Seasonal Community Structure of Mesozooplankton in the Daya Bay, South China Sea
LIU Huaxue1), 2), LI Kaizhi2), HUANG Honghui1), SONG Xingyu2), YIN Jianqiang2),and HUANG Liangmin2), *
Seasonal Community Structure of Mesozooplankton in the Daya Bay, South China Sea
LIU Huaxue, LI Kaizhi, HUANG Honghui, SONG Xingyu, YIN Jianqiang,and HUANG Liangmin
1),,,,510300,2),,,510301,
Mesozooplankton are key components of coastal ecosystems, linking the microbial food web to the classic food chain. In this study, species composition and abundance of mesozooplankton is studied for the Daya Bay in April (spring) and October (fall), 2006. A total of 27 species of mesozooplankton were identified in spring and 58 species in fall. Dominant species were,,andin spring, shifting to,andin fall. Higher mesozooplankton abundance was found at Aotou Cove and Dapeng’ao Cove compared to other stations, indicating the influence of eutrophication on mesozooplankton community in the Daya Bay. The outbreak ofbloom in spring reduced the species diversity and abundance of mesozooplankton.
community structure; mesozooplankton; Noctiluca; Daya Bay
1 Introduction
Mesozooplankton (MZ, 0.2–2mm) are key components of the marine food web. As the primary or secondary consumers, MZ transfer materials and energy to higher trophic levels and link the microbial food web to the classic food chain (Dussart, 1965; Calbet, 2001; Chen., 2011). Hydrographic features of water masses may influence the distribution pattern of MZ, as temperature and salinity directly affect the growth conditions of phytoplankton and MZ (Sabates., 1989; Badylak and Phlips, 2008). Other parameters and associated interactions (such as predation, natural mortality, tidal advection, eddies and mixing) also contribute to the dynamics of MZ (Greve, 1994; Fernandez., 1993). For example, nutrients determine phytoplankton standing stocks and the community structure of phytoplankton, and consequently control the dynamics of MZ through a bottom-up effect (Chen., 2011). This pathway is enhanced by the increased nutrient loadings from aquaculture and sewage, which particularly favors the large-sized phytoplankton (>20μm), a major food source of MZ (Liu and Dagg, 2003). Overall, the effect of environmental parameters and food conditions on MZ is complicated, and the response of MZ to enhanced eutrophication in coast waters remains unclear (Lian., 2011; Chen., 2011).
The Daya Bay (DYB), a semi-enclosed coastal inlet, is one of the main aquaculture areas,, Dapeng’ao Cove due to its rich biological resources (Song., 2009; Wang., 2008). The eutrophication level of DYB has been increasing in some areas (, Aotou Cove) since the 1980s (Liu., 2012; Wu., 2009). For example, the average ratio of N/P increased from 1.377 in 1985 to 49.090 in 2004. The limiting factor of nutrients has changed from N to P (Wang., 2008), mainly resulting from the anthropogenic activities (., aquaculture and sewage drainage). In cage-culture areas of Dapeng’ao Cove and Aotou Cove, harmful algal blooms (HABs) have frequently occurred in spring or early summer nearly every year since 2000 (Song., 2009; Wang., 2006). The environment parameters and food web also change when HABs occur. In this study, the composition, abundance and biomass of MZ in DYB are investigated in order to understand the influence of environmental factors and food web change on the community composition and distribution of MZ.
2 Materials and Methods
2.1 Sampling Site
The DYB on the Guangdong coast is a subtropical drowned valley bay with an area of about 600km(Fig.1). Two cruises were conducted during spring (April 8−10) and fall (October 28−29) in 2006. Samples were collected at 12 and 11 sites in spring and fall, respectively (Table 1).
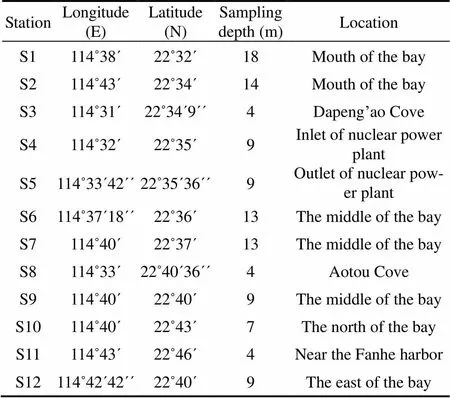
Table1 Sampling stations in the DYB, South China Sea

Fig.1 Sampling sites in the Daya Bay, South China Sea.
2.2Analytical Methods
Seawater temperature and salinity were measuredusing a YSI 6600 multi-parameter water quality monitor (Yellow Springs Instrument Co., USA). The concentrations of nitrate, nitrite, ammonia, phosphate and silicate were analyzed with a nutrients-autoanalyzer (Quickchem 8500, USA) according to Kirkwood. (1996).Water samples for chlorophyll(Chl) analysis were collected from surface layer using a 5L Niskin bottle, and then filtered through a GF/F filter (0.7µm). The Chlconcentration was measured using a Turner Designs 10-AU fluorometer according to Parsons. (1984).
The MZ samples were collected by vertical tows from 1m above the sediment column to the water surface with a plankton net (0.5m diameter, 169µm mesh size). Samples were preserved with formalin (5% final concentration) and identified to species level when possible. Abundance was counted under a light microscope (Chen and Zhang, 1965, 1974; Chen., 1974).
2.3 Data Analysis
The temporal changes in environmental factors were tested by one-way analysis of variation (ANOVA). For statistical analysis, all measurements of the environmental factors and percentage of MZ abundance were transformed by log(), and those of the abundance were transformed by log(+1). The temporal distribution pattern of MZ and its relationship with environmental factors were elucidated by means of canonical correspondence analysis (CCA) according to Chen. (2011). The CCA analysis was processed using CANOCO 4.5.
3 Results
The environmental parameters showed significant seasonal variations between spring and fall in 2006 (Table 2). Sea surface temperature and salinity were significantly lower in spring than in fall (<0.01 and<0.05, respectively) (Fig.2). Dissolved inorganic nitrogen (DIN) and silicate concentrations (<0.05) were also lower in spring than in fall (<0.01) (Table 2). Differently, the average concentration of surface Chlwas higher in spring than in fall (<0.01), with high values mainly observed at S3 and S8 (Fig.2).
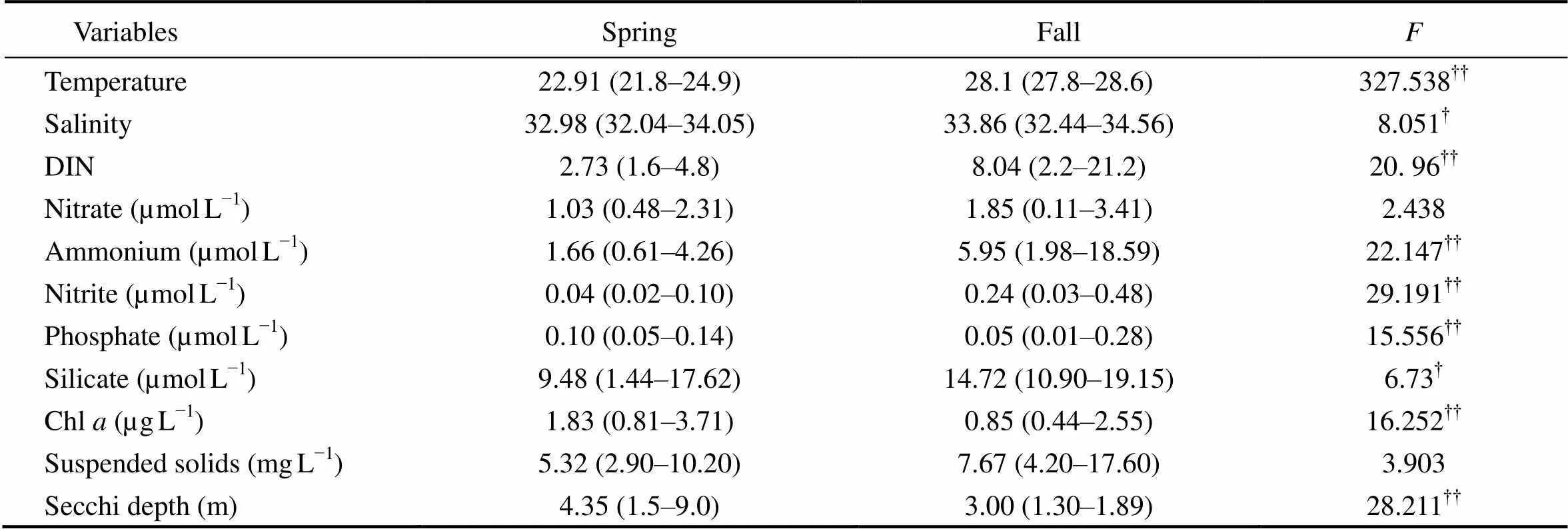
Table 2 Comparison of major environmental variables of DYB between spring and fall, 2006
Notes: Data are spatial averages with the range shown in parentheses; comparison of each factor between two seasons tested by one-way ANOVA with-value provided;andindicate significance levels at<0.05 and<0.01, respectively.
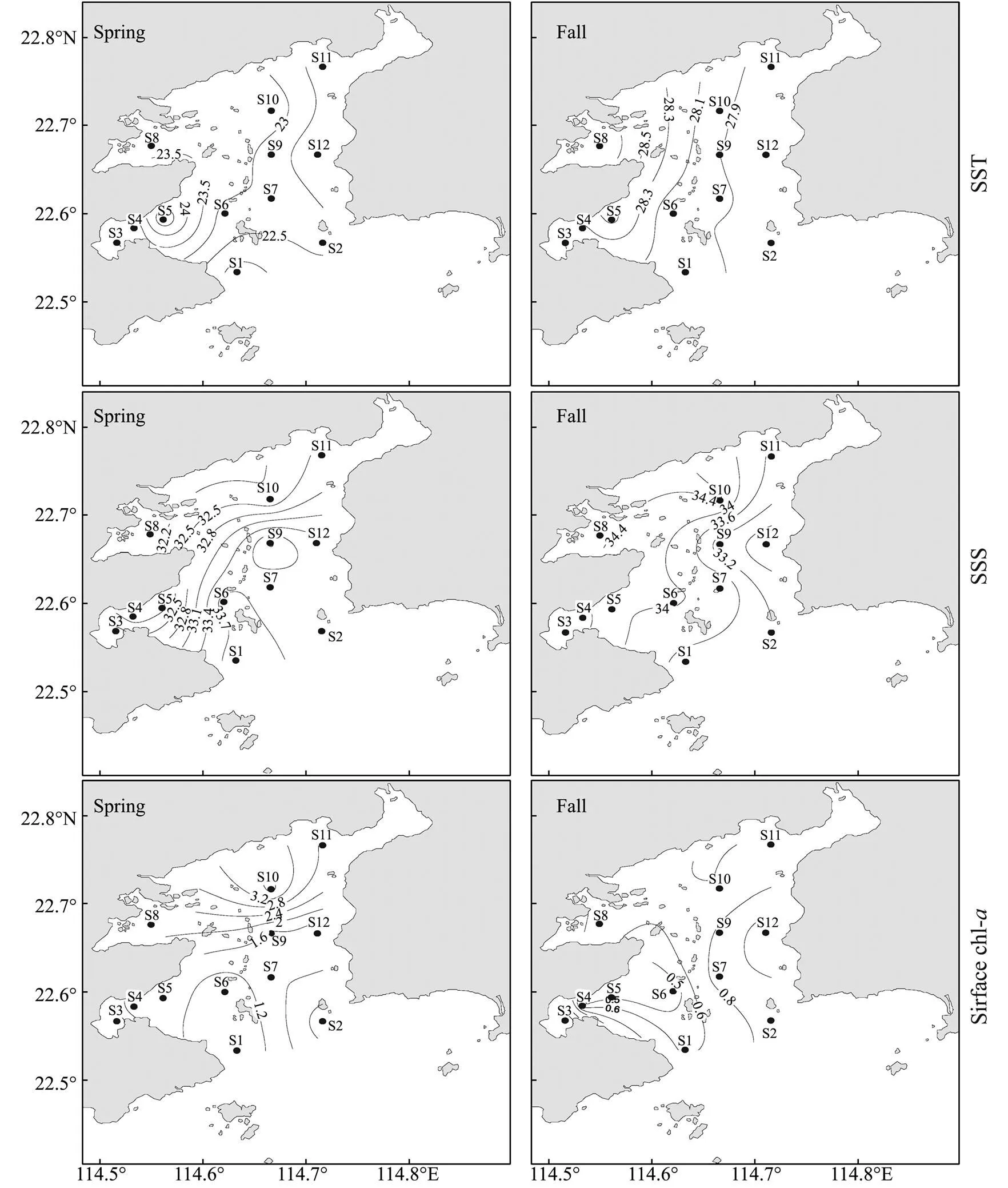
Fig.2 Spatial and temporal variations in the environmental variables of DYB in spring and fall (2006).
A total of 27 species of MZ were identified in spring (2006), with species number ranging from 4to 13 (Table 3). A total of 59 species of MZ were identified in fall, with species number ranging from 14 to 31. As shown in Fig.4, the diversity index ranged from 1.77 to 2.63 (2.13 ±0.27) in spring, andfrom 1.40 to 4.33 (2.84±0.96) in fall.
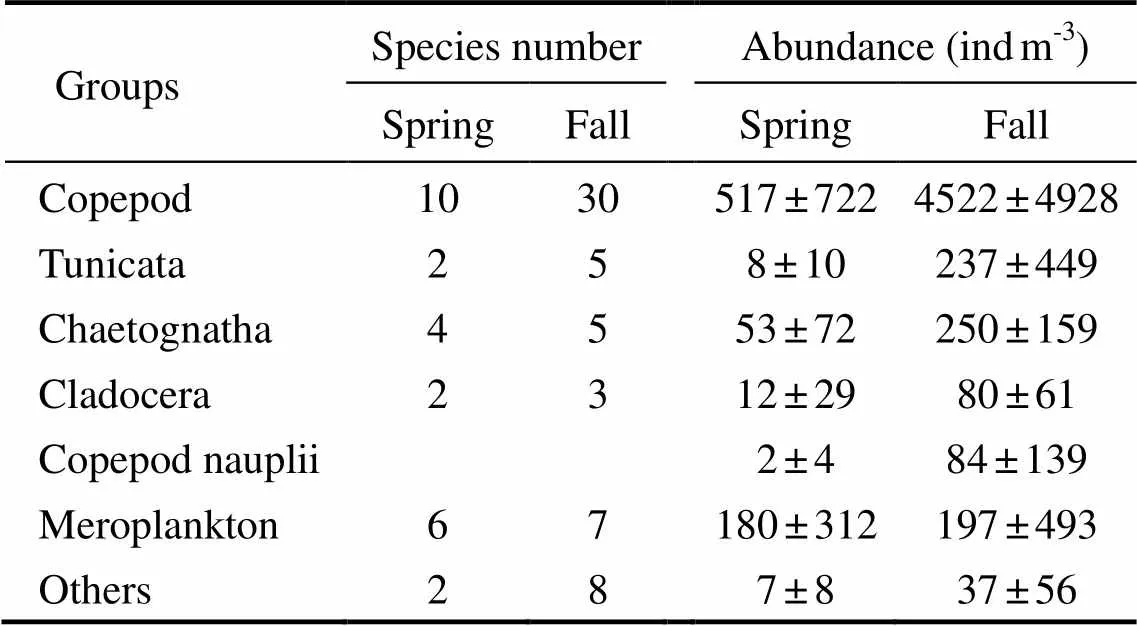
Table 3 Species number and abundance of major groups of mesozooplankton in spring and fall (2006)in DYB, South China Sea

Fig.3 Spatial variations of Noctiluca scintillans abundance (103 ind m−3) in DYB in spring (2006).
The abundance ofranged from 2.3 to 220.7×10indmin spring, with maximum values observed at S8 (Fig.3).was rarely found in fall (42±73indm). Copepods dominated the MZ community in both seasons. Dominant species of MZ were,,andin spring, shifting to,,andin fall.
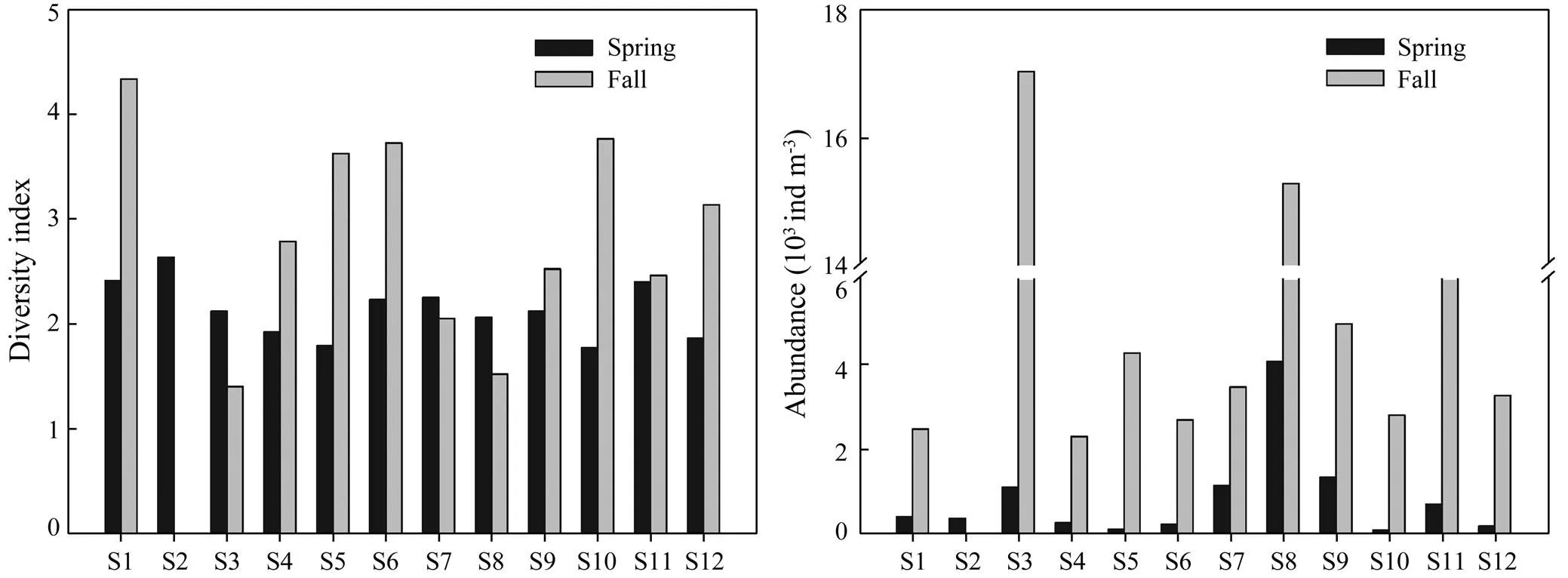
Fig.4 Comparison of mesozooplankton diversity index and abundance in spring and fall (2006) in DYB, South China Sea.
The MZ abundance in spring was significantly lower than in fall (0.05), with high levels mainly distributed at S8 and S3. At most sites,the abundance ofandin the spring were less than in the fall (0.05). The abundance ofand(0.01) in spring were less than in fall (0.05 and0.01, respectively),with maximum values observed at S8 (Fig.5).
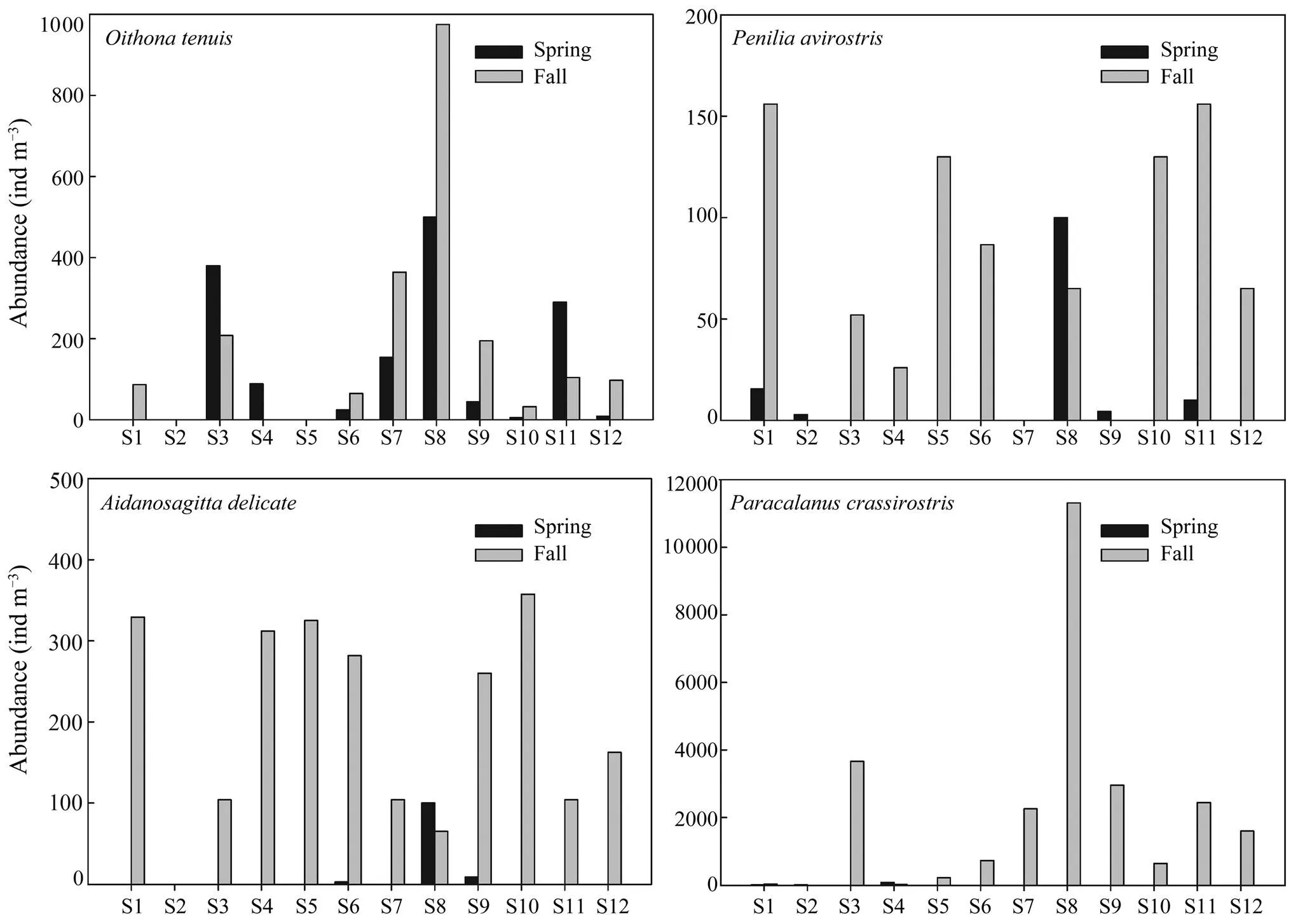
Fig.5 Abundance of dominant mesozooplankton species (Penilia avirostris, Parvocalanus crassirostris and Aidanosagitta delicate) in spring and fall (2006) in DYB, South China Sea.
As shown in Table 4, the two main axes cumulatively explained 54.4% and 46.6% of MZ variations in spring and fall, respectively. In the spring, secchi depth and temperature were the main determinants of axis 1, while nitrate and ammonia were the main determinants of axis 2 (Fig.6). CCA analysis revealed that thewas related to temperature, whileandwere related to phosphate concentration. In the fall, temperature and nitrite were the main determinants of axis 1, while suspended solids, nitrate and nitrite were the main determinants of axis 2 (Fig.6). CCA analysis revealed that thewas related to suspended solids, whilewas adapted to high temperature.

Table 4 Results of CCA analysis for spring and fall (2006) in DYB, South China Sea
4 Discussion
4.1 Spatial and Temporal Variations in MZ Community
The variability of environment conditions, such as hydrography, nutrients and suspended solids, has measureable effects on MZ distribution and species composition in northern Svalbard waters (Daase and Eiane, 2007; Dvoretsky and Dvoretsky, 2010). In the DYB, MZ community was dominated by copepods in spring and fall in 2006, consistent with previous studies (Lian., 2011; Fang., 2010). During the spring and fall, major environment parameters showed significant changes whereas MZ species number and abundance varied with an opposite trend. This could be related to thebloom in the spring, which influenced zooplankton community by reducing their egg/larva numbers or competition for food resources. The MZ abundance substantially fluctuated and showed distinct heterogeneity, primarily due to spatial difference of environment conditions in the DYB (Wang., 2006; Song., 2009), particularly at S8 and S3. Peaks in MZ abundance were recorded at S8. The MZ abundance was positively correlated to Chlconcentration, suggesting that the zooplankton abundance was elevated due to increased food availability. Due to the presence of petro chemistry and aquaculture industry, the environment conditions at S8 differed substantially from other areas. Consequently, MZ community at S8 differed from other sites in the spring.
4.2Bloom
Thelarge dinoflagellateforms conspicuous red tides worldwide (Uhlig and Sahling, 1990; Huang and Qi, 1997; Yin., 2003; Nakamura, 1998; Fonda- Umani., 2004). In the adjacent areas of South China Sea,, Hong Kong,bloom frequently occurs in spring (Fung and Trott, 1973) and shows obvious seasonal changes (Liu and Wong, 2006). In the present study, the outbreak ofbloom during the sampling period in spring was mainly caused by monsoon change and rainfall. The green Noctiluca is highly restricted to a temperature range of 25–30℃ and mainly occurs in tropical waters of Southeast Asia (Harrison., 2011), as well as other similar regions (Miyaguchi., 2006; Tada., 2004).
Previous work indicates that densein surface water can reduce the light penetration in spring. The bloom ofalso influences the MZ composition (Nakamura, 1998; Quevedo., 1999; Murray and Suthers, 1999). In special cases,can overcome zooplankton biomass by feeding on their eggs and competing for food resources, which leads to blooms. Thus, the outbreak ofin DYB in the spring of 2006 was likely the main cause of significant seasonal differences in MZ community composition between spring and fall.
5 Conclusions
The MZ composition showed substantial changes in spring and fall (2006) in the DYB, South China Sea. Highest MZ abundance was concentrated at Aotou Cove and Dapeng’ao Cove in both seasons. The outbreak ofbloom in the spring reduced the species number and abundance of MZ.
Acknowledgements
This research was supported by the National Nature Science Foundation of China (Nos. 41276159, 41130855), and the Special Fund of Basic Research for Centre Commonweal Scientific Research Institute (Nos. 2007ZD07, 2011TS06, 2013TS07).
Badylak, S., and Phlips, E. J., 2008. Spatial and temporal distributions of zooplankton in Tampa Bay, Florida, including observations during a HAB event.,30: 449-465.
Calbet, A., 2001. Mesozooplankton grazing effect on primary production: A global comparative analysis in marine ecosystems.,46: 1824-1830.
Chen, M. R., Chen, B. Z., Harrison, P., and Liu, H. B., 2011. Dynamics of mesozooplankton assemblages in subtropical coastal waters of Hong Kong: A comparative study between a eutrophic estuarine and a mesotrophic coastal site., 31: 1075-1086.
Chen, Q. C., and Zhang, S. Z., 1965. The planktonic copepods of the Yellow Sea and the East China Sea. I. Calanoida., 7: 20-131 (in Chinese).
Chen, Q. C., and Zhang, S. Z., 1974. The pelagic copepods of the South China Sea. I., 9: 101-116 (in Chinese).
Chen, Q. C., Zhang, S. Z., and Zhu, C. S., 1974. The planktonic copepods of the Yellow Sea and the East China Sea. II. Cyclopoida and Harpacticoida., 9: 27- 76 (in Chinese).
Daase, M., and Eiane, K., 2007. Mesozooplankton distribution in northern Svalbard waters in relation to hydrography., 30: 969-981.
Dussart, B. H., 1965. Les différentes catégories de plancton., 26: 72-74.
Dvoretsky, V. G., and Dvoretsky, A. G., 2010. Mesozooplankton structure in Dolgaya Bay (Barents Sea)., 33: 703-708.
Fang, L., Li, C. H., Du, F. Y., Jia, X. P., and Zhang, W., 2010. Ecological characteristics of zooplankton in Daya Bay., 11: 2981-2991 (in Chinese with English abstract).
Fernandez, E., Cabal, J., Acuna, J. L., Bode, A., Botas, A., and Garcia-Soto, C., 1993. Plankton distribution across a slope current induced front in the Southern Bay of Biscay., 15: 619-641.
Fonda-Umani, S., Beran, A., Parlato, S., Virgilio, D., Zollet, T., De Olazabal, A., Lazzarini, B., and Cabrini, M., 2004.(Macartney) in the Northern Adriatic Sea: long-term dynamics, relationships with temperature and eutrophication, and role in the food web., 5: 545-561.
Fung, Y. C., and Trott, L. B., 1973. The occurrence of a(Macartney) induced red tide in Hong Kong., 18: 472-476.
Greve, W., 1994. The 1989 German Bight invasion of., 51: 355-358.
Harrison, P. J., Furuya, K., Glibert, P. M., Xu, J., Liu, H. B., Yin, K. D., Lee, J. H. W., Anderson, D. M., Gowen, R., Al-Azri, A. R., and Ho, A. Y. T., 2011. Geographical distribution of red and green., 29 (4): 807-831
Huang, C. J., and Qi, Y. Z., 1997. The abundance cycle and influence factors on red tide phenomena of(Dinophyceae) in Dapeng Bay, the South China Sea.,19: 303-318.
Kirkwood, D. S., Aminot, A., and Carlberg, S. R., 1996. The 1994 quasimeme laboratory performance study: Nutrients in seawater and standard solutions., 32: 640-645.
Lian, X. P., Tan, Y. H., Huang, L. M., Chen, Q. C., Li, K. Z., and Liu, Y. H., 2011. Space-time variations and impact factors of macro-mesozooplankton in Daya Bay., 5: 640-645 (in Chinese with English abstract).
Liu, H. X., Song, X. Y., Huang, L. M., and Zhong, Y., 2012. Using primary productivity as an index of coastal eutrophication: A case study in Daya Bay., 2: 235-240.
Liu, H., and Dagg, M. J., 2003. Interactions between nutrients, phytoplankton growth, and micro- and mesozooplankton grazing in the plume of the Mississippi River.s, 258: 31-42.
Liu, X. J., and Wong, C. K., 2006. Seasonal and spatial dynamics ofin a semi-enclosed bay in the northeastern part of Hong Kong., 49: 145- 150.
Miyaguchi, H., Fujiki, T., Kikuchi, T., Kuwahara, V., and Toda, T., 2006. Relationship between the bloom ofand environmental factors in the coastal waters of Sagami Bay, Japan., 28: 313-324.
Murray, S., and Suthers, M., 1999. Population ecologyMacartney, a red-tide-forming dinoflagellate., 50: 243-252.
Nakamura, Y., 1998. Biomass, feeding and production ofin the Seto Inland Sea, Japan., 20: 2213-2222.
Parsons, T. R., Maita, Y., and Lalli, C, M., 1984.. Pergamon Press, Oxford, 173pp.
Quevedo, M., Gonzales-Quiros, R., and Anadon, R., 1999. Evidence of heavy predation byon(Copepoda) eggs of the central Cantabrian coast., 22: 127-131.
Song, X. Y., Huang, L. M., Zhang, J. L., Huang, H. H., Li, T., and Su, Q., 2009. Harmful algal blooms (HABs) in Daya Bay, China: Anstudy of primary productivity and environmental impacts., 58: 1310-1318.
Tada, K., Pithakpol, S., and Montani, S., 2004. Seasonal variation in the abundance ofin the Seto Inland Sea, Japan., 51: 7-14.
Uhlig, G., and Sahling, G., 1990. Long-term studies on. German Bight population dynamics and red tide phenomena 1968–1988., 25: 101-112.
Wang, Y. S., Lou, Z. P., Sun, C. C., and Sun, S., 2008. Ecological environment changes in Daya Bay, China, from 1982 to 2004., 56: 1871-1879.
Wang, Z. H., Qi, Y. Z., and Chen, J. F., 2006. Phytoplankton abundance, community structure and nutrients in cultural areas of Daya Bay, South China Sea., 62: 85-94.
Wu, M. L., Wang, Y. S., Sun, C. C., Wang, H. L., Dong, J. D., and Han, S. H., 2009. Identification of anthropogenic effects and seasonality on water quality in Daya Bay, South China Sea., 90: 3082-3090.
Yin, K. D., 2003. Influence of monsoons and oceanographic processes on red tides in Hong Kong waters.s, 262: 27-41.
(Edited by Qiu Yantao)
10.1007/s11802-013-1991-5
ISSN 1672-5182, 2013 12 (3): 452-458
. E-mail: hlm@scsio.ac.cn
(April 3, 2012; revised May 15, 2012; accepted December 29, 2012)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2013
 Journal of Ocean University of China2013年3期
Journal of Ocean University of China2013年3期
- Journal of Ocean University of China的其它文章
- Evaluation of Antitumor, Immunomodulatory and Free Radical Scavenging Effects of A New Herbal Prescription Seaweed Complex Preparation
- Effects of Exposure to Four Endocrine Disrupting-Chemicals on Fertilization and Embryonic Development of Barbel Chub (Squaliobarbus curriculus)
- A Preliminary Phylogenetic Analysis of Luidia (Paxillosida:Luidiidae) from Chinese Waters with Cytochrome Oxidase Subunit I (COI) Sequences
- The Effect of Three Culture Methods on Intensive Culture System of Pacific White Shrimp (Litopenaeus vannamei)
- Effects of Dietary Corn Gluten Meal on Growth Performance and Protein Metabolism in Relation to IGF-I and TOR Gene Expression of Juvenile Cobia (Rachycentron canadum)
- Comparison of Lipids in Organs of the Starfish Asterias amurensis Associated with Different Treatments
