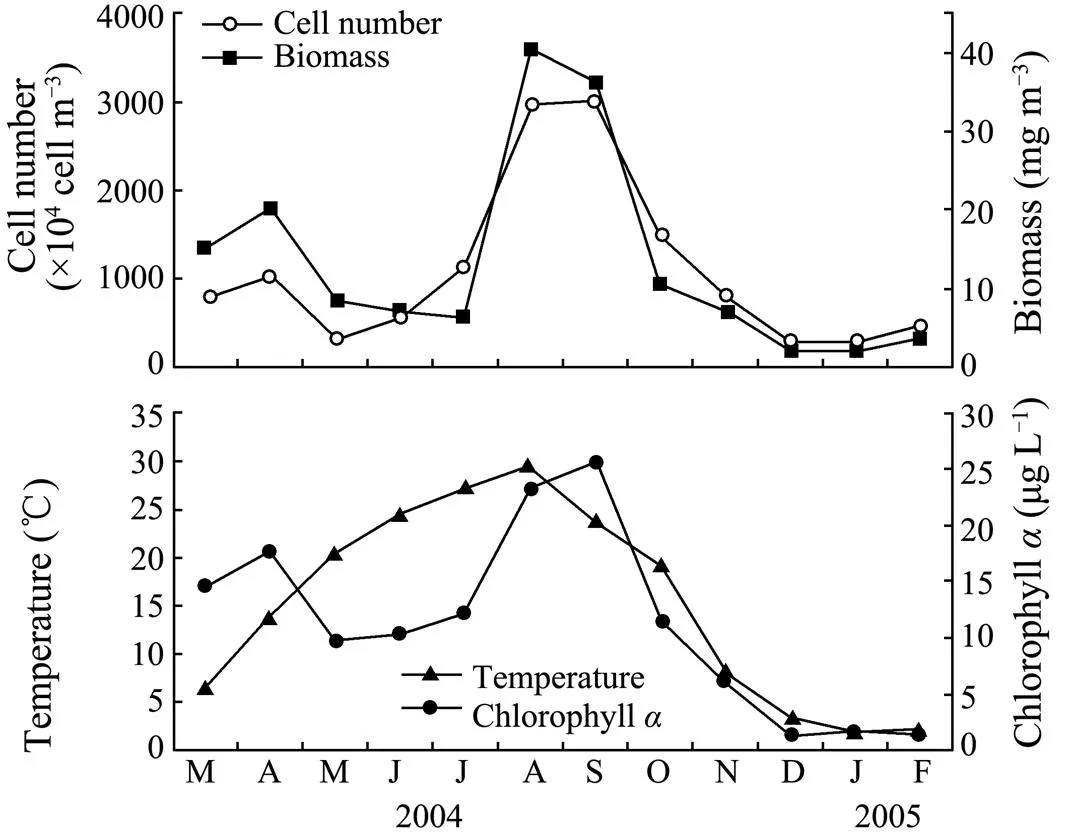
Fig.1 Monthly variations in phytoplankton cell number, biomass, water temperature and chlorophyll a concentrations in Weihai during March 2004–February 2005.
As shown in Fig.1, there is a seasonal cycle in water temperature during the study period. The maximum temperature was observed in summer (28℃ in August 2004); then the temperature gradually decreased towards winter (0.3℃ in January 2005). The concentration of chlorophyllexhibited a clear seasonal pattern characterized by two peaks,., the lower one in April 2004 (17.7μgL) and the higher one in September 2004 (25.8μgL). The chlorophyllconcentration was low in winter, and averaged 11.3±8.2μgLduring the entire experimental period. Regression analysis showed that chlorophyllconcentration was significantly correlated with phytoplankton biomass and cell number (<0.05) (Table 1). Pearson correlation analysis showed that the lipid content was significantly correlated with water temperature in June, October, January and February. A significant positive correlation was observed between the lipid content and chlorophyllconcentration in June–August, January and February (<0.05) (Table 2).

Table 1 Multiple regression relationships between chlorophyll a concentration and the phytoplankton biomass and cell number
Note:<0.05.
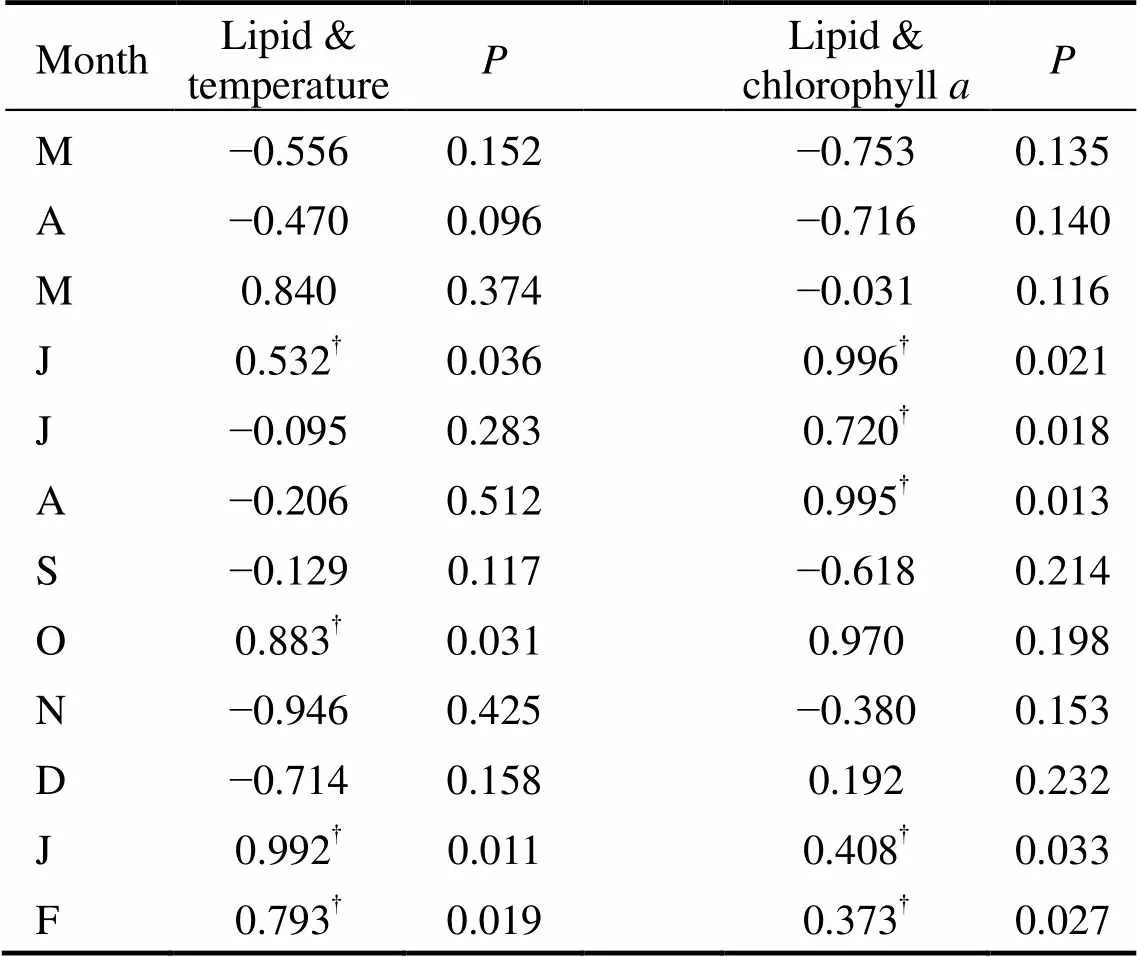
Table 2 Pearson correlation relationship between lipid content and the two environmental factors (sea water temperature and chlorophyll a concentration)
Note:<0.05.
3.2 Gametogenesis
The reproductive cycle ofdisplayed a clear seasonal pattern (Fig.2). The mean oocyte diameter showed significant monthly variations (<0.01), in- creasing from 20.8μm in November, reached a maximum value of 68.6μm in May, and then decreased after June. During July–October, the gonad ofwas spent, and thus oocyte diameter was not estimated. Gonad development started in November and peaked during March to April (Fig.2). Ripe gonads were found in February– May. Histological observations suggested thatpartially spawned between May and June. After spawning, there was a sexual resting period from July to October (Fig.3).

Fig.2 Monthly variations in oocyte diameter and gonadal development of F. mutica in Weihai during March 2004–February 2005. Data analyzed by one-way ANOVA and post hoc Tukey test. Mean values with different letters are significantly different (P<0.05). Bars represent standard errors.
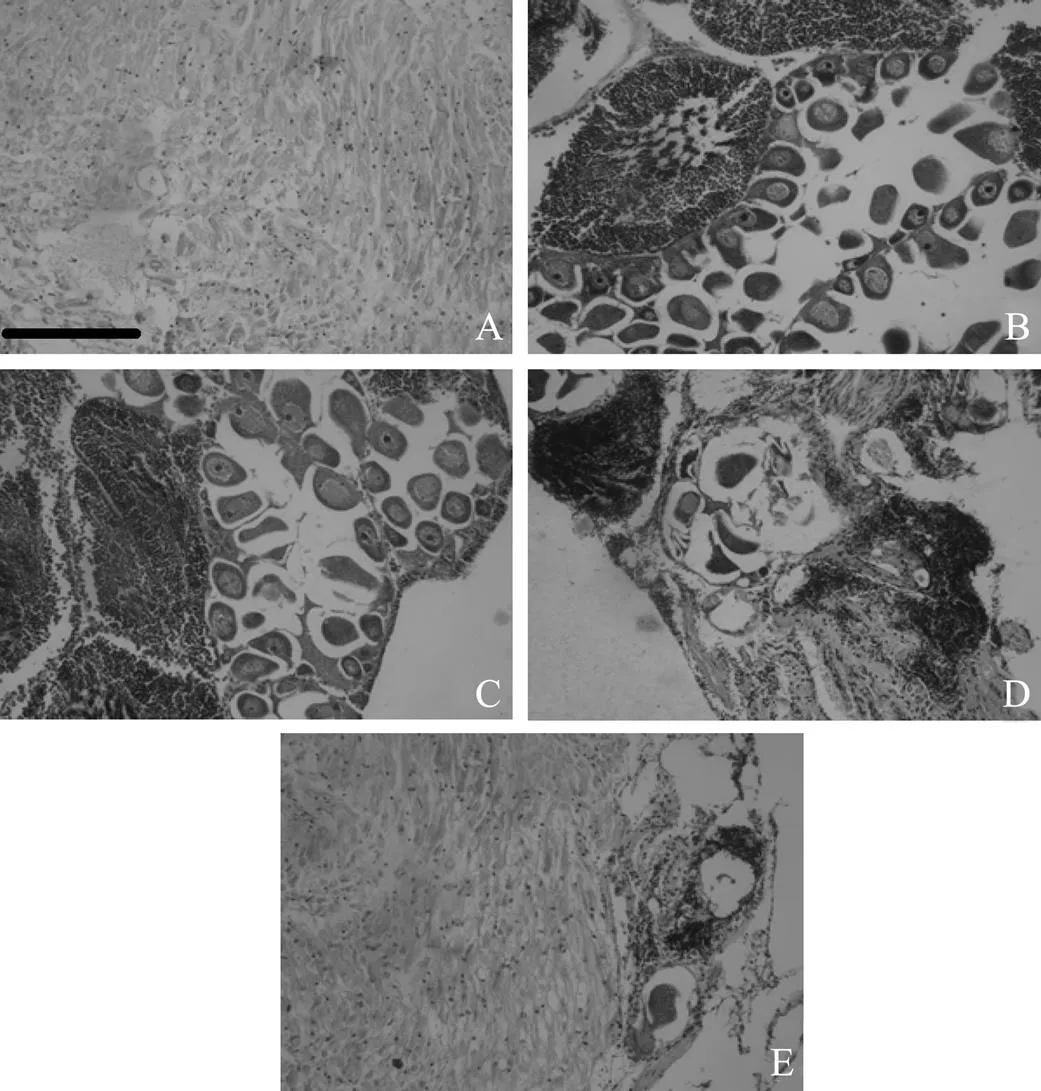
Fig.3 Photomicrographs showing the five stages of reproductive cycle in gonad of Fulvia mutica. (A) Undifferentiated; (B) Developing; (C) Ripe; (D) Partially spawned; and (E) Spent. Bar=50µm.
3.3 Lipid Classes and Fatty Acid Composition
As shown in Fig.4, the lipid content of gonad ofsignificantly varied among months (<0.01). It ranged from 1.5% to 3.0% of wet weight, with a gradual increase in January–March followed by a decrease during spawning. The lipid content increased again in July–August.
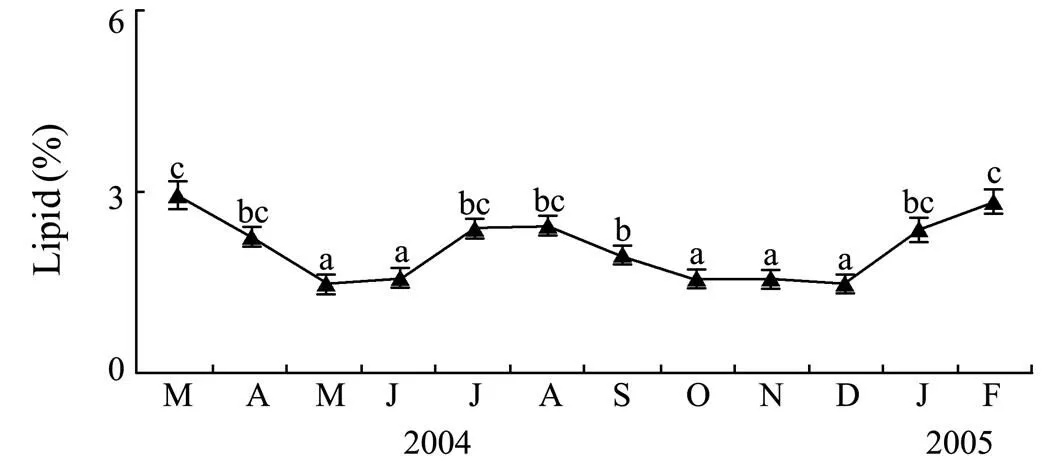
Fig.4 Monthly variations in lipid content of gonad of F. mutica in Weihai during March 2004–February 2005 Data analyzed by one-way ANOVA and post hoc Tukey test. Mean values with different letters are significantly different. Bars represent standard errors.
Regarding the fatty acid composition of neutral lipids in the gonad (Table 3), the PUFA was found predominant (29.7%–42.3% of total fatty acid), followed by the SFA (15.5%–28.5%) and MUFA (4.9%–9.8%). The changes in the PUFA content followed a similar pattern to the reproductive cycle, whereas the variations in the SFA were consistent with that of the PUFA. The palmitic (16:0), eicosapentaenoic (20:5n-3) and docosahexaenoic (22: 6n-3) were the most abundant fatty acids (Table 3). The ∑(n-3) values were relatively high in January–May, and the ∑(n-6) values were low in January–March. The DHA/ EPA ratio was significantly lower in January–March than in other months, whereas the UI was significantly higher in January–May than in other months (Table 3).
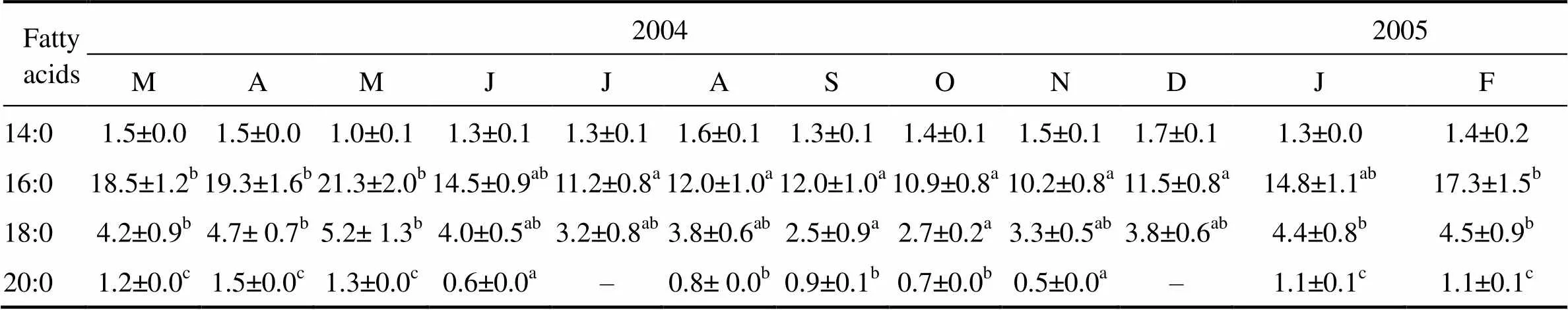
Table 3 Seasonal variations of the fatty acid composition in neutral lipids of Fulvia mutica gonad (Results expressed as percent of total phospholipid fatty acids)

Fattyacids20042005 MAMJJASONDJF ∑SFA25.4±2.8c27.0±1.1c28.5±1.8c20.4±1.3b15.7±1.4a18.2±1.8b16.7±2.2a15.7±2.0a15.5±1.4a16.9±1.2a21.6±1.6b24.2±2.4c 16:13.1±0.53.5±0.33.4±0.72.5±0.42.5±0.22.2±0.92.3±0.62.5±0.52.0±0.42.3±0.82.2±0.32.5±0.9 18:1n-93.9±0.8ab3.5±0.8ab4.9±0.3b2.8±0.4a2.5±0.5a2.0±0.7a2.5±0.7a2.4±0.9a2.4±0.3a3.3±0.9ab3.7±0.5ab4.7±0.2b 18:1n-7–0.7±0.6a1.7±0.1b1.2±0.3ab0.9±0.3a0.5±0.1a1.7±0.1b1.0±0.2ab0.8±0.1a–1.5±0.1b1.1±0.2ab ∑MUFA7.0±1.5ab7.8±1.2b9.8±1.2b6.5±0.8ab5.8±1.2a4.9±0.9a6.5±1.2ab5.9±1.4a5.2±1.5a5.6±1.1a7.4±1.4ab8.4±1.6b 18:2n-61.0±0.0b0.9±0.0b0.9±0.2b0.7±0.1ab0.6±0.1ab0.9±0.0b0.8±0.1b0.7±0.1ab0.6±0.1ab––0.4±0.0a 20:4n-6–1.1±0.1a1.7±0.1ab2.3±0.1b1.5±0.1ab1.2±0.1a1.1±0.1a1.1±0.1ab1.9±0.2b1.7±0.1ab1.5±0.1ab0.9±0.1a 18:3n-31.4±0.11.8±0.11.4±0.11.4±0.11.1±0.11.1±0.01.2±0.11.0±0.11.3±0.2–1.3±0.11.3±0.1 20:5n-315.5±1.5b15.2±1.4b12.3±1.5ab9.6±1.3a9.9±1.1a10.3±1.0a11.3±1.4ab11.5±1.0ab10.1±1.5a10.9±1.4ab15.6±1.6b15.1±1.1b 22:6n-324.6±1.6c25.4±2.0c22.4±1.7b15.8±1.6a16.8±1.8a20.8±1.8b15.7±1.0a19.3±2.0b19.7±1.8b18.4±1.6b22.0±1.3b21.0±1.7b ∑PUFA42.5±4.0b44.4±4.4b38.7±5.1b29.8±3.6a29.9±3.6a34.3±4.3ab30.1±3.6a33.6±4.1ab33.2±2.9ab31.0±3.7a40.4±3.1b38.7±2.8b ∑(n-3)41.5±2.4a42.4±1.2a36.1±1.1ab26.8±1.0b27.8±1.0b32.2±0.9b28.2±0.8b31.8±1.0b31.1±1.2b29.3±1.5b36.9±1.0ab37.4±1.0ab ∑(n-6)1.0±0.0a2.0±0.1b2.6±0.2c3.0±0.1c2.1±0.1b2.1±0.1b1.9±0.1b1.8±0.1b2.1±0.2b1.7±0.1b1.5±0.1ab1.3±0.1a DHA/EPA1.2±0.1a1.7±0.2b1.8±0.1b1.7±0.1b1.7±0.1b2.2±0.2b1.4±0.1b1.7±0.1b2.0±0.1b1.7±0.1b1.4±0.1a1.4±0.1a UI238.3±5.4a247.4±7.8a228.5±6.9a154.1±6.2b166.6±5.3b192.1±4.7ab166.8±9.0b188.0±8.1ab186.6±7.9ab177.3±7.2ab227.3±9.4a218.2±9.6a
Notes: ∑SFA represents the summation of saturated fatty acids; ∑MUFA represents the summation of monosaturated fatty acids; ∑PUFA represents the summation of polysaturated fatty acids; ‘–’ means not detected; Means not sharing the same superscript are significantly different; UI, unsaturation index was calculated as the sum of the percentage of each unsaturated FA multiplied by the number of double bonds within that FA.
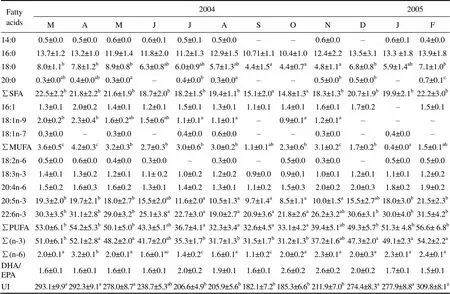
Table 4 Seasonal variations of the fatty acid composition in polar lipids of Fulvia mutica gonad (Results expressed as percent of total phospholipid fatty acids)
Notes: The same as those of Table 3.
4 Discussion
It has been reported that the timing and duration of gametogenesis are different among bivalves in different regions of the world (Pazos., 1997; Bayne, 1976; Brokordt., 2004). In the present study, we observed that the gametogenesis ofbegan the period November–December when water temperature was low (6.6℃). It occurred at the expense of the stored reserves because of the poor food availability, as indicated by the low chlorophylllevels. Similar results have been observed inin the west coast of France(Berthelin., 2000). The mean oocyte diameter reached a maximum value in May. A single spawning occurred over a short period and might be restricted to May and June when water temperature is 18.9–23.1℃. After spawning,had a resting phase in summer–autumn.
As the major energy source and/or membrane constituents of cells, lipids play an important role in the biochemistry, metabolism and reproduction of marine bivalves (Galap., 1997; Alkanani., 2007). Cycles in the lipid content ofshowed annual patterns of accumulation and use of reserves. The lipids accumulated by the developing oocytes provide the necessary energy for the biosynthetic processes of oogenesis and vitellogenesis (Palacios., 2007). The lipid reserves in the gonad decreased during gametogenesis, suggesting that they were used to provide energy when food was scarce.
Several studies have proposed that (n-3) and (n-6) HUFA are involved in the reproductive processes of bivalves and 22:6n-3 plays a major role at structural and functional level of cell membranes (Uki., 1986; Pazos., 2003). The decrease in 22:6n-3 in the cockle lipid content suggested that the cockle had low ability for elongation and desaturation of dietary PUFAs. A large part of the changes in HUFA composition oflipids can be explained by a direct transformation of algae fatty acids into neutral and polar lipids. This result was in good agreement with those obtained from oysters (Langdon and Waldock, 1981). It was also consistent with the findings of Waldock and Holland (1984), who demonstrated that juvenile oysters are able to elongate and further desaturate dietary long chain fatty acids but not in sufficient amounts to sustain optimum growth.
The decrease in PUFA levels ofduring June–July likely reflected a combination of 1) reduced dietary intake of PUFA due to substantially low phytoplankton biomass in June–July; 2) energetic stress as evidenced by a relative increase in the saturated fatty acids with energetic-type functions, such as C14:0 and C16:0 (Freites., 2002); and 3) the cockle having made a greater investment in PUFA to egg. These hypotheses are supported by the documented lower level of total PUFA, eicosapentaenoic acid (EPA) and particularly DHA in the cockle. Of these fatty acids, DHA plays a major role in gametogenesis.
Our work demonstrats that the lipid content shows a seasonal variation similar to that of the mean oocyte diameter, and that the fatty acids play an important role in gametogenesis of. These results provide the first hand evidence for evaluating the mechanisms of the reproductive physiology of.
Acknowledgements
The study was supported by the National Marine Public Welfare Research Program (201205023), and the Scientific and Technical Supporting Program (2011BAD 13B03).
Alkanani, T., Parrish, C. C., Thompson, R. J., and McKenzie, C. H., 2007. Role of fatty acids in cultured mussels,, grown in Notre Dame Bay, Newfoundland., 348: 33-45.
Allan, E. L., Ambrose, S. T., Richoux, N. B., and Froneman, P. W., 2010. Determining spatial changes in the diet of nearshore suspension-feeders along the South African coastline: Stable isotope and fatty acid signatures.,,87: 463-471.
Barber, B. J., and Blake, N. J., 1981. Energy storage and utilization in relation to gametogenesis in(Say)., 52: 121-134.
Bayne, B. L., 1976. Aspects of reproduction in bivalve molluscs. In:. Wiley, M. L., ed.,Academic Press, New York, 432-448.
Beninger, P. G., and Lucas, A., 1984. Seasonal variations in condition, reproductive activity, and gross biochemical composition of two species of adult clam reared in a common habitat:L. (Jeffreys) and(Adams & Reeve)., 79: 19-37.
Berthelin, C., Kellner, K., and Mathieu, M., 2000. Storage metabolism in the Pacific oyster () in relation to summer mortalities and reproductive cycle (West Coast of France).,125B: 359-369.
Brokordt, K. B., and Guderley, H. E., 2004. Energetic requirements during gonad maturation and spawning in scallops: sex differences in(Muller 1776)., 23: 25-32.
China State Bureau of Technical Supervision (CSBTS), 1991. The Specification for Oceanographic survey: Marine Biological Survey (GB12763.6-91), 17-22.
Dalsgaard, J., St. John, M., Kattner, G., Muller-Navarra, D., and Hagen, W., 2003. Fatty acid trophic markers in the pelagic marine environment., 46: 225- 340.
Delaunay, F., Marty, Y., Moal, J., and Samain, J.-F., 1993. The effect of monospecific algal diets on growth and fatty acid composition of(L.) larvae., 173: 163-179.
Folch, J., Lees, M., and Stanley-Sloane, G. H., 1957. A simple method for the isolation purification of total lipids from animal tissues., 226: 497- 507.
Freites, L., Fernández-Reiriz, M. J., and Labarta, U., 2002. Lipid classes of mussel seedsof subtidal and rocky shore origin., 207: 97-111.
Gabbott, P. A., 1983. Developmental and seasonal metabolic activities in marine molluscs. In:, Vol. 2. Hochachka, P. W., ed., Academic Press, New York, 165-217.
Galap, C., Leboulenger, F., and Grillot, J. P., 1997. Seasonal variations in biochemical constituents during the reproductive cycle of the female dog cockle., 129: 625-634.
Gallucci, V. F., and Gallucci, B. B., 1982. Reproduction and ecology of the hermaphroditic cockle(Bivalvia: Cardiidae) in Garrison Bay., 7: 137-145.
Holland, D. L., 1978. Lipid reserves and energy metabolism in the larvae of benthic marine invertebrates. In:.Malin, D. C., and Sargent, J. R., eds., Academic Press, London, 85-123.
Humason, G. L., 1979.. W. H. Freeman and Company, San Francisco, 661pp.
Kharlamenko, V. I., Kiyashko, S. I., Imbs, A. B., and Vysh- kvartzev, D. I., 2001. Identification of food sources of invertebrates from the seagrasscommunity using carbon and sulfur stable isotope ratio and fatty acid analyses.,220: 103-117.
Langdon, C. J., and Waldock, M. J., 1981. The effect of algal and artificial diets on the growth and fatty acid composition ofspat.,61: 431-448.
Li, Q., Liu, W. G., Shirasu, K., Chen, W. M., and Jiang, S. X., 2006. Reproductive cycle and biochemical composition of the Zhe oysterGmelin in an eastern coastal bay of China., 261: 752-759.
Li, Q., Osada, M., and Mori, K., 2000. Seasonal biochemical variations in Pacific oyster gonadal tissue during sexual maturation., 66: 502-508.
Li, S. Y., Lu, N., Jiang, S., and Bi, Y. P., 1994. Early development ofReeve., 13: 3-5 (in Chinese).
Li, S. Y., Lu, N., Jiang, S., and Bi, Y. P., 1999. Effect of temperature and salinity on survival and growth of the veliger of head clam (Reeve)., 14: 66-69 (in Chinese).
Liu, W. G., Li, Q., Yuan, Y. D., and Zhang, S. H., 2008. Seasonal variations in reproductive activity and biochemical composition of the cockle(Reeve) from the eastern coast of China., 27: 405-411.
Mann, R., 1979. Some biochemical and physiological aspects of growth and gametogenesis inandgrown at sustained elevated temperature., 59: 95-110.
Napolitano, G. E., and Ackman, R. G., 1993. Fatty acid dynamics in sea scallops(Gmelin, 1791) from George bank, Nova Scotia.,12: 267-277.
Palacios, E., Racotta, I. S., Arjona, O., Marty, Y., Le Coz, J. R., Moal, J., and Samain, J. F., 2007. Lipid composition of the pacific lion-paw scallop,, in relation to gametogenesis 2. Lipid classes and sterols., 266: 266-273.
Parsons, T. R., Maita, Y., and Lalli, C. M., 1984.. Pergamon Press, New York, 173pp.
Pazos, A. J., Román, G.., Acosta, C. P., Abad, M., and Sánchez, J. L., 1997. Seasonal changes in condition and biochemical composition of the scallopL. from suspended culture in the Ria de Arousa (Galicia, N.W. Spain) in relation to environmental conditions., 211: 169-193.
Pazos, A. J., Sánchez, J. L., Román, G., Luz Pérez-Parallé, M., and Abad, M., 2003. Seasonal changes in lipid classes and fatty acid composition in the digestive gland of., 134B: 367- 380.
Pernet, F., Malet, N., Pastoureaud, A., Vaquer, A., Quéré, C., and Dubroca, L., 2012. Marine diatoms sustain growth of bivalves in a Mediterranean lagoon., 68: 20-32.
Pernet, F., Pelletier, C., and Milley, J., 2006. Comparison of three solid-phase extraction methods for fatty acid analysis of lipid fractions in tissues of marine bivalves., 1137A: 127-137.
Soudant, P., Marty, Y., Moal, J., Robert, R., Quéré, C., Le Coz, J. R., and Samain, J. F., 1996. Effect of food fatty acid and sterol quality ongonad composition and reproduction process., 143: 361-378.
Soudant, P., Moal, J., Marty, Y., and Samain, J. F., 1996. Impact of the quality of dietary fatty acids on metabolism and the composition of polar lipid classes in female gonads of(L.)., 205: 149-163.
Soudant, P., Moal, J., Marty, Y., and Samain, J. F., 1997. Composition of polar lipid classes in male gonads of(L.). Effect of nutrition., 215: 103-114.
Soudant, P., Van, R. K., Marty, Y., Moal, J., Samain, J. F., and Sorgeloos, P., 1999. Comparison of the lipid class and fatty acid composition between a reproductive cycle in nature and a standard hatchery conditioning of the Pacific oyster.,123B: 209-222.
Taylor, A. C., and Venn, T. J., 1979. Seasonal variation in weight and biochemical composition of the tissues of the queen scallopfrom the Clyde sea area., 59: 605-621.
Uki, N., Sugiura, M., and Watanabe, T., 1986. Requirement of essential fatty acids in the abalonehannai., 52: 1013-23.
Waldock, M. J, and Holland, D. L., 1984. Fatty acid metabolism in young oysters,: polyunsaturated fatty acids., 19: 332-336.
Xu, K., Li, C. L., and Xu, Y. F., 1995. Biology studies in., 1-2: 12-18 (in Chinese).
Xue, C., Wang, Y., Li, Z., and Lou, W., 1995. Changes in lipids of mussel during storage., 2: 56-63 (in Chinese).
(Edited by Qiu Yantao)
10.1007/s11802-013-1979-1
ISSN 1672-5182, 2013 12 (3): 427-433
. Tel: 0086-532-82031622 E-mail: qili66@ouc.edu.cn
(March 17, 2012; revised May 15, 2012; accepted November 27, 2012)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2013
 Journal of Ocean University of China2013年3期
Journal of Ocean University of China2013年3期
- Journal of Ocean University of China的其它文章
- Evaluation of Antitumor, Immunomodulatory and Free Radical Scavenging Effects of A New Herbal Prescription Seaweed Complex Preparation
- Effects of Exposure to Four Endocrine Disrupting-Chemicals on Fertilization and Embryonic Development of Barbel Chub (Squaliobarbus curriculus)
- A Preliminary Phylogenetic Analysis of Luidia (Paxillosida:Luidiidae) from Chinese Waters with Cytochrome Oxidase Subunit I (COI) Sequences
- Seasonal Community Structure of Mesozooplankton in the Daya Bay, South China Sea
- The Effect of Three Culture Methods on Intensive Culture System of Pacific White Shrimp (Litopenaeus vannamei)
- Effects of Dietary Corn Gluten Meal on Growth Performance and Protein Metabolism in Relation to IGF-I and TOR Gene Expression of Juvenile Cobia (Rachycentron canadum)
