Effects of Exposure to Four Endocrine Disrupting-Chemicals on Fertilization and Embryonic Development of Barbel Chub (Squaliobarbus curriculus)
NIU Cuijuan*, WANG Wei, GAO Ying, and LI Li
Effects of Exposure to Four Endocrine Disrupting-Chemicals on Fertilization and Embryonic Development of Barbel Chub ()
NIU Cuijuan, WANG Wei, GAO Ying, and LI Li
,,,100875,
The toxicities of 4 common endocrine-disrupting chemicals (EDCs), 17b-estradiol (E2), p,p’-dichlorodiphenyldi- chloro-ethylene (DDE), 4-nonylphenol (NP) and tributyltin (TBT), to sperm motility, fertilization rate, hatching rate and embryonic development of Barbel chub () were investigated in this study. The duration of sperm motility was significantly shortened by exposure to the EDCs at the threshold concentrations of 10ngLfor E2 and TBT, 1μgLfor NP and 100μgLfor DDE, respectively. The fertilization rate was substantially reduced by the EDCs at the lowest observable effect concentrations (LOECs) of 10ngLfor E2 and TBT and 10μgLfor DDE and NP, respectively. Of the tested properties of, larval deformity ratewas most sensitive to EDC exposure and was significantly increased by DDE at the lowest experimental level of 0.1μgL. Other EDCs increased the larval deformity rate at the LOECs of 1ngLfor E2, 10ngLfor TBT and 1μgLfor NP, respectively. Despite their decreases with the increasing EDC concentrations, the hatching rate and larval survival rate ofwere not significantly affected by the exposure to EDCs. The results indicated that all the 4 EDCs affected significantly and negatively the early life stages of the freshwater fish. Overall, E2 and TBT were more toxic than NP and DDE, while DDE might be more toxic to larval deformity rate than to other measured parameters. Thus, the 4 EDCs showed potential negative influences on natural population dynamics of. Our findings provided valuable basic data for the ecological risk assessment of E2, DDE, NP and TBT.
17β-estradiol; p,p’-dichlorodiphenyldichloro-ethylene; 4-nonylphenol; tributyltin;; early life stage
1 Introduction
Endocrine-disrupting chemicals (EDCs) are synthetic chemicals or natural compounds that interfere with endocrine in animals (Diamanti-Kandarakis., 2009). Common EDCs in water environments include 4-Non- ylphenol (NP), dichlorodiphenyldichloro-ethylene (DDE), tributyltin (TBT) and 17β-estradiol (E2) (Gao., 2012). NP is a degradation product of nonylphenol ethoxylates (NPEO) which is extensively used for production of plastics, pesticides and cleaning agents. In China, NP is found ubiquitous in aquatic environments with a concentration range from <0.01 to 180μgL−1 in freshwater ecosystems (Shao ., 2002). DDE, including two isomers p,p’-DDE and o,p’-DDE, is the primary metabolite of dichlorodiphenyltrichloroethane (DDT), a widely used pesticide in the last century, which is capable of remaining in the environment for over a decade (Milston., 2003). Xue. (2005) reported that DDE level ranged from 3.25nggto 17.2ngg−1 in the substrate of Guanting Reservoir, Beijing. TBT is another popular chemical utilized in antifouling paints and plastic products (Ferraro ., 2004). Gao (2004) reported that the mean TBT concentration was as high as 93.8ngLseawater in Bohai, China. Other research indicated that exposure of eggs and yolk sac fry of the minnowto 0.82–19.51µgLTBT led to mortality and behavioral, morphological and histopathological effects (Fent and Meier, 1992). The synthetic E2is commonly used for contraception and related pharmaceutical purposes, which enters the aquatic environmenteffluent discharges from sewage treatment works (Lange., 2001). In the sediments of Wenyu River, Beijing, E2 level has been found to range from 0.39nggto 36.6ngg(Lei., 2008). Nimrod and Benson (1998) reported that the sexual differentiation and fecundity of Japanese medaka () was altered by exposure to environmentally relevant concentrations of E2 (<10ngL). It has been shown that all of the 4 EDCs have either developmental or reproductive toxicities to fish, especially at their early life stages (Labadie and Budzinski, 2006).
Several studies showed that wild freshwater fish are facing significant risk of EDCs exposure (Rolland, 2000; Diamanti-Kandarakis., 2009). For example, the concentrations of DDTs (containing 54%–100% p,p’-DDE) and TBT in muscle of wild Chinese sturgeon () in Yangtze River have reached 15.9µgglipid weight (Wan., 2007) and 4.3nggwet weight (Hu., 2009), respectively. Associated female-to-male sex ratio ofpopulation increased 8 times while the motility of sperm substantially decreased in the last 2 decades (Hu., 2009).
The Barbel chub () is a freshwater fish with great economical value. It is widespread in natural rivers and lakes in China. Presently, little is known about the effects of EDCs on early life stages of.. Thus, the present work examined the toxicities of E2, NP, p,p’-DDE (hereafter referred to as DDE) and TBT to sperm activity, fertilization and embryonic development ofat environmentally relevant concentrations. The results can be used for ecological risk assessment of EDCs to the fish
2 Materials and Methods
2.1 Fish
The experiment was conducted in Huanxin Hatchery, Tianjin, China. A pair of healthy mature male (600g) and female fish (800g) cultured in a farm pond in the hatchery was selected as the parents to obtain sperms and eggs.
2.2 Chemicals and EDC Treatment Solutions
The 4 EDCs (E2, DDE, NP, and TBT) and the co-sol- vent (dimethyl sulfoxide, DMSO) were purchased from Sigma Company (Beijing, China). The EDCs were prepared in 0.005% DMSO at different concentrations (Table 1). A blank control (BC) of distilled water, a 0.005% DMSO control (DMSOC) of 0.005% DMSO solution and 4 treatments of a specific EDC solution at increasing concentrations (G1, G2, G3, and G4) were prepared, 3 repeats each. All solutions were adjusted to pH 7.4 in accordance with the blank control. The experimental concentrations of EDCs were chosen based on the literature and the values reported in natural water bodies (Rolland, 2000; Segner., 2003; Yang., 2006; Shen., 2005; Liu., 2005).

Table 1Concentrations of b-estradiol (E2), p,p’-dichlorodi- phenyldichloro-ethylene (DDE), 4-nonylphenol (NP), and tributyltin (TBT) in different treatment groups
Notes: BC, blank control; DC, 0.005% DMSO solution control; G1–G4, treatment groups with graded EDCs dose levels dissolved in 0.005% DMSO solution.
2.3 Experimental Procedure
The parent fish were collected gently from the pond and brought to the laboratory. Water around the gonopore was removed with a clean towel. Semen and eggs were then collected with a clean Petri dish by gently pressing the abdomen of the fish.
For examination of the sperm motility,2μL of semen was added to 200μL of treatment solution on a single concave glass slide and gently mixed by pipetting. The sperm were quantified under a light microscope (Axioskop 2, Zeiss). According to Ruan. (2004), the duration of sperm motility was taken as the period from the mix of semen and treatment solution to 70% of the sperm losing normal motility in one field of view (100´magnification, 6 fields of view checked for each sample). Treatments each were quantified in triplicates.
For examination of the fertilization process, glass Petri dishes were pre-soaked in different treatment solutions for 24h. To each Petri dish was added about 100 eggs, 100mL of semen, and 100mL of specific treatment solution, and groups each were prepared in triplicate (Billard, 1978). All treatment solutions were replaced 3 times per day to maintain comparatively constant EDC concentrations during the experiment. The developmental processes of fertilized eggs were continually checked by examining the mortality at each developmental stage until the end of the yolk-sac stage. The number of deformed larva was counted after hatching. Deformed larva was defined based on at least one of the following characteristics: malformation of the vertebral axis; imbalance in yolk sac absorption; and abnormal swimming (Fig.1).
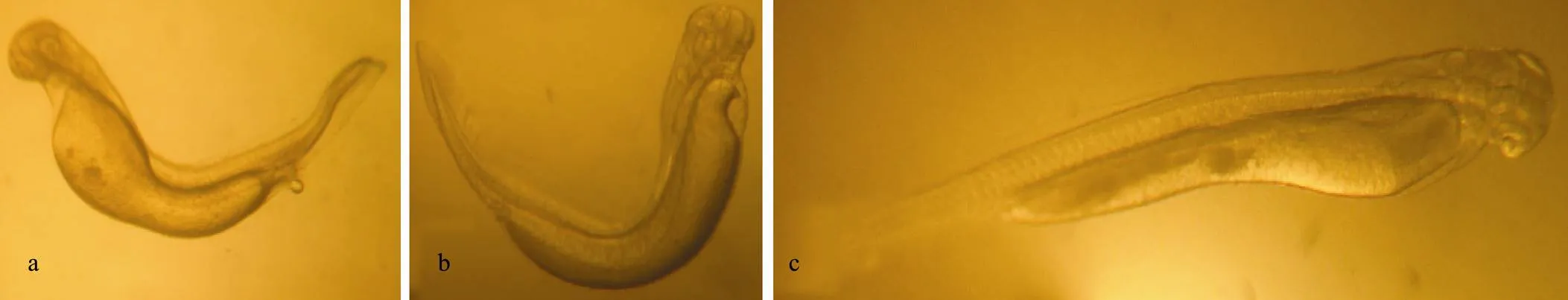
Fig.1 Deformed larvae of Squaliobarbus curriculus exposed to different EDCs. a, b: Scoliosis; c: Unbalanced absorption of yolk sac.
The following parameters were calculated as:
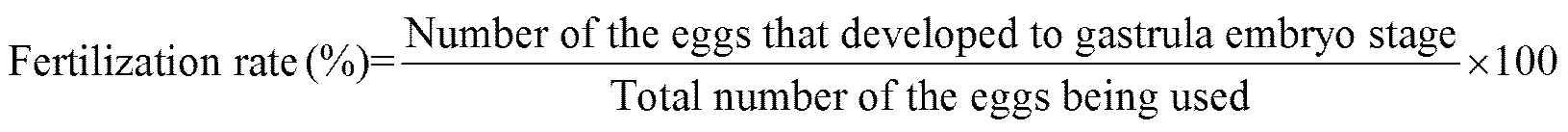
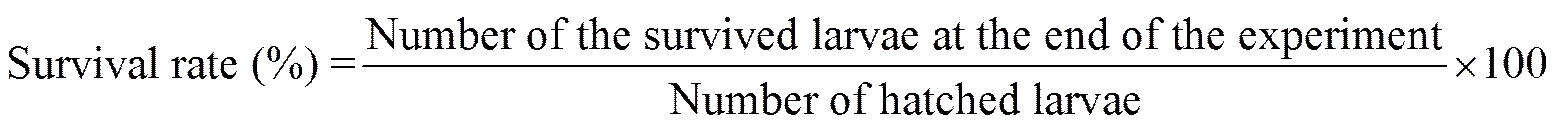
2.4 Statistical Analyses
The data were presented as mean value ± SE. All data were examined for normality and homogeneity of variances prior to statistical analyses. For data with homogenous variances, one-way analysis of variance (ANOVA) was utilized to compare the means among different groups. Significant difference among groups was further evaluated using theTukey’s multiple comparison tests. For data with non-homogeneous variances, the Kruskal-Wallis test followed by the Dunnett’s T3 test was applied. If a positive concentration-dependent effect was observed, the no-observed-effect concentration (NOEC) and the lowest-observed-effect concentration (LOEC) were evaluated by multiple comparisons with the control. The maximum acceptable toxic concentration (MATC) was indicated by the Geometric mean of NOEC and LOEC. All statistical analyses were carried out using SPSS 16.0 for Windows (SPSS Inc., USA).<0.05 was considered to be statistically significant different.
3 Results
Two-Independent-Samples-Test showed thathad no statistically significant differences in the measured properties between BC and DMSOC control groups (>0.05). Therefore, the data of DMSOC group were used as the control in the following analyses.
3.1 EDC Effects on the Sperm Motility of
All of the 4 chemicals significantly affected the duration of sperm motility of(<0.05 for E2;<0.01 for DDE, NP and TBT) in a negative concentration-dependent manner (Figs.2 and 3). Compared to the control, groups treated with ≥10ngLE2 or TBT, ≥1μgLNP, or ≥100μgLDDE had significantly shorter duration of sperm motility (Fig.2). Accordingly, NOEC, LOEC and MATC were estimated within the experimental concentration ranges (Table 2).
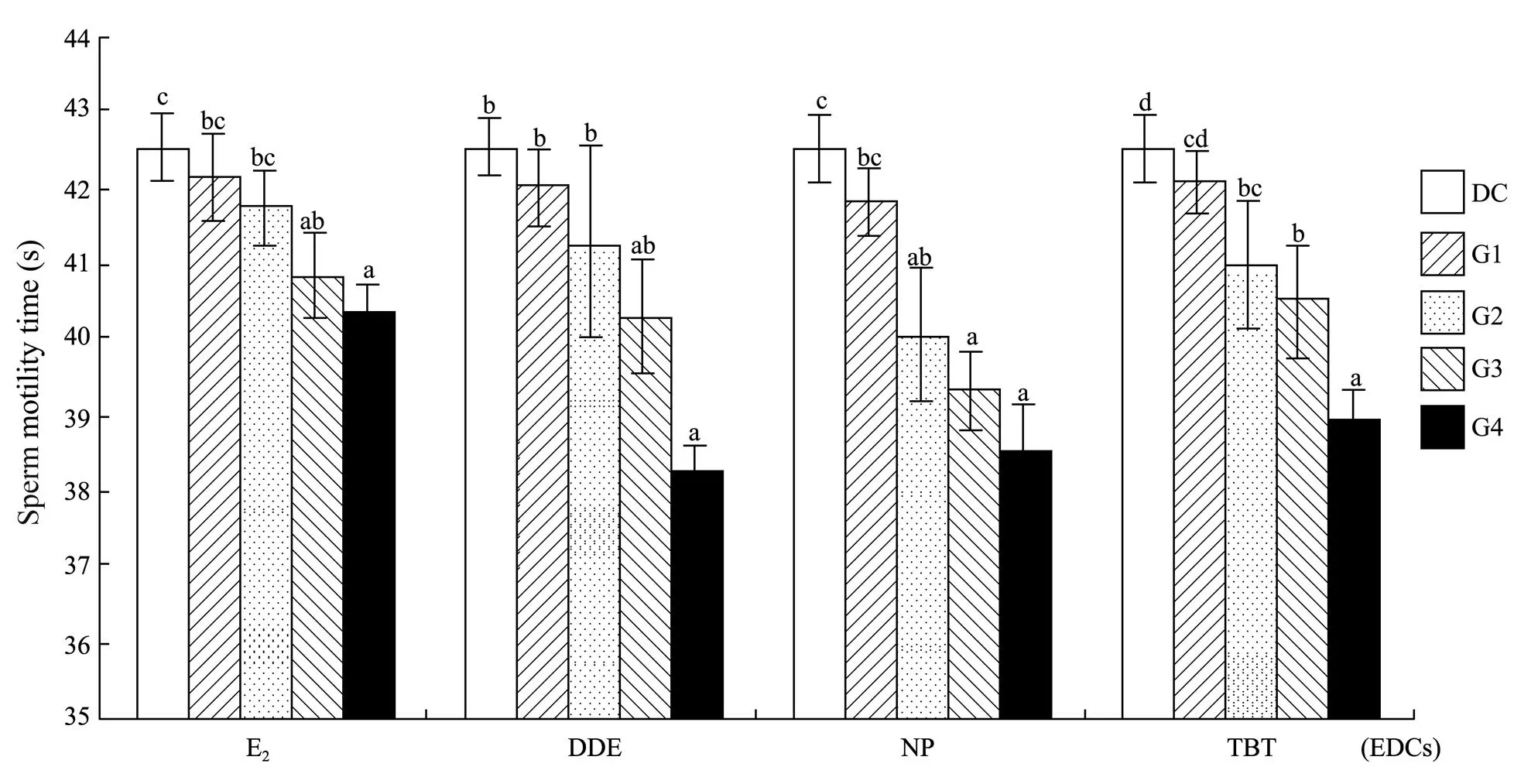
Fig.2 Duration of sperm motility of Squaliobarbus curriculus exposed to different concentrations of b-estradiol (E2), p,p’-dichlorodiphenyldichloro-ethylene (DDE), 4-nonylphenol (NP), and tributyltin (TBT). Data with different letters on the bar are significantly different (P<0.05). DC, DMSO control group; G1–G4, Experimental groups with graded chemical concentrations shown in Table 1.
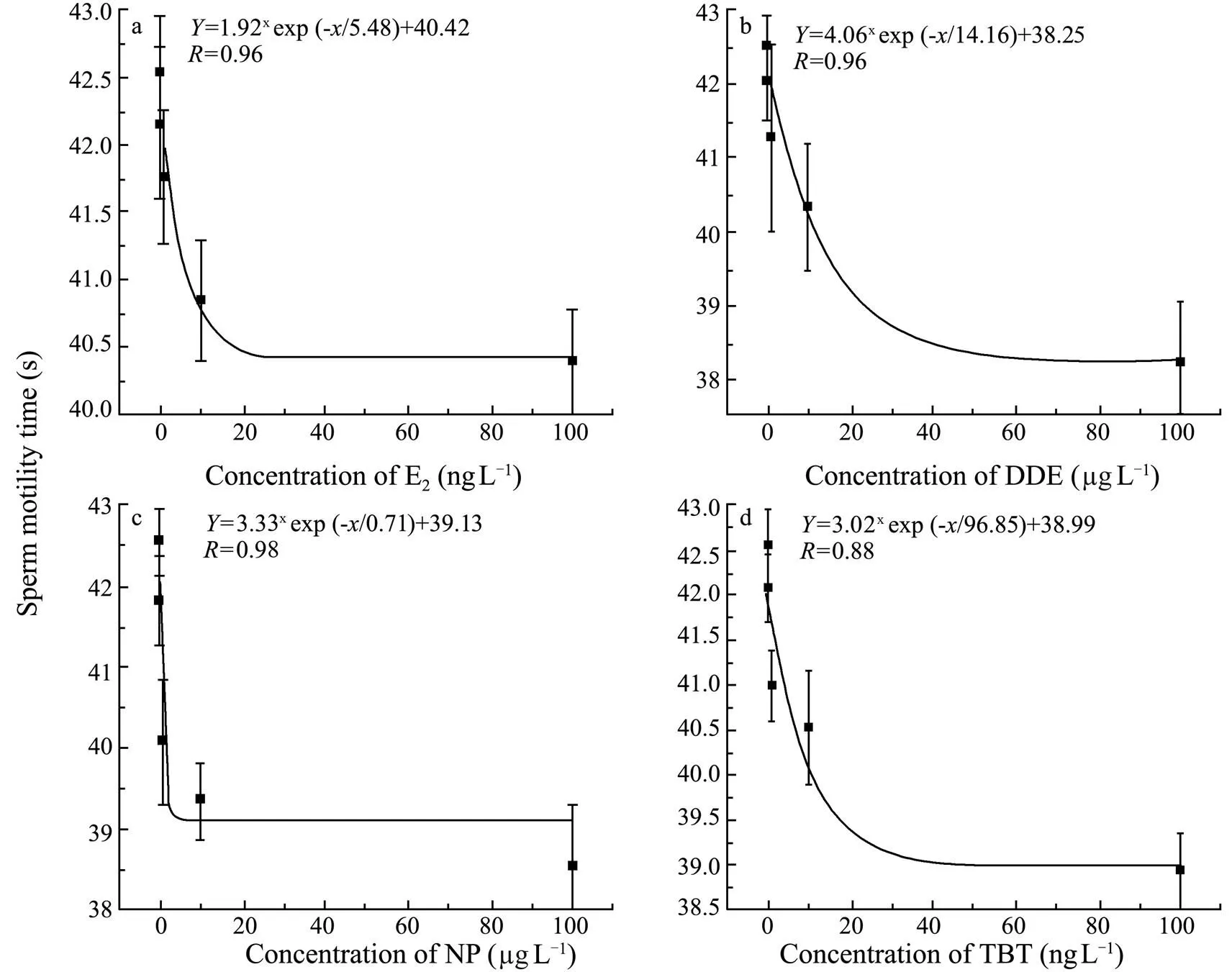
Fig.3 Concentration-effect curve for duration of sperm motility of Squaliobarbus curriculus exposed to different concentrations of E2, DDE, NP, and TBT.
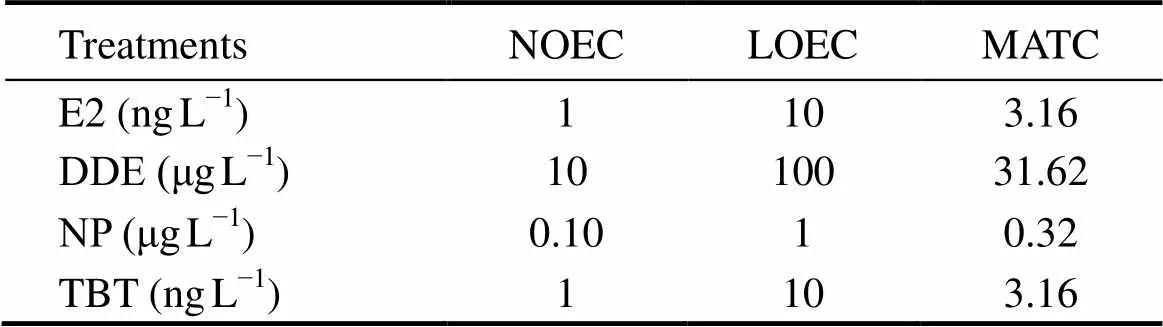
Table 2Parameters obtained from the sperm motility of Squaliobarbus curriculus exposed to differentconcentrations of E2, DDE, NP, and TBT
Notes: NOEC, no-observed-effect concentration; LOEC, lowest- observed-effect concentration; MATC, maximum acceptable concentration.
3.2 EDC Effects on the Fertilization Rate of
The fertilization rate ofwas negatively affected clearly by the tested EDCs (<0.05 for E2, DDE, and NP;<0.01 for TBT). Compared to the control, groups treated with ≥10ngLE2 or TBT and those treated with ≥10μgLDDE or NP had significantly lower fertilization rates (Fig.4). The relationship between EDC concentration and the fertilization rate ofwas indicated by a concentration-response curve (Fig.5). Accordingly, NOEC, LOEC and MATC were estimated for the fertilization rate within the present experimental concentration ranges (Table 3).
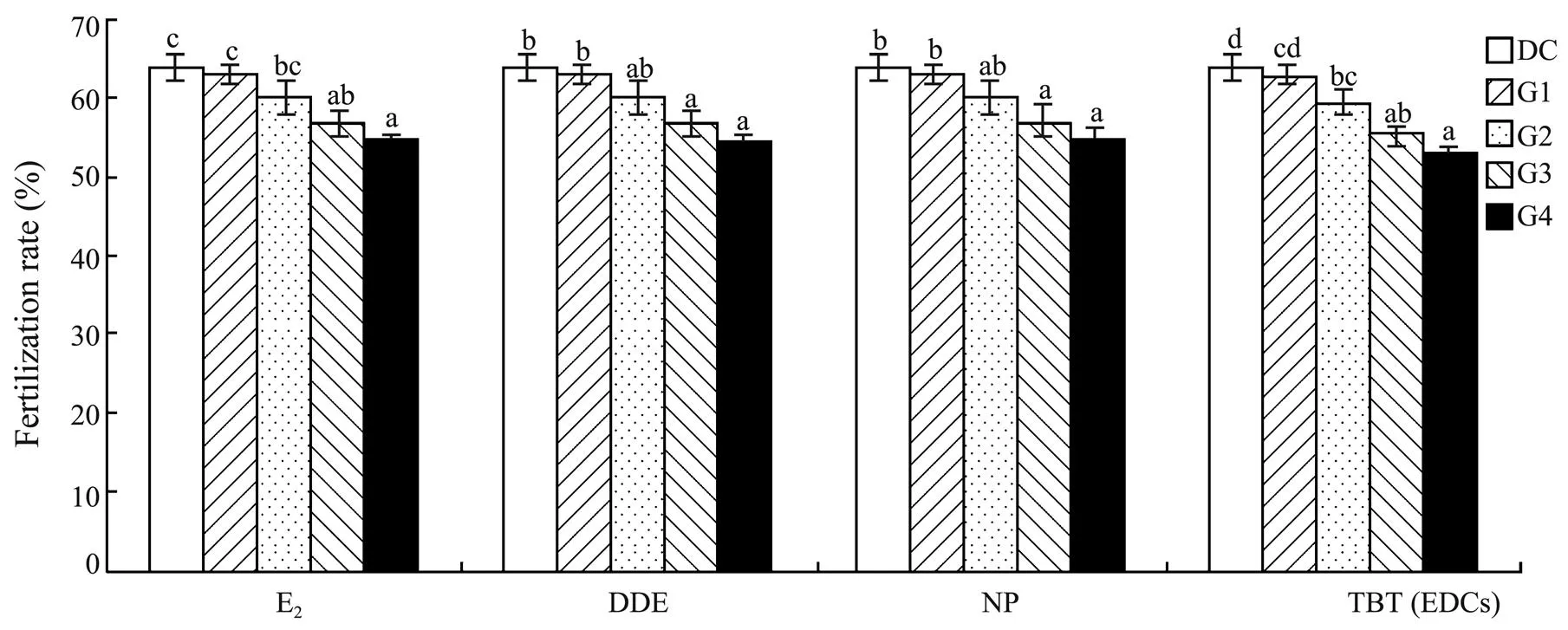
Fig.4 Fertilization rate of Squaliobarbus curriculus exposed to different concentrations of E2, DDE, NP, and TBT. Data with different letters on the bar are significantly different (P<0.05). DC: DMSO control group; G1-G4: Experimental groups with graded chemical concentrations shown in Table 1.
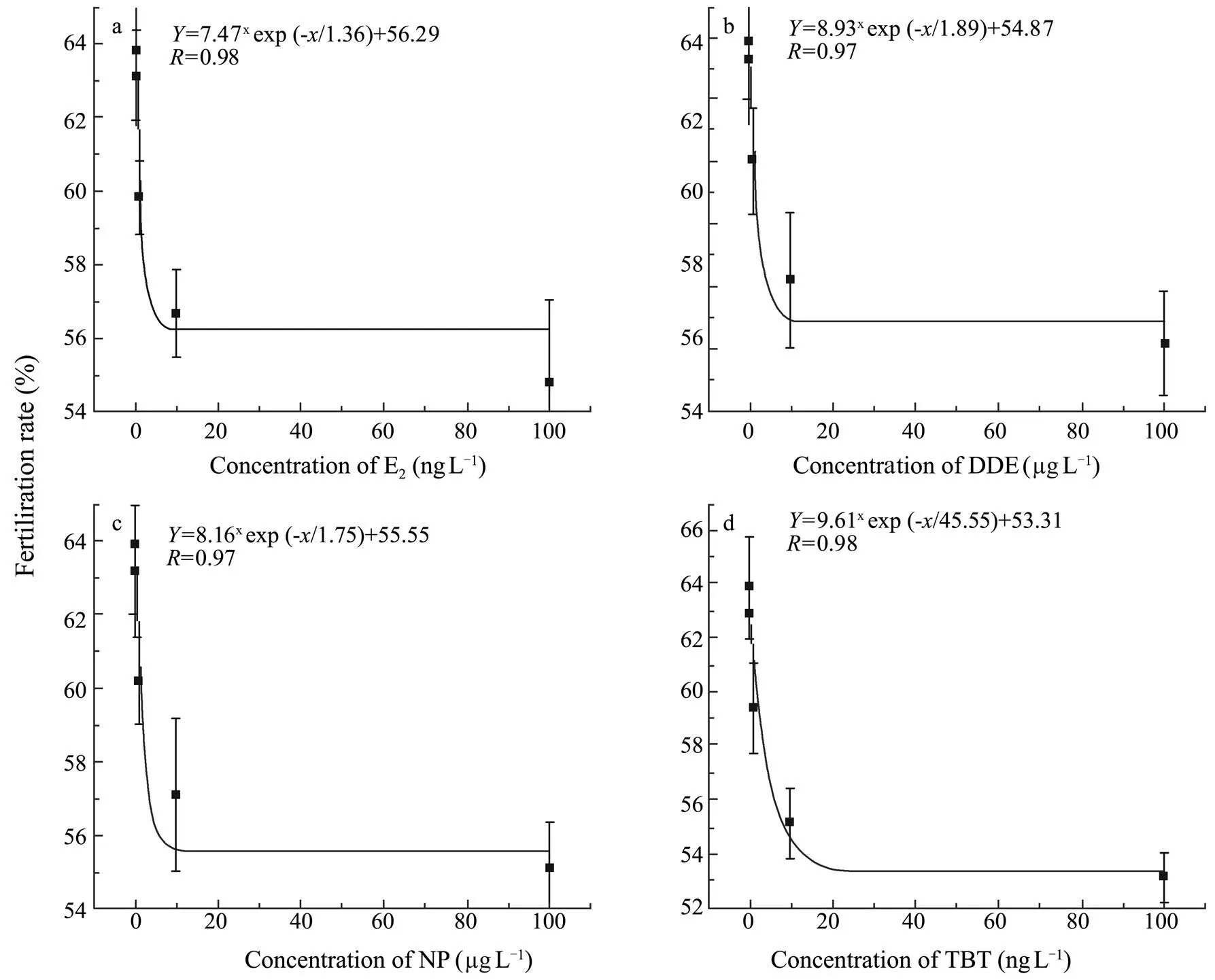
Fig.5 Concentration-response curve for the fertilization rate of Squaliobarbus curriculus exposed to different concentrations of E2, DDE, NP, and TBT.
concentrations of E2, DDE, NP, andTBT

Table 3Parameters obtained from the fertilization rate of Squaliobarbus curriculus exposed to different
Notes: NOEC: no-observed-effect concentration; LOEC: lowest-observed-effect concentration; MATC: maximum acceptable concentration.
3.3 EDC Effects on the Hatching Rate of
Despite decreasing with the increasing concentration of a specific EDC, the hatching rate ofwas similar among different treatments in the same group (corresponding to different concentrations of the same EDC) (>0.05, Fig.6).
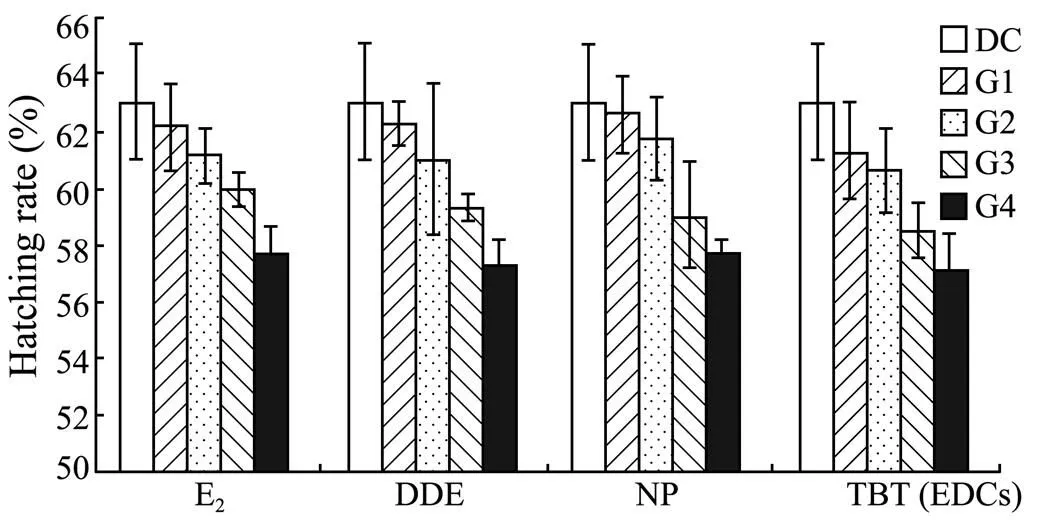
Fig.6 Hatching rate of Squaliobarbus curriculus exposed to different EDCs.DC: DMSO control group; G1–G4: Experimental groups with graded chemical concentrations shown in Table 1.
3.4 EDC Effects on the Larval Deformity Rate
The average heart rate of deformed fish was 14.52± 0.25 beats per min, significantly higher than that of the normal fish (13.34±0.32 beats per min) (<0.01). The 4 EDCs significantly affected the larval deformity rate (< 0.01), showing a distinct positive concentration-depend- ent effect (Fig.7). Compared with the control, groups treated with ≥0.1μgLDDE, ≥1μgLNP, ≥1ngLE2,or ≥10ngLTBT had significantly higher larval deformity rates. These EDC effects on larval deformity were described in a concentration-response curve (Fig.8). Accordingly, NOEC, LOEC and MATC were estimated for the deformity rate within the experimental concentration ranges (Table 4). These parameters were not estimated for DDE because of the narrow concentration range.

Fig.7 Larval deformity rate of Squaliobarbus curriculus exposed to different concentrations of E2, DDE, NP, and TBT. Data with different letters on the bar are significantly different (P<0.05).DC: DMSO control group; G1–G4: Experimental groups with graded chemical concentrations shown in Table 1.

Fig.8 Concentration-response curve forlarval deformity rate of Squaliobarbus curriculus exposed to different concentrations of E2, DDE, NP, and TBT.
concentrations of E2, DDE, NP, and TBT
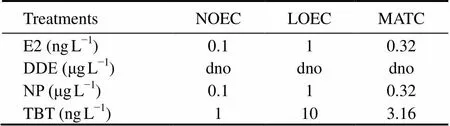
Table 4Parameters obtained from larval deformity rate of Squaliobarbus curriculus exposed to different
Notes: NOEC, no-observed-effect concentration; LOEC, lowest-observed-effect concentration; MATC, maximum acceptable concentration; dno, data not obtained.
3.5 EDC Effects on the Larval Survival Rate of
The 4 EDCs showed no significant effect on the larval survival rate of.(>0.05) (Fig.9).
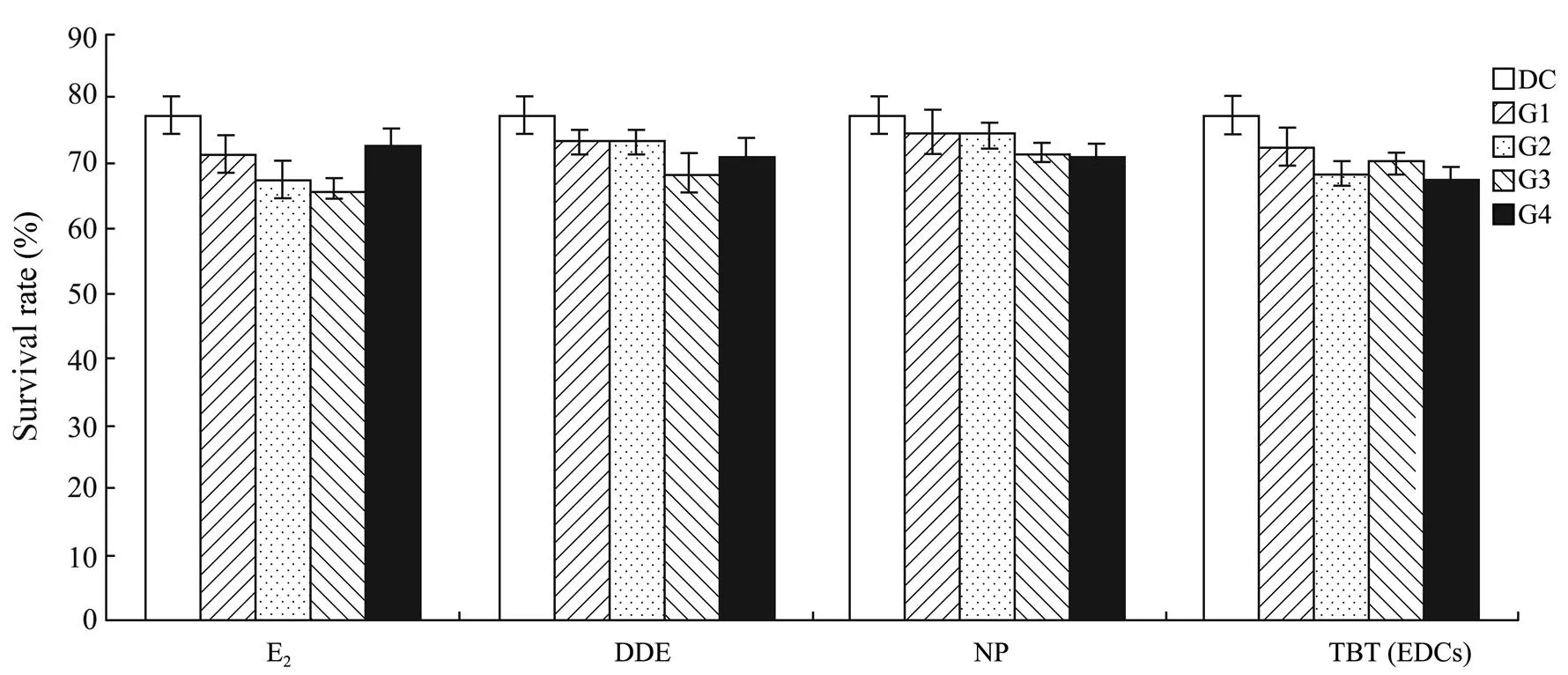
Fig.9 Survival rate of larval Squaliobarbus curriculus exposed to different concentrations of E2, DDE, NP, and TBT. DC, DMSO control group; G1–G4, Experimental groups with graded chemical concentrations shown in Table 1.
3.6 EDC Effects on the Final Survival Rate ofat Embryo Development Stage
To assess the cumulative EDC effect on the embryo development of, the final survival rate of the fish at the embryo development stage (from unfertilized egg to the end of the yolk-sac stage of the larva) was calculated (Table 5). Results showed that the 4 EDCs distinctly reduced the survival rate ofat embryo development stage (<0.01). EDC treatments (G3 and G4 groups shown in Table 1) resulted in about 10% mortality of.at embryo development stage.
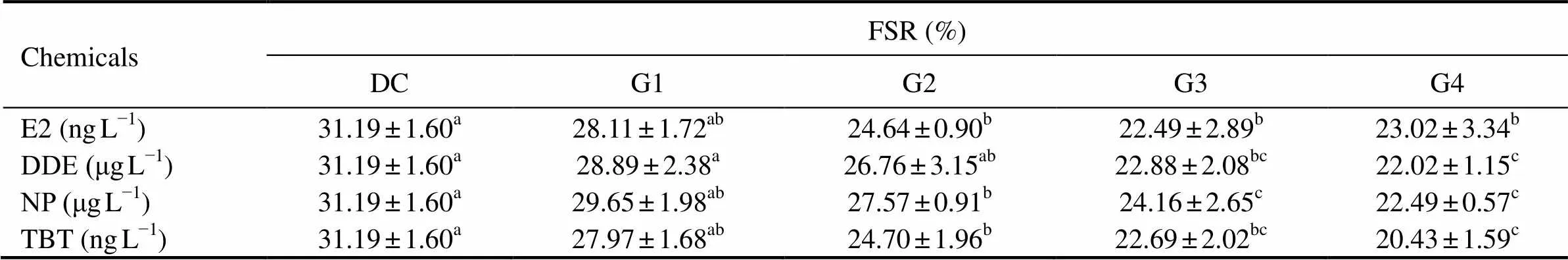
Table 5Final survival rate (FSR) of Squaliobarbus curriculusin the embryo development stage in different treatment groups
Notes: DC, DMSO control group; G1–G4, experimental groups with graded chemical concentrations shown in Table 1. Data (mean ± S.D.,=3) not sharing a common letter in the same row are significantly different (<0.05).
4 Discussion
This study demonstrated that the 4 well-known EDCs, E, DDE, NP and TBT, had distinct negative effects on freshwater fish.regarding fertilization and embryo development. In the experimental concentration ranges, the 4 EDCs yielded good concentration-response curves, showing the toxicities positively increasing with chemical concentrations. The 4 EDCs distinctly affectedat early life stages as was indicated by duration of sperm motility, fertilization rate and larval deformity rate. Larval deformity rate was most sensitive to EDC exposure. Four EDCs showed no apparent effect on hatching rate and larval survival rate ofin the concentration ranges investigated (up to 100ngLfor E2, 100mgLfor DDE and NP, and 1000ngLfor TBT) or at specific life stages (from the beginning of fertilization of the egg to the end of yolk-sac stage of the larva). We suggested that the relatively sensitive parameters such as larval deformity rate and fertilization rate were more suitable biomarkers for evaluation of EDC impacts.
The duration of sperm mobility in the fish was distinctly shortened by exposure to any of the 4 EDCs in a clearly negative concentration-dependent pattern (Figs.1, 2). The toxic effects of the 4 EDCs onfollowed the order from E2 and TBT to NP and to DEE (Table 2 for LOEC). In a study (Zuo., 2010), mature male black sleeperwere exposed to 10mgLE2 and 1mgL, 10mgL, 100mgL, and 1000mgLNP, respectively for 28d. The authors found that when the sperms were activated in diluted seawater (salinity 15), their motility was significantly inhibited by 10mgLE2 or 100mgLNP. In addition, Rurangwa(2002) reported that the duration and intensity of sperm motility significantly decreased in African catfishwhich were exposed to 0.27mgLTBT for 24h. However, the sperm motility of the common carpwas significantly reduced by exposure to 2.7mgLTBT but not affected by exposure to 0.27mgLTBT. In present study, the sperm mortality ofwas clearly affected by TBT at very low concentrations,.., 0.01mgL, indicating the high sensitivity ofsperms to TBT.
A number of studies have reported that the exposure of parent fish to EDCs can cause substantial decreases in the fertilization rate and the hatching rate while increases the larval mortality (Rolland, 2000; Segner., 2003; Nakayama,., 2004). This can be attributed to the endocrine-disrupting effect of EDCs. However, few researches have examined the toxic effect of EDCs on fish sperms or eggs, and as a final effect, on the fertilization rate from the egg exposure. In present study, the fertilization rate of.was substantially decreased by the 4 EDCs exposure, partly because of the suppressed sperm motility. The toxic effects of the 4 EDCs on the fertility of.were E2 and TBT>NP and DEE (Table 3 for LOEC). Previous adult exposure experiments (the exposure starts from the parent fish) have shown that the hatching rate and larval mortality are sensitive to EDC exposure, showing decreases and increases, respectively (Nirmala., 1999; Rolland, 2000). However, the present waterborne exposure (the exposure starts from the sperm and egg) to the 4 EDCs had no significant negative influence on the hatching rate and larval mortality of. Maternal transfer of EDCs into eggs may play an important role in the larval development of the first filial generation. Mac. (1985) reported that the source of eggs (from females exposed to different EDC- containing habitats) imposed more influences on the fry mortality than the quality of the water in which the fry was reared. On the other hand, different fish species might have different sensitivities to various contaminants. Segner. (2003) reported that exposure to 0.05–10ng estrogenic chemical ethynylestradiol (EE2) had no clear effect on the mortality of zebra fishin both a whole life cycle experiment and partial life cycle tests (0–3 and 0–21d post fertilization, respectively). In addition, these authors found that EE2 had no effect on the hatching rate but significantly reduced the fertilization success of zebra fish in the whole life span experiment. Nimrod and Benson (1998) reported that NP had no clear toxic effect on the hatching rate and mortality of the Japanese medaka at concentrations lower than 1.9mgL.
In present study, larval survival rate ofwas not clearly affected by the waterborne exposure to EDCs. However, larva deformity rate was obviously higher in the EDC-treated groups than in the control group. The toxic effects of E2, NP and TBT on the deformity rate of.were E2>TBT>NP (Table 4 for LOEC). The toxic effect of DDE on the deformity rate ofwas significantly greater than those on the other parameters, as LOEC was not observed for DDE at the lowest experimental concentration of 0.1mgL. Research indicated that the period of enhanced vulnerability to EDC exposure was likely from fertilization to the yolk-sac embryo stage for many teleost species (Rolland, 2000). Except for the distinctly higher larval deformity rate at the environmentally-relevant EDC concentrations at this life stage, a cumulative toxic effect of EDCs was observed at higher concentrations (G3 and G4, >10% higher than that of control, Table 5). Considering the endocrine-disrupting effects of the 4 EDCs on sex differentiation and reproduction, further studies should be conducted to assess the successive long-term EDC effects onand evaluate the ecological risk of EDCs to natural fish.
In summary, this work demonstrated that waterborne exposure of.sperms and eggs to E2, DDE, NP or TBT at environmentally-relevant concentrations led to substantial reduction of sperm mortality and fertilization rate and increase of larval deformity rate. Of the tested parameters, larval deformity rate was found to be most sensitive to the EDC exposure, thus can be used as a good candidate biomarker. Among the 4 EDCs, the toxic effects at early life stages offollowed the order of E2≥TBT≥DDE≥NP. This study indicated that in aquatic systems, E2, DDE, NP and TBT at environmentally-relevant concentrations potentially affect the natural population dynamics of..
Acknowledgements
This research was supported by the National Science Foundation of China (No. 40632009). We would like to thank Drs. Junbao Shen, Wankun Jin and Shaoquan Wang (Huanxin High-quality Fish Farm, Tianjin) for technical supports and Mr. Luyin Sun (our labortory) for his assistance in data processing. We also thank Profs. Jianying Hu and Zhaobin Zhang in Beijing University and Yunwei Dong in Xiamen University for their valuable comments on the manuscript.
Billard, R., 1978. Changes in structure and fertilizing ability of marine and freshwater fish spermatozoa diluted in media of various salinities., 14 (3): 187-198.
Diamanti-Kandarakis, E., Bourguignon, J. P., Giudice, L. C., Hauser, R., Prins, G. S., Soto, A. M., Zoeller, R. T., and Gore, A. C., 2009. Endocrine-Disrupting chemicals: an endocrine society scientific statement.,30 (4): 293- 342.
Ferraro, M. V. M., Fenocchio, A. S., Mantovani, M. S., Ribeiro, C. D. O., and Cestari, M. M., 2004. Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fishas evaluated using the comet assay and the piscine micronucleus and chromosome aberration tests.,: 103-107.
Gao, L., Yu, Z., Yang, F., Ru, X., Huang, J., Xu, T., and Sun, L., 2012. Distribution level and removal methods of 17β-estra- diol in water environment., 35 (2): 84-89.
Guillette, L. J., Crain, D. A., Gunderson, M. P., Kools, S. A. E., Milnes, M. R., Orlando, E. F., Rooney, A. A., and Woodward, A. R., 2000. Alligators and endocrine disrupting contaminants: a current perspective., 40 (3): 438- 452.
Hu, J. Y., Zhang, Z. B., Wei, Q. W., Zhen H. J., Zhao, Y. B., Peng, H., Wan, Y., Giesy, J. P., Li, L. X., and Zhang, B., 2009. Malformations of the endangered Chinese sturgeon,, and its causal agent.,106 (23): 9339-9344.
Labadie, P., and Budzinski, H., 2006. Alteration of steroid hormone profile in juvenile turbot () as a consequence of short-term exposure to 17α-ethynylestradiol., 64 (8): 1274-1286.
Lange, R., Hutchinson, T. H., Croudace, C. P., Siegmund, F., Schweinfurth, H., Hampe, P., Panter, G. H., and Sumpter, J. P., 2001. Effects of the synthetic estrogen 17α-ethinylestra- diol on the life-cycle of the fathead minnow ()., 20 (6): 1216-1227.
Lei, B. L., Huang, S. B., Wang, D. H., Luo, J. P., Wang, Z. J., and Liu, G., 2008. Present state of six estrogens in the sediment of Wenyu River., 29 (9): 2419- 2424.
Liu, W., Chen, J., Lin, X., and Tao, S., 2005. Spatial distribution characteristics of DDTs, PCBs and phthalates in surface sediments from Bohai Sea, China., 25 (1): 58-63.
Mac, M. J., Edsall, C. C., and Seelye, J. G., 1985. Survival of lake trout eggs and fry reared in water from the upper Great Lakes.,11: 520-529.
Milston, R. H., Fitzpatrick, M. S., Vella, A. T., Clements, S., Gundersen, D., Feist, G., Crippen, T. L., Leong, J., and Schreck, C. B., 2003. Short-term exposure of Chinook salmon () to o,p’-DDE or DMSO during early life-history stages causes long-term humoral immunosuppression., 111 (13): 1601-1607.
Nakayama, K., Oshima, Y., Yamaguchi, T., Tsuruda, Y., Kang, I. J., Kobayashi, M., Imada, N., and Honjo, T., 2004. Fertilization success and sexual behavior in male medaka,, exposed to tributyltin., 55: 1331-1337.
Nimrod, A. C., and Benson, W. H., 1998. Reproduction and development of Japanese medaka following an early life stage exposure to xenoestrogens., 44: 141-156.
Nirmala, K., Oshima, Y., Lee, R., Imada, N., Honjo, T., and Kobayashi, K., 1999. Transgenerational toxicity of tributyltin and its combined effects with polychlorinated biphenyls on reproductive processes in Japanese medaka ()., 18: 717-721.
Rolland, R. M., 2000. Ecoepidemiology of the effects of pollution on reproduction and survival of early life stages in teleosts., 1 (1): 41-72.
Ruan, G., Feng, J., and Yang, D., 2004. Effects of osmolality, pH and temperature on sperm motility and fertilization of loach ()., 24 (1): 22-25.
Rurangwa, E., Biegniewska, A., Slominska, E., Skorkowski, E. F., and Ollevier, F., 2002. Effect of tributyltin on adenylate content and enzyme activities of teleost sperm: a biochemical approach to study the mechanisms of toxicant reduced spermatozoa motility.:, 131 (3): 335-344.
Segner, H., Caroll, K., Fenske, M., Janssen, C. R., Maack, G., Pascoe, D., Schfers, C., Vandenbergh, G. F., Watts, M., and Wenzel, A., 2003. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: report from the European IDEA project., 54 (3): 302-314.
Shao, B., Hu, J. Y., and Yang, M., 2002. A survey of nonylphenol in aquatic environment of Chongqing valley., 22 (1): 12-16.
Shen, G., Zhang, Z. L., Yu, G., Li, F. S., and Li, X., 2005. The pollution state of nonylphenol and octylphenol in Haihe River and Bohai Bay in summer.,25 (6): 733-736.
Wan, Y., Wei, Q., Hu, J. Y., Jin, X., Zhang Z. B., Zhen, H. J., and Liu, J., 2007. Levels, tissue distribution, and age-related accumulation of synthetic musk fragrances in Chinese sturgeon (): comparison to organochlorines., 41 (2): 424-430.
Xue, N. D., Xu, X. B., and Jin, Z. L., 2005. Screening 31 endocrine-disrupting pesticides in water and surface sediment samples from Beijing Guanting reservoir., 61 (11): 1594-1606
Yang, R. Q., Zhou, Q. F., Zhang, Q. H., and Jiang, G. B., 2006. Study on the contamination status of butyltins in Taihu Lake., 27 (4): 661-664.
Zuo, M. J., Hong, W. S., and Zhou, L. B., 2010. Effects of Nonylphenol on spermatogenesis and sperm motility inLadpde., 49 (4): 579-584.
(Edited by Qiu Yantao)
10.1007/s11802-013-2102-3
ISSN 1672-5182, 2013 12 (3): 491-499
. Tel: 0086-10-58807966 E-mail: cjniu@bnu.edu.cn
(July 12, 2012; revised October 8, 2012; accepted February 23, 2013)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2013
 Journal of Ocean University of China2013年3期
Journal of Ocean University of China2013年3期
- Journal of Ocean University of China的其它文章
- Evaluation of Antitumor, Immunomodulatory and Free Radical Scavenging Effects of A New Herbal Prescription Seaweed Complex Preparation
- A Preliminary Phylogenetic Analysis of Luidia (Paxillosida:Luidiidae) from Chinese Waters with Cytochrome Oxidase Subunit I (COI) Sequences
- Seasonal Community Structure of Mesozooplankton in the Daya Bay, South China Sea
- The Effect of Three Culture Methods on Intensive Culture System of Pacific White Shrimp (Litopenaeus vannamei)
- Effects of Dietary Corn Gluten Meal on Growth Performance and Protein Metabolism in Relation to IGF-I and TOR Gene Expression of Juvenile Cobia (Rachycentron canadum)
- Comparison of Lipids in Organs of the Starfish Asterias amurensis Associated with Different Treatments
