Effects of Dietary Corn Gluten Meal on Growth Performance and Protein Metabolism in Relation to IGF-I and TOR Gene Expression of Juvenile Cobia (Rachycentron canadum)
LUO Yiwen, AI Qinghui*, MAI Kangsen, ZHANG Wenbing, XU Wei, ZHANG Yanjiao,and LIUFU Zhiguo
Effects of Dietary Corn Gluten Meal on Growth Performance and Protein Metabolism in Relation to IGF-I and TOR Gene Expression of Juvenile Cobia ()
LUO Yiwen, AI Qinghui, MAI Kangsen, ZHANG Wenbing, XU Wei, ZHANG Yanjiao,and LIUFU Zhiguo
,,,266003,
A growth experiment was conducted on cobia (, initial weight 108.2g±3.0g) to investigate the effects of dietary corn gluten meal (CGM) levels on the fish growth, whole body composition and protein metabolism in relation to specific gene expression. Five isonitrogenous (crude protein 45%) and isoenergetic (gross energy 20kJg) practical diets were formulated by replacing 0% (the control), 17.5%, 35.0%, 52.5%, and 70.0% of fish meal (FM) protein with CGM protein. No significant differences were observed in the survival, feed intake (FI), specific growth rate (SGR), feed efficiency (FE) and protein productive value (PPV) among fish fed diets with 0%, 17.5%, 35.0%, and 52.5% of CGM protein. However, these indices were significantly lower in fish fed the diet with 70.0% of CGM protein than those in fish fed the control diet (<0.05). The whole-body crude protein and lipid contents were significantly lower while the whole-body moisture content was significantly higher in fish fed the diet with 70.0% of CGM protein compared with the control group (<0.05). When 70.0% of FM protein was replaced by CGM, plasma total protein and cholesterol contents were significantly lower than those in the control group (<0.05). Fish fed the diet with 70.0% of CGM protein had significantly lower hepatic insulin-like growth factor I (IGF-I) expression levels than those in the control group (<0.05). However, no significant differences were observed in hepatic target of rapamycin (TOR), dorsal muscle IGF-I and TOR expression levels among dietary treatments. Results of the present study indicated that 52.5% of FM protein could be replaced by CGM in the diets without significant influences on the growth, feed utilization and protein metabolism of juvenile cobia. The present results might be useful for developing cost effective and sustainable cobia dietary formulations.
Cobia; corn gluten meal; growth performance; protein metabolism; gene expression
1 Introduction
Fish meal (FM) is a major protein source for most carnivorous fish. However, the production of FM has been nearly constant while the demand for FM is rapidly growing, resulting in substantially increased cost of FM. Thus, it is necessary to identify alternative protein sources for aquaculture (Hardy, 2010). Corn gluten meal (CGM) is an important plant protein source with high protein content (60%–70% dry matter), low anti-nutritional factors (ANFs) contents, and a favorable essential amino acid (EAA) profile except for lysine and arginine (Pereira and Oliva-Teles, 2003). Research indicates that CGM can be a potential protein source alternative to FM protein for diets of rainbow trout (Gomes., 1995), turbot (Regost., 1999), Japanese flounder (Kikuchi, 1999), red sea bream (Takagi., 2000), gilthead sea bream (Pereira and Oliva-Teles, 2003), Atlantic salmon (Mente., 2003), and Atlantic cod (Hansen., 2007a). However, these studies also demonstrated that fish growth performance was generally inversely related to the dietary CGM levels in relevant species.
Fish growth is controlled largely by the growth hormone (GH)/insulin-like growth factor-I (IGF-I) axis (Duan., 2010), and the IGF-I is a major anabolic agent responsible for tissue growth (Thissen., 1999). Nutritional signals can have profound effects on hepatic IGF-I expression levels in fish. Several studies have indicated that hepatic IGF-I expression levels are positively correlated to growth rates of fish (Matthews., 1997; Gómez-Requeni., 2004; Pedroso., 2006; Hevrøy., 2008). Binding of IGF-I to its receptor in cell membrane can activate the target of rapamycin (TOR) protein via an IRS-PI3K-Akt pathway. Being a conserved Ser/Thr kinase, the TOR protein is a central protein in the TOR signaling pathway. Activated TOR protein can regulate phosphorylation of downstream targets that modulates multiple growth-related processes, such as translation, ribosome biogenesis, macroautophagy, transcription, and metabolism (Wullschleger., 2006). In addition to growth factors (insulin/IGFs), major upstream regulators of TOR protein include nutrients (especially amino acids), energy and stress. Despite the extensive research of the TOR signaling pathway in mammals, few relevant studies have been conducted on fish, such as rainbow trout (Seiliez., 2008; Lansard., 2009; Lansard., 2010), and Jian carp (Chen., 2011; Wu., 2011), which indicates that nutrition status can regulate the TOR signaling pathway in fish as in mammals.
Being a large carnivorous marine fish, cobia () can grow from fingerling to 4–6kg marketable size in one year with high feed efficiency (Chou., 2004). Cobia culture has been developed rapidly in coastal provinces in southern China with the success of artificial propagation and larval production (Zhou., 2005). Several studies have been reported on the replacement of FM protein in cobia diets with alternative protein sources(Chou., 2004; Zhou., 2005; Lunger., 2007; Salze., 2010). However, no studies have been reported on the use of CGM in cobia diets. Zhou. (2004a) reported that the digestibility coefficients of CGM, including dry matter, crude protein, crude lipid and amino acids, are similar for juvenile cobia to those of FM, indicating the great potential of CGM as an alternative to FM protein in diets of cobia. Therefore, the present study was to evaluate CGM as an alternative protein source for FM protein in cobia diets by examining feed intake, growth, survival and protein metabolism in relation to gene expression of the fish.
2 Materials and Methods
2.1 Experimental Diets
As shown in Table 1, five isonitrogenous (crude protein 45%) and isoenergetic (gross energy 20kJg) experiment- tal diets were formulated by replacing 0% (the control), 17.5%, 35.0%, 52.5% and 70.0% of FM (Peruvian) protein with CGM protein. In all diets, crystalline amino acids were supplemented to simulate the EAA composi- tion in the whole body of cobia (Table 2), and monocalcium phosphate was supplemented to meet the phosphorous requirement of cobia (Zhou., 2004b).
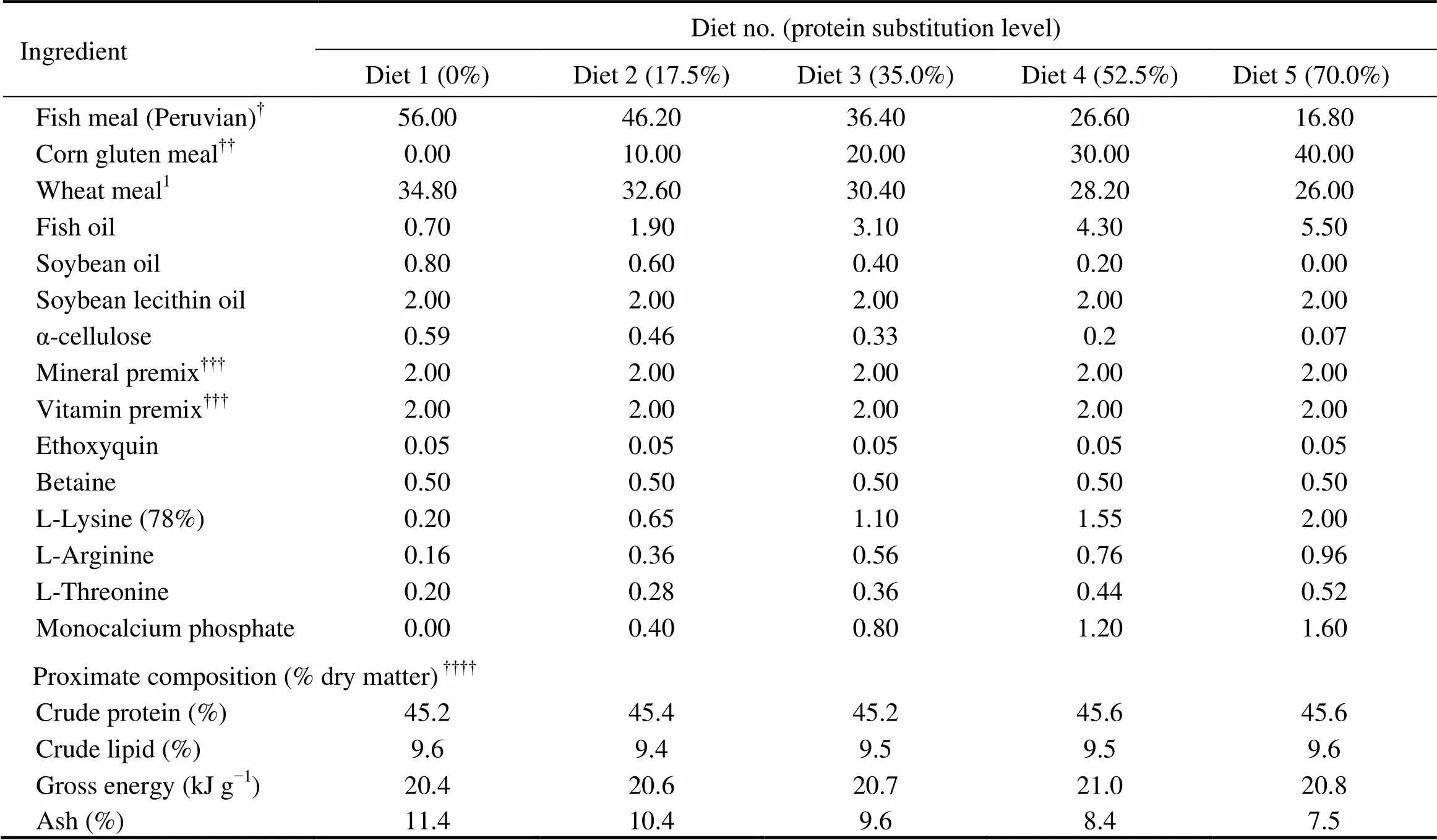
Table 1Formulation and proximate composition of the experimental cobia diets (% dry matter)
Notes:Guangdong Yuehai Feed Group Co. Ltd., Guangdong,China. Fish meal composition (dry matter basis): crude protein, 70.6%; and crude lipid, 11.9%. Wheat meal composition (dry matter basis): crude protein, 15.2%; and crude lipid, 1.0%;Evergreen Feed Group Co. Ltd., Guangdong,China.Corn gluten meal composition (dry matter basis): crude protein, 66.2%; and crude lipid, 1.8%;According to Ren.(2011);Means of three analyses.
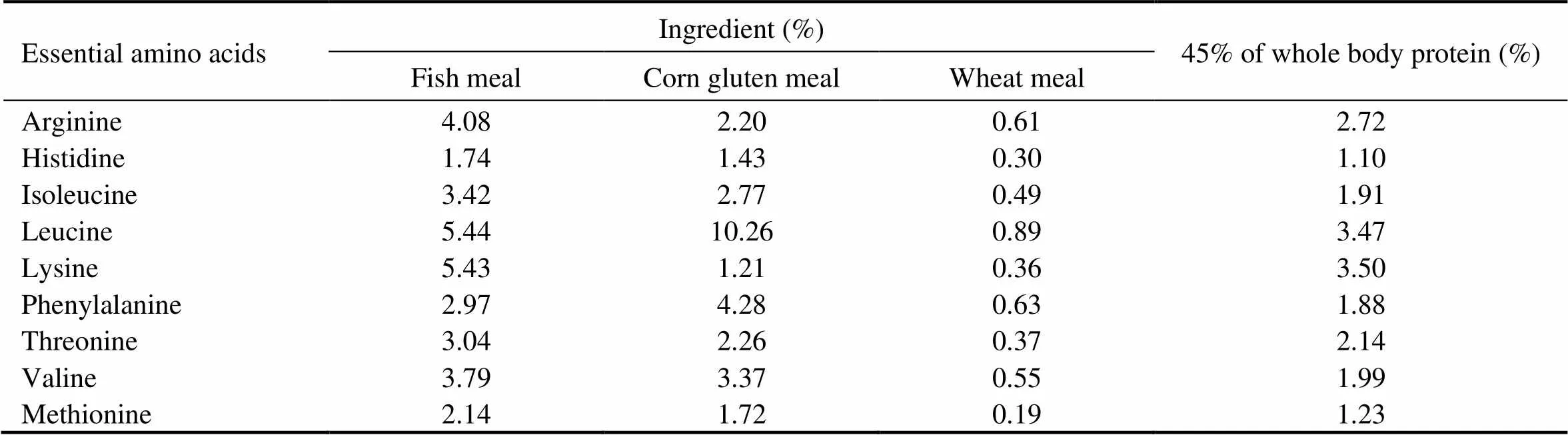
Table 2 Amino acid composition of the ingredients and 45% of whole body protein of cobia (% dry weight)
Ingredients were ground into fine powder and passed through a 246-μm mesh. They were thoroughly mixed with oil, to which was added water to produce stiff dough. The dough was then pelleted with an experimental feed mill (F-26 (II), South China University of Technology, China). Pellets (4.0mm diameter) were air-dried to less than 10% moisture, and then stored at −20℃ prior to analysis.
2.2 Feeding Trial Procedures
Disease-free cobia juveniles were obtained from Jiufu Fish Hatchery in Sanya (Hainan, China). The fish were fed the control diet during a 1-week conditioning period after collection. Thereafter, the fish were fasted for 24h and then weighed after anesthetized with eugenol (1: 10000) (Shanghai Reagent Corporation, China). Fish of homogenous size (average weight 108.2g±3.0g) were randomly distributed into 15 seawater floating cages (1.5m×1.5m×2.5m), and each cage was stocked with 20 fish. Each diet was randomly assigned to three replicate cages, and the fish were hand-fed to apparent satiation twice daily (07:00 and 17:00) for 7 weeks. During the experi- mental period, rearing water temperature ranged from 27.0 to 29.5℃, the salinity was 24 to 26, and the dissolved oxygen was approximately 7mgL.
2.3 Analyses and Measurement
2.3.1 Sample collection
At the start of the experiment, five fish were randomly selected for determination of the initial whole body composition, and fasted for 24h before harvest. Total number and body weight of fish in each cage were determined. Four fish per cage were randomly collected and stored at −20℃prior to determination of the whole- body composition. Another four fish from each cage were anesthetized with eugenol (1:10000), with samples collected from the fish heart using 2-mL heparinized syringes. Subsequently, plasma samples were obtained via centrifugation (4000g for 10min) of blood samples at 4℃. The liver, intestinal tract and dorsal muscle were obtained from four fish per cage, and chyme was removed from the gut using distilled water. Both plasmid and tissue samples wereimmediately stored at −80℃until analysis.
2.3.2 Composition analyses of ingredients, diets and fish body
Composition analyses of ingredients, diets and fish body were conducted for duplicate samples following the standard procedures (AOAC, 1995): dry matter was determined by 105℃ oven-drying to a constant weight; crude protein by measuring nitrogen (N×6.25) using the Kjeldahl method (Kjeltec TM 8400, FOSS, Tecator, Sweden); crude lipid by ether extraction using the Soxhlet method; ash by combustion at 550℃ for 16h; and energy by an adiabatic bomb calorimeter (PARR1281, USA).Essential amino acids, except methionine, were determined according to Cheng. (2010) using a Biochrom 30 amino acid analyzer (Biochrom Ltd, UK), and methionine was determined using reverse-phase high-per- formance liquid chromatography (HPLC, HP1100, USA).
2.3.3 Enzyme activity assays
Protease activity of intestine was analyzed according to Kumar. (2006). Tyrosine was used as the standard, and one unit of enzyme activity was defined as the amount of enzyme needed to catalyse the formation of 1μg of tyrosine per min. Activities of alpha-amylase, alanine aminotransferase (ALT) and aspartate amino- transferase (AST) were determined following the methods described by Cheng. (2010) using commercially available kits(Jiancheng Bioengineering Institute, Nanjing, China). The lysozyme activity was determined according to Ellis (1990).
2.3.4 Plasma metabolite assay
Plasma total protein concentration was assayed according to Bradford (1976);plasma cholesterol content determined spectrophotometrically using commercially available kits (Zhejiang Dongou bioengineering Co., Zhejiang, China); and plasma ammonia, urea and total amino acids determined according to Melo. (2006) using Multiskan spectrum (Thermo, USA).
2.3.5 RNA extraction, cDNA synthesis and partial sequence cloning of tor and IGF-1 genes
Total RNA was extracted from cobia liver using Trizol Reagent (Invitrogen, USA). Three μg of RNA extract was used for reverse transcription by PrimerScript® RT Enzyme using Oligo-dT primers (Takara, Japan) in a volume of 10μL following reagent’s instructions. The degenerate primer pairs of TOR and IGF-I were designed using CODEHOP (Table 3). The polymerase chain reaction (PCR) was conducted on an Eppendorf Mastercycler gradient (Eppendorf, German) to amplify cDNA fragment of TOR and IGF-I. The PCR conditions were as follows: for TOR, initial denaturation at 94℃ for 2min, followed by 35 cycles of denaturation at 94℃ for 30s, annealing at 59℃ for 30s, and extension at 72℃ for 1min, and final extension at 72℃ for 5min; and for IGF-I, initial denaturation at 94℃ for 2min, followed by 35 cycles of denaturation at 94℃ for 30s, annealing at 57℃ for 30s, and extension 72℃ for 30s, and final extension at 72℃for 5min. The PCR products were electrophoresed to determine the length, cloned into the pEASY-T1 vector (TransGen Biotech, China), and then transformed into the competent cells ofTOP10. The recombinants were identified by PCR, and three positive clones were sequenced for each PCR fragment. Then, these resulting sequences were verified in NCBI.

Table 3 Sequences of the primers used in this study
2.3.6 Real-time quantitative PCR analysis of TOR and IGF-I mRNA expression
Total RNA was extracted from individual liver and dorsal muscle samples (n=4 per cage) using Trizol Reagent (Invitrogen, USA), and electrophoresed to test the integrity. The RNA extracts were treated with Recombinant DNase I (RNase-free) (Takara, Japan) to remove possible DNA contamination. The quantity and quality of RNA extracts were assessed using the Nano Drop® ND-1000 spectrophotometer (Nano-Drop Technologies, Wilmington, DE, USA). The 260/280nm absorbance ratios of all samples ranged from 1.86 to 2.00, indicating a favorable purity of the RNA samples. Purified RNA was reversely transcribed to cDNA using PrimeScriptTM RT reagent Kit (Takara, Japan). The real-time quantitative PCR primer pairs were designed using Primer Premier 5.00 based on nucleotide sequences of TOR and IGF-I genes of cobia in order to evaluate the mRNA levels of TOR and IGF-I in liver and dorsal muscle (Table 3). Reference β-actin gene (EU266530) was used as internal control (Table 3). Real-time PCR assays were conducted in a quantitative thermal cycler (Mastercycler ep realplex, Eppendorf, German). The 25μL reaction contained 12.5µL of 2×SYBR® Premix Ex TaqTM (Perfect Real Time) (Takara, Japan), 0.5μL of each of the primers (10μmol L), 1μL of cDNA mix. The real-time quantitative PCR amplification began with 30s at 95℃, followed by 35 cycles of 5s at 95℃, 25s at 60.2℃, and 30s at 72℃. No-template controls were run for each PCR assay. Standard curves were made with five different dilutions (in triplicate) of the cDNA samples and amplification efficiency was analyzed according to the following equation
=10−1.
In liver, the primer amplification efficiency was 0.9768 for TOR, 0.9804 for IGF-I and 0.9810 for β-actin, and the absolute ΔCvalue (TOR–β-actin) and ΔCvalue (IGF-I–β-actin) of the slope were 0.0486 and 0.0277, respectively. In dorsal muscle, the primer amplification efficiency was 0.9792 for TOR, 0.9747 for IGF-I and 0.9728 for β-actin, and the absolute ΔCvalue (TOR–β-actin) and ΔCvalue (IGF-I–β-actin) of the slope were 0.0615 and 0.0312, respectively. These indicated that ΔΔCcalculation could be used for the relative quantification of target genes. No significant differences were observed in β-actin expression levels among dietary treatments using the 2method. The expression levels of TOR and IGF-I were calculated by the 2method, and the value stood for n-fold difference relative to the calibrator. The relative expression levels of IGF-I and TOR in control group were used as calibrator (Livak and Schmittgen, 2001).
2.4 Calculations and Statistical Methods
The following variables were calculated:
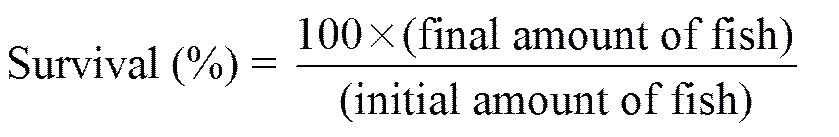
,
,

Protein productive value (PPV)=
,
where Wand Ware final and initial body weights, respectively; andis duration of experimental days.
All data were subjected to analysis of variance using SPSS 17.0 for Windows. Differences among the mean values were tested by the Tukey's multiple range test. The level of significance chosen was<0.05.
3 Results
3.1 Survival and Growth Performance
There were no significant differences in the survival, FI, final body weight (FBW), SGR, FE and PPV among fish fed diets with 0% (the control), 17.5%, 35.0% and 52.5% of CGM protein (>0.05) (Table 4). However, these indices were significantly lower in fish fed the diet with 70.0% of CGM protein compared with the control group (<0.05).
3.2 Whole Body Composition
Compared with the control group, fish fed the diet with 70.0% of CGM protein had significantly higher whole- body moisture content, with significantly lower crude protein and crude lipid contents (<0.05). When the substitution level was < 70%, no significant differences were observed in the whole-body moisture, crude protein and crude lipid contents(>0.05) (Table 5). There was no significant difference in whole-body ash (3.6%–3.9%) among dietary treatments (>0.05).
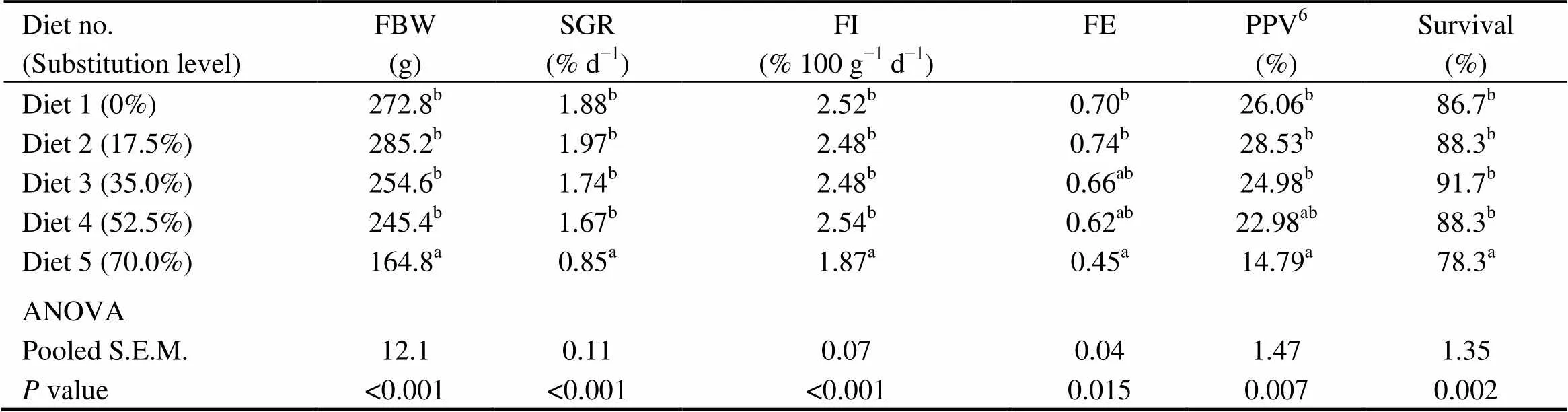
Table 4 Growth performanceof cobia fed the diets with graded levels of corn gluten meal†
Notes:Values are means of three replicates. Means in each column with the same superscript have no significant differences (> 0.05). FBW, final body weight; SGR, specific growth rate; FI, Feed intake; FE, feed efficiency; PPV, Protein productive value; ANOVA, One-way analysis of variance; and S.E.M., standard error of mean.
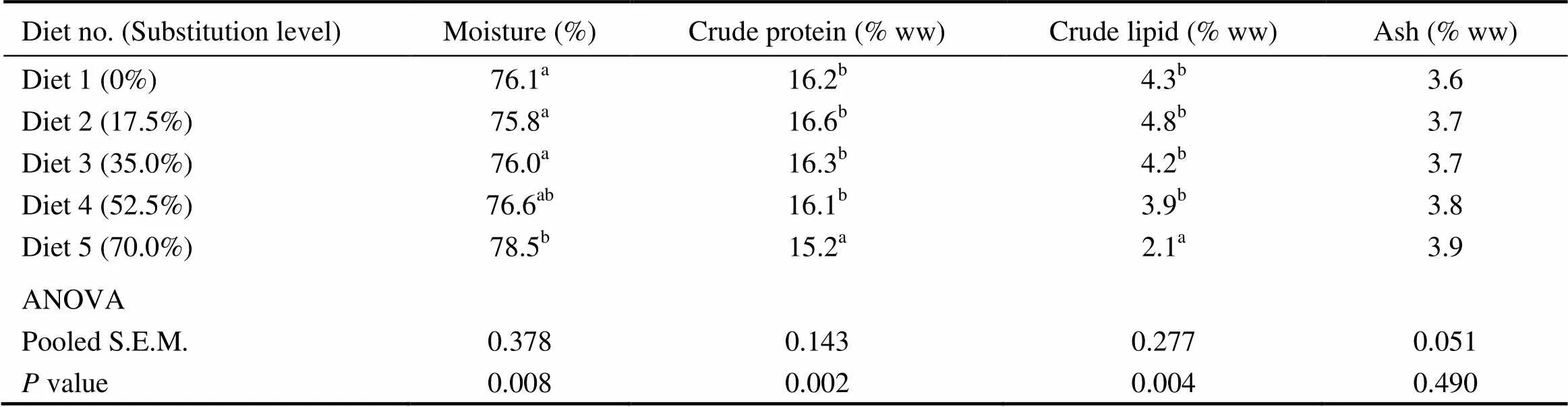
Table 5 Whole-body composition of cobia fed the diets with graded levels of corn gluten meal†
Notes:Values are means of one composite sample from four fish in each of the triplicate groups. Means in each column with the same superscript have no significant differences (>0.05); ww, wet weight; ANOVA, One-way analysis of variance; and S.E.M., standard error of mean.
3.3 Activities of Digestive Enzymes, Protein Metabolism Enzymes and Lysozyme
There were no significant differences in the activities of protease (1.50–2.01U (mg protein)) and alpha-amy- lase (0.63–0.76U (mg protein)) in intestine, AST (21.96 –36.28U (mg protein)) and ALT (18.39–30.23U (mg protein)) in liver among dietary treatments (>0.05) (Table 6). However, the activities of ALT and AST generally decreased with the increasing dietary CGM levels.So did the activity of lysozyme in plasma. When 70.0% of FM protein was replaced by CGM, lysozyme activity was significantly lower than that in the control group (<0.05).

Table 6 The activities of digestive enzymes, protein metabolism enzymes and lysozyme of cobia fed the diets with graded levels of corn gluten meal†
Notes:Values are means of one composite sample from four fish in each of the triplicate groups. Means in each row with the same superscript have no significant differences (>0.05). ANOVA, One-way analysis of variance; S.E.M., standard error of mean.
3.4 Plasma Metabolites
Fish fed the diet with 70.0% of CGM protein had significantly lower plasma total protein and cholesterol than the control group (<0.05). When the substitution level was <70%, there were no significant differences in plasma total protein and cholesterol.No significant differences were observed in plasma ammonia (0.209–0.224μmolmL), urea (1.39–1.57μmolmL), and amino acids (11.36–14.02μmolmL) among dietary treatments (>0.05) (Table 7).
3.5 Cloning of Partial cDNA Sequence of TOR and IGF-I Gene
The PCR product of TOR is 596bp in length, and its nucleotide sequence is related to those of tilapia(XM003449131) (identity 93%), zebra fish(AB290031) (identity 83%) and common carp(FJ899680) (identity 83%).
The PCR product for IGF-I is 256 bp in length, and its nucleotide sequence is related to red striped snapper(JN383435) (identity 98%), yellowtail(AB439208) (identity 97%), Atlantic halibut(EU682475) (identity 97%), and Asian bass(EU- 136126) (identity 97%).
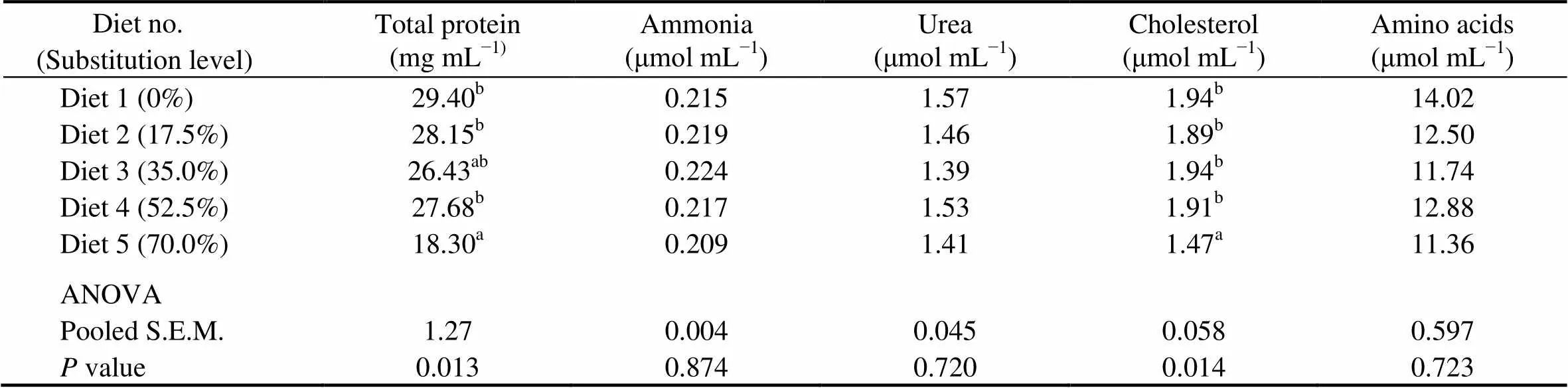
Table 7 Plasma metabolites of cobia fed the diets with graded levels of corn gluten meal†
Notes:Values are means of one composite sample from four fish in each of the triplicate groups. Means in each column with the same superscript have no significant differences (>0.05); ANOVA, One-way analysis of variance; and S.E.M., standard error of mean.
3.6 Expression of TOR and IGF-I Gene in Liver and Dorsal Muscle
With the increasing dietary CGM levels, hepatic IGF-Iexpression levels generally decreased. Fish fed the diet with 70.0% of CGM protein had significantly lower IGF-Iexpression level in liver compared with the control group (<0.05). However, no significant difference was observed in the dorsal muscle IGF-I expression level (0.86–1.01) among dietary treatments (>0.05) (Fig.1). In addition, there were no significant differences in hepatic TOR expression (0.86–1.01) and dorsal muscle TOR expression levels (0.89–1.02) among dietary treatments(>0.05).
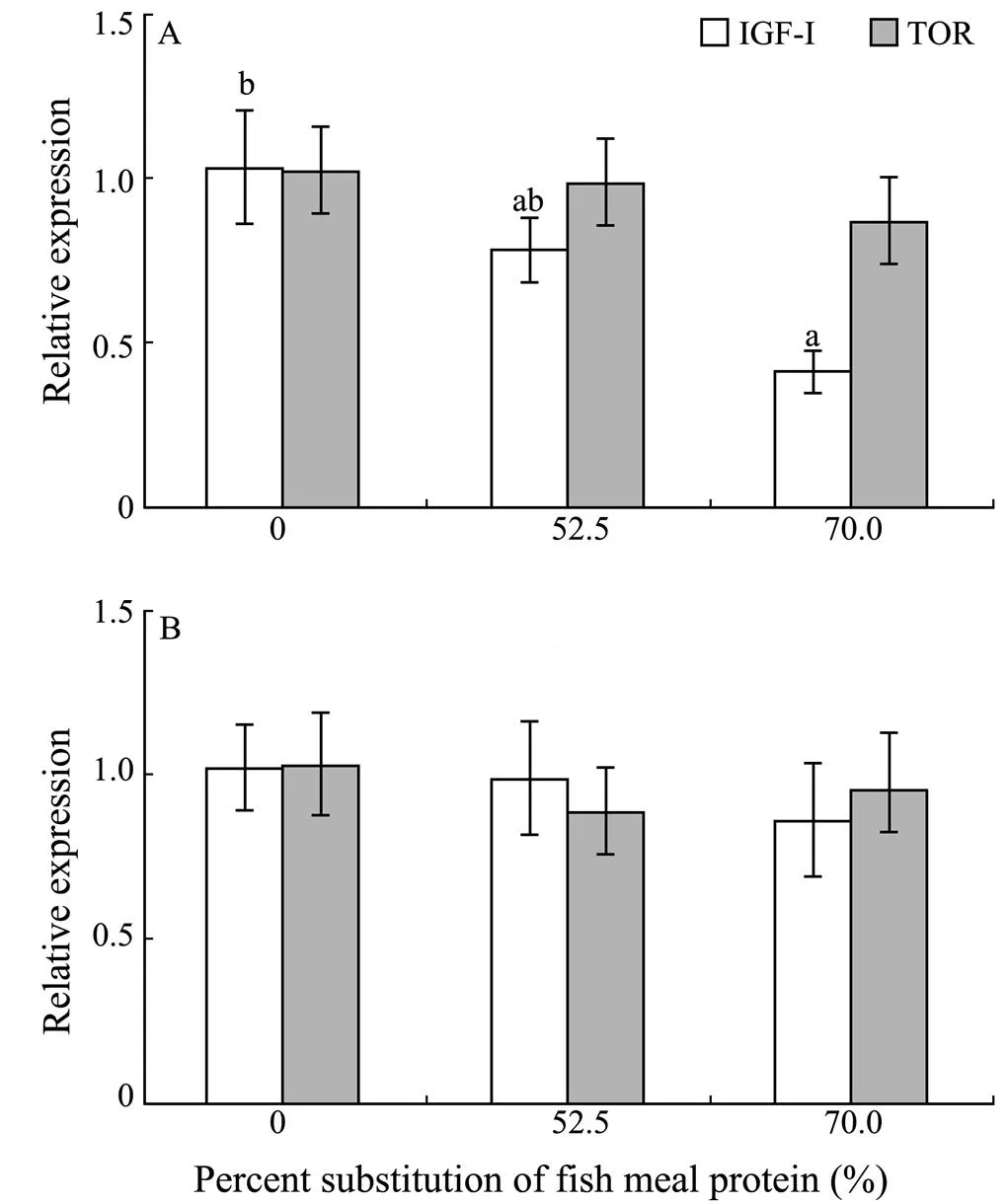
Fig.1 Relative mRNA expression of IGF-I and TOR in the liver (A) and dorsal muscle (B) of cobia fed diets with graded levels of corn gluten meal for 7 weeks.Relative mRNA expression was evaluated by real-time quantitative PCR; values are means±S.E.M. (n=3); bars of the same gene with the same letters are not significantly different by Tukey’s test (P>0.05).
4 Discussion
In the present study, no significant differences were observed in SGR and FE of cobia with up to 52.5% of FM protein replaced by CGM protein, that is, 30% of CGM content in the diet. However, when the substitution level reached 70.0%, the SGR and FE were significantly decreased in cobia compared with the control group. This indicated that the cobia had good tolerance for dietary CGM, and up to 52.5% of FM protein could be replaced by CGM protein without significant effects on the growth performance and feed utilization of cobia supplemented with crystalline amino acids. Our result was in agreement with previous findings in Atlantic salmon (Mente., 2003), red sea bream (280g) (Takagi., 2000) and Atlantic cod (Hansen., 2007a). However, the result obtained here was higher than those reported in Japanese flounder (Kikuchi, 1999), turbot (Regost., 1999), red sea bream (53g) (Takagi., 2000), sunshine bass (Lewis and Kohler, 2008) and puffer (Zhong., 2011), which showed significantly decreasedgrowth when CGM replaced >15%–40% of FM protein. Such differences could be attributed to multiple factors, such as different fish species, ages, dietary compositions, feeding strategies, and processing technologies.
The limitations of using alternative plant protein sources can be related to the relatively low FI of fish. In the present study, fish fed the diet with 70.0% of CGM protein had significantly lower FI than the control group, consistent well with the results of fish growth performance. Thus, the lowered FI was likely an important factor decreasing the growth of tested cobia in this study, and to some extent feed palatability was affected when CGM protein replaced 70.0% of FM protein in cobia diets. Similar results had been observed in rainbow trout (Gomes., 1995), gilthead sea bream (Pereira and Oliva- Teles, 2003) and sunshine bass (Lewis and Kohler, 2008), which showed significantly decreased FI with an in- creasing dietary CGM level.
The IGF-I was a major anabolic agent responsible for tissue growth (Thissen., 1999). In this study, fish fed the diet with 70.0% of CGM protein had significantly lower hepatic IGF-Iexpression level than the control group, consistent with the results of SGR and PPV. This result was confirmed by previous studies on gilthead sea bream (Gómez-Requeni., 2004) and Atlantic salmon (Hevrøy., 2008), which showed significantly lower hepatic IGF-I expression when fed the diet with high plant protein compared with those fed the whole FM diet. It has been shown that feed restriction decreases hepatic IGF-I expression levels in eel (Duan and Hirano, 1992), coho salmon (Duan and Plisetskaya, 1993), barramundi (Matthews., 1997), and grouper (Pedroso., 2006) compared with the control group, whereas refeeding of the starved fish leads to a rise in hepatic IGF-I mRNA levels (Duan and Plisetskaya, 1993; Pedroso., 2006). In the present study, FI of cobia significantly decreased with the increasing dietary CGM levels. Hence, feed restriction likely contributed to the decrease in hepatic IGF-Iexpression of cobia. Lysine and arginine are the limiting amino acids in CGM. When FM protein was replaced by CGM, lysine and arginine levels correspondingly decreased in the diet. Hevrøy. (2007) demonstrated that lower lysine intake resulted in a significant down-regulation of hepatic IGF-I expression levels compared to Atlantic salmon on higher lysine intake. In the present study, crystalline amino acids were supplemented to simulate the EAA composition in the whole body of cobia fed different diets. However, due to absorption imbalance of crystalline amino acids and protein amino acids in diets, imbalance of essential amino acids perhaps still existed among dietary treatments. This could have decreased the growth performance of fish fed the diet with 70.0% of CGM protein. Regost. (1999) also suggested that when FM was replaced by high levels of CGM protein, supplemented crystalline amino acids could not be efficiently utilized by turbot. Hence,the imbalance of essential amino acids could be another factor decreasing the hepatic IGF-Iexpression. The GH is a primary stimulus for synthesis of liver IGF-I as well as its release into the circulation. The biological effects of IGF-I were regulated mainly by the interaction of IGF-I ligand with IGF-I receptor (IGF-IR) modulated through IGF-bind- ing proteins (IGFBPs) (Duan., 2010). Further studies are needed to examine the effects of dietary CGM levels on GH, IGF-IR and IGFBPs expression levels in cobia.
Wu. (2011) reported that choline supplementation decreased the hepatic and muscle TOR expression levels in Jian carp. Chen. (2011) found that arginine supplementation increased the hepatic and muscle TOR expression levels in Jian carp. These indicated that nutritional signals could affect the TOR expression in fish. However, in the present study, no significant differences were observed in TOR expression in liver and dorsal muscle of cobia fed different experimental diets. Hence, it was concluded that the protein source in diet did not significantly affect the TOR protein of the tested fish species at the transcriptional level, in agreement with previous findings in mammals. The kinase activity of TOR protein was regulated by a variety of factors, such as phosphorylation. Research has demonstrated that refeeding can enhance the phosphorylated TOR protein level in liver of rainbow trout (Seiliez., 2008; Lansard., 2009). Insulin and amino acid supplementation can also increase the phosphorylated TOR protein in liver cells of rainbow trout (Lansard., 2010). Together these findings indicate that the nutritional signals can modulate phosphorylation of TOR protein. Whether the dietary CGM levels affect phosphorylated TOR protein levels in liver and muscle of cobia needs to be further investigated.
In the present study, fish fed the diet with 70.0% of CGM protein had significantly lower plasma cholesterol content than fish fed the control diet, in agreement with previous findings in turbot (Regost., 1999; Yun., 2011), gilthead sea bream (Sitja-Bobadilla., 2005), and Atlantic cod (Hansen., 2007b). At present, the causing factors of the hypocholesterolemic effect of plant protein in fish remain unknown. Cholesterol is rich in FM, but deficient in most plant-based ingredients (Yun., 2011). Hence, when FM in diet was replaced by plant protein sources, cholesterol correspondingly decreased in diet. It had been demonstrated that cholesterol supple- mentation in plant-based diets significantly improved the FI and growth performance of fish, such as channel catfish (Twibell and Wilson, 2004), Japanese flounder (Deng., 2010), and turbot (Yun., 2011). Whether cholesterol supplementation in diet with 70.0% of FM protein replaced by CGM protein can improve the FI and growth performance of cobia needs further study.
In conclusion, the present study showed that up to 52.5% of FM protein could be replaced by CGM protein without significant negative effects on the growth per- formance of cobia and their feed utilization of different diets. Reduced growth performance of fish fed the diet with 70.0% of FM protein replaced by CGM was likely resulted from the suppression of feed intake, decreases in protein synthesis activities related to lower hepatic IGF-I gene expression, and deficiency of cholesterol.
Acknowledgements
This research was supported by the National Department Public Benefit Research Foundation (Ministry of Agriculture of the People’s Republic of China, No.2010- 03020), the National Natural Science Foundation of China (No. 30901108), and Agricultural Scientific and Technological Achievements into Capital 2010GB23600673. We thank Tan, B. P., Chi, S. Y., Zhang, L., Liu, Y. L., and Liu, K. for their experimental help, and Tan, P., and Li, S. L. for their assistance with RNA extraction.
Association of Official Analytical Chemists (AOAC), 1995., 16th Edn. Association of Official Analytical Chemists, Arlington, VA, USA.
Bradford, M. M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding., 72: 248-254.
Chen, G. F., Feng, L., Kuang, S. Y., Liu, Y., Jiang, J., Hu, K., Jiang, W. D., Li, S. H., Tang, L., and Zhou, X. Q., 2011.Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (var. Jian)., 107: 1-13.
Cheng, Z. Y., Ai, Q. H., Mai, K. S., Xu, W., Ma, H. M., li, Y., and Zhang, J. M., 2010. Effects of dietary canola meal on growth performance, digestion and metabolism ofJapanese seabass,., 305: 102-108.
Chou, R. L., Her, B. Y., Su, M. S., Hwang, G., Wu, Y. H., and Chen, H. Y., 2004. Substituting fish meal with soybean meal in diets of juvenile cobia,., 229: 325-333.
Deng, J. M., Mai, K. S., Ai, Q. H., Zhang, W. B., Wang, X. J., Tan, B. P., Xu, W., Liufu, Z. G., and Ma, H. M., 2010. Interactive effects of dietary cholesterol and protein sources on growth performance and cholesterol metabolism of Japanese flounder ()., 16: 419-429.
Duan, C., and Hirano, T., 1992. Effects of insulin-like growth factor-I and insulin on the in vitro uptake of sulfate by eel branchial cartilage: evidence for the presence of independent hepatic and pancreatic sulfation factors., 133: 211-219.
Duan, C., and Plisetskaya, E. M., 1993. Nutritional regulation of insulin-like growth factor I mRNA expression in salmon tissues., 139: 243-252.
Duan, C., Ren, H., and Gao, S., 2010. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation., 167: 344-351.
Ellis, A. E., 1990. Lysozyme assays. In:. Stolen, J. S.,., eds., SOS Publications, Fair Haven, NJ, 101-103.
Gomes, E. F., Rema, P., and Kaushik, S. J., 1995. Replacement of fish meal by plant proteins in the diet of rainbow trout (): digestibility and growth performance., 130: 177-186.
Gómez-Requeni, P., Mingarro, M., Calduch-Giner, J. A., Médale, F., Martin, S. A., Houlihan, D. F., Kaushik, S. J., and Perez-Sanchez, J., 2004. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream ()., 232: 493-510.
Hansen, A. C., Karlsen, Ø., Rosenlund, G., Rimbach, M., and Hemre, G., 2007a. Dietary plant protein utilization in Atlantic cod,L., 13: 200-215.
Hansen, A. C., Rosenlund, G., Karlsen, Ø., Koppe, W., and Hemre, G. I., 2007b. Total replacement of fish meal with plant proteins in diets for Atlantic cod (L) I: effects on growth and protein retention., 272: 599-611.
Hardy, R. W., 2010. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal.,41: 770-776.
Hevrøy, E. M., El-Mowafi, A., Taylor, R., Olsvik, P. A., Norberg, B., and Espe, M., 2007. Lysine intake affects gene expression of anabolic hormones in Atlantic salmon ()., 152: 39-46.
Hevrøy, E. M., El-Mowafi, A., Taylor, R., Norberg, B., and Espe, M., 2008. Effects of a high plant protein diet on the somatotropic system and cholecystokinin in Atlantic salmon (L.)., 151: 621-627.
Kikuchi, K., 1999. Partial Replacement of fish meal with corn gluten meal in diets for Japanese Flounder ()., 30: 357-363.
Kumar, S., Sahu, N. P., Pal, A. K., Choudhury, D., and Mukherjee, S. C., 2006. Studies on digestibility and digestive enzyme activities in(Hamilton) juveniles: effect of microbial α-amylase supplementation in non-gelatinized or gelatinized corn based diet at two protein levels., 32: 209-220.
Lansard, M., Panserat, S., Seiliez, I., Polakof, S., Plagnes-Juan, E., Geurden, I.,Medale, F.,Kaushik, S., Corraze, G., and Skiba-Cassy, S., 2009. Hepatic protein kinase B(Akt)-targetof rapamycin (TOR)-signalling pathways and intermediary metabolism in rainbow trout() are not significantly affected by feedingplant-based diets.,102: 1564-1573.
Lansard, M., Panserat, S., Plagnes-Juan, E., Seiliez, I., and Skiba-Cassy, S., 2010. Integration of insulin and amino acid signals that regulate hepatic metabolism-related gene expression in rainbow trout: role of TOR., 39: 801-810.
Lewis, H. A., and Kohler, C. C., 2008. Corn gluten meal partially replaces dietary fish meal without compromising growth or fatty acid composition of sunshine bass., 70: 50-60.
Livak, K. J., and Schmittgen, T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2method., 25: 402-408.
Lunger, A. N., McLean, E., and Craig, S. R., 2007. The effects of organic protein supplementation upon growth, feed conversion and texture quality parameters of juvenile cobia ()., 264: 342-352.
Matthews, S. J., Kinhult, A. K., Hoeben, P., Sara, Y. R., and Anderson, T. A., 1997. Nutritional regulation of insulin-like growth factor-I mRNA expression in barramundi,., 18: 273-276.
Melo, J. F. B., Lundstedt, L. M., and Metón, I., 2006. Effects of dietary levels of protein on nitrogenous metabolism of Rhamdia quelen ()., 145: 181-187.
Mente, E., Deguara, S., Santos, M. B., and Houlihan, D., 2003. White muscle free amino acid concentrations following feeding a maize gluten dietary protein in Atlantic salmon (L.)., 225: 133-147.
Pedroso, F. L., de Jesus-Ayson, E. G., Cortado, H. H., Hyodo, S., and Ayson, F. G., 2006. Changes in mRNA expression of grouper () growth hormone and insulin-like growth factor I in response to nutritional status., 145: 237-246.
Pereira, T. G., and Oliva-Teles, A., 2003. Evaluation of corn gluten meal as a protein source in diets for gilthead sea bream (L.) juveniles., 34: 1111-1117.
Regost, C., Arzel, J., and Kaushik, S. J., 1999. Partial or total replacement of fish meal by corn gluten meal in diet for turbot ().,180: 99-117.
Ren, M. C., Ai, Q. H., Mai, K. S., Ma, H. M., and Wang, X. J., 2011.Effects of dietary carbohydrate level on growth performance, body composition, apparent digestibility coefficient and digestive enzyme activities of juvenile cobia,L., 42: 1467- 1475.
Salze, G., McLean, E., Battle, P. R., Schwarz, M. H., and Craig, S. R., 2010. Use of soy protein concentrate and novel ingredients in the total elimination of fish meal and fish oil in diets for juvenile cobia,., 298: 294-299.
Seiliez, I., Gabillard, J.C., Skiba-Cassy, S., Garcia-Serrana, D., Gutierrez, J., Kaushik, S.,Panserat, S., and Tesseraud, S., 2008. Anandassessment of TORsignalingcascade in rainbow trout ().,, 295: 329-335.
Sitja-Bobadilla, A., Pena-Llopis, S., Gomez-Requeni, P., Me- dale, F., Kaushik, S., and Perez-Sanchez, J., 2005. Effect of fish meal replacement by plant protein sources on non-speci- ficdefence mechanisms and oxidative stress in gilthead sea bream ()., 249: 387-400.
Takagi, S., Hosokawa, H., Shimeno, S., and Ukawa, M., 2000. Utilization of corn gluten meal in a diet for red sea bream.i,66: 417-427.
Thissen, J. P., Underwood, L. E., and Ketelslegers, J. M., 1999. Regulation of insulin-like growth factor-I in starvation and injury., 57: 167-176.
Twibell, R. G., and Wilson, R. P., 2004. Preliminary evidence that cholesterol improves growth and feed intake of soybean meal-based diets in aquaria studies with juvenile channel catfish,., 236: 539-546.
WU, P., Feng, L., Kuang, S. Y., Liu, Y., Jiang, J., Hu, K., Jiang, W. D., Li, S. H., Tang, L., and Zhou, X. Q., 2011. Effect of dietary choline on growth, intestinal enzyme activities and relative expressions of target of rapamycin and eIF4E-binding protein 2 gene in muscle, hepatopancreas and intestine of juvenile Jian carp (var. Jian)., 317: 107-116.
Wullschleger, S., Loewith, R., and Hall, M. N., 2006. TOR signaling in growth and metabolism., 124: 471-484.
Yun, B., Mai, K. S., Zhang, W. B., and Xu, W., 2011. Effects of dietary cholesterol on growth performance, feed intake and cholesterol metabolism in juvenile turbot (L.) fed high plant protein diets., 319: 105-110.
Zhong, G., Hua, X., Yuan, K., and Zhou, H., 2011. Effect of CGM on growth performance and digestibility in puffer ()., 19: 395-403.
Zhou, Q. C., Tan, B. P., Mai, K. S., and Liu, Y. J., 2004a. Apparent digestibility of selected feed ingredients for juvenile cobia., 241: 441-451.
Zhou, M., Cao, J. M., Wu, J. K., Liang, H. O., Zhu, W. M., Zhao, H. X., Zhang, H. T., Yang, D. W., Ma, L., and Lan, H. B., 2004b. Dietary phosphorus requirement of juvenile cobia,., 17: 62 (in Chinese with English abstract).
Zhou, Q. C., Mai, K. S., Tan, B. P., and Liu, Y. J., 2005. Partial replacement of fishmeal by soybean meal in diets for juvenile cobia,., 11: 175-182.
(Edited by Qiu Yantao)
10.1007/s11802-013-2021-3
ISSN 1672-5182, 2013 12 (3): 418-426
. Tel/Fax: 0086-532-82031943 E-mail: qhai@ouc.edu.cn
(April 23, 2012; revised May 15, 2012; accepted August 20, 2012)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2013
 Journal of Ocean University of China2013年3期
Journal of Ocean University of China2013年3期
- Journal of Ocean University of China的其它文章
- Evaluation of Antitumor, Immunomodulatory and Free Radical Scavenging Effects of A New Herbal Prescription Seaweed Complex Preparation
- Effects of Exposure to Four Endocrine Disrupting-Chemicals on Fertilization and Embryonic Development of Barbel Chub (Squaliobarbus curriculus)
- A Preliminary Phylogenetic Analysis of Luidia (Paxillosida:Luidiidae) from Chinese Waters with Cytochrome Oxidase Subunit I (COI) Sequences
- Seasonal Community Structure of Mesozooplankton in the Daya Bay, South China Sea
- The Effect of Three Culture Methods on Intensive Culture System of Pacific White Shrimp (Litopenaeus vannamei)
- Comparison of Lipids in Organs of the Starfish Asterias amurensis Associated with Different Treatments
