Evaluation of Antitumor, Immunomodulatory and Free Radical Scavenging Effects of A New Herbal Prescription Seaweed Complex Preparation
LIU Xin1), 2), SHAO Changlun1), KONG Wenwen1), FANG Yuchun1), and WANG Changyun1), *
Evaluation of Antitumor, Immunomodulatory and Free Radical Scavenging Effects of A New Herbal Prescription Seaweed Complex Preparation
LIU Xin, SHAO Changlun, KONG Wenwen, FANG Yuchun, and WANG Changyun
1),,,,266003,2),250014,
Seaweed Complex Preparation (SCP) is a clinical traditional Chinese medicine preparation which is composed of seven traditional Chinese herbs, and it has been used for treatment of lung cancer, liver cancer and digestive cancer. However, little information is available about the pharmacodynamic basis. The antitumor, immunomodulatory and free radical scavenging effects of SCP were evaluated in this study. Transplanted tumormethod was used to determine the antitumor effect. The effects on splenocyte proliferation and phagocytosis of macrophages in tumor-bearing mice were measured by the MTT method and the phagocytizing cock red blood cell (CRBC) method respectively. The scavenging activities of SCP on DPPH and hydroxyl radicalswere investigated. It was found that the medium-dose and high-dose of SCP could significantly inhibit the growth of transplanted hepatic tumor of murine hepatocarcinoma cell line H22, and promote proliferation of splenocytes and phagocytosis of macrophages. SCP possessed noticeable scavenging activities on DPPH and hydroxyl radicals. The antitumor effects of SCP might be achieved by improving immune system and scavenging free radicals, which is in accordance with the viewpoint of traditional Chinese medicine in promoting the body resistance and eliminating pathogenic factors for cancer treatment.
traditional Chinese medicine preparation; Seaweed complex preparation; antitumor; immunomodulatory; free radical scavenging
1 Introduction
Traditional Chinese medicine preparation (TCMP) is a main form of drugs for Chinese medical practice, and it is composed of several single herbs with an intrinsic mass ratio according to the compatibility theory and dialectical view. It has been widely used in China as well as many Asian countries for thousands of years because of its high effectiveness against many diseases and its low toxicity.
According to the Chinese medicine theory, deficiency of healthy energy is a primary factor for cancers because it makes the body vulnerable to toxin and pathogen. Then, accumulation of toxin in human body leads to deficiency of organs, which gives tumor a chance to grow (Cao., 2011). On the other hand, one of the major drawbacks in current cancer therapeutic practices, such as chemotherapy and radiation therapy, is suppressing the immune system (Ehrke, 2003). In recent years, it has been found that some TCMPs possess antitumor and immunomodulatory properties with a low risk of toxicity in normal tissues (Lee., 2003). The study on the pharmaco- dynamic effects and chemical constituents of TCMP has attracted more and more attentions in the biochemical and medical fields because of their multi-components, multi- targets and multi-approaches effects.
Seaweed Complex Preparation (SCP) is a clinical traditional Chinese medicine preparation. It consists of seven herbs including(Turn.) C. Ag., radix of(Fisch.) Bge., fruit ofAit., rhizome ofRoxb., radix ofL., rhizome ofOsbeck and sclerotium ofWolf. As a clinical preparation, it has been used to treat lung cancer, liver cancer and gastric cancer, as well as to relieve the pain caused by chemotherapy. It was reported that SCP could significantly inhibit the growth of malignant tumor S180(Fang., 2000). The anticancer effect of SCP follow and uphold the traditional thinking of promoting the body resistance and eliminating pathogenic factors. SCP emphasizes the regulation of human body functions by diverse modalities, which is fundamentally different from the conception in chemotherapeutic drugs. The antitumor effect on H22-bearing mice, the immunomodulatory effect and free radical scavenging activities of SCP were evaluated in this study.
2 Materials and Methods
2.1 Materials
The herbs,(Turn.) C. Ag., radix of(Fisch.) Bge., fruit ofAit., rhizome ofRoxb., radix ofL., rhizome ofOsbeck and sclerotium ofWolf, were purchased from Qingdao Shandatianyuan Company, Shan- dong, China in June 2006 and identified by Prof. F. Q. Zhou at Shandong University of Traditional Chinese Medicine, Jinan, China. All the voucher specimens have been deposited in the Key Laboratory of Marine Drugs, Ministry of Education, Ocean University of China, Qingdao, China.
1,1-Diphenyl-2-picrylhydrazyl radical (DPPH), buty- lated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazo- lium bromide (MTT), concanacalin (Con A) and lipo- polysaccharide (LPS) were purchased from Sigma Chemical Co. Saint Louis, Missouri, USA. RPMI-1640 medium was purchased from, Grand Island, NY. Cyclophosphamide (CTX) was provided by Jiangsu Hengrui Co., China. H22 cell lines were provided by the Center for New Drugs Evaluation of Shandong University. All other chemicals and solvents used were of analytical grade.
2.2 Animals
Male ICR mice (5 weeks old, 18–22g each) were purchased from the Center for New Drugs Evaluation of Shandong University (Certificate No. SKXK. 20030004, Jinan, China) and acclimatized for 1 week before experiment. Rodent laboratory chow and tap water were providedand maintained under controlled conditions with a temperature of 24℃±1℃, humidity of 50% ±10%, and a 12/12h light/dark cycle. All the procedures were in strict accordance with the P. R. China legislationon the use and care of laboratory animals and with the guidelines established by the Institute for Experimental Animals of Shandong University, and were approved by the University Committee for animal experiments.
2.3 Preparation of SCP Decoction
The formula of SCP include.(450g),.(450g),.(600g),.(600g),.(450g),.(600g) and(450g). The powdered mixture of above herbs was decocted three times in boiling water (1:10, for 2h; 1:8, 1.5h; and 1:6, 1h). The filtrate of the decoction was concentrated under vacuum to yield SCP extract with a ratio of herb material to solution as 2:1 (g:mL), and then was stored at 4℃ for future analysis.
2.4 H22-Bearing Mice Model and Treatment for Antitumor Effect
The antitumor effect was determined based on transplanted tumormethod with minor modifications (Qi and Xu, 2006; Zhao., 2012; Zhang., 2009). Ascites of the H22-bearing mice were drawn out under aseptic conditions and then diluted 4-fold with aseptic saline. The tested male mice were randomly divided into five groups, each group consisting of twelve animals. The diluted H22 cell suspension (10per mL) was inoculated subcutaneously (s.c.) into the armpit of the tested mice for 0.2mL per mouse (2×10). After 24h, the inoculated mice were orally administered with SCP at the doses of 10, 20 and 40gkgdonce daily for 10d or injected intraperitoneally (i.p.) with CTX at a dose of 20mgkgdonce daily for 10d. The dose volume was 0.2mL per 10g body weight. The control group received the same volume of saline. At the end of the experiment, mice were weighed, and sacrificed by cervical dislocation. The solid tumors and spleen were collected and weighed. Then the inhibitory rate against the growth of tumor was calculated as:
The inhibitory rate (%)=(−)/×100%,
whereis the average tumor weight of the control group;is the average tumor weight of tested groups.
2.5 Splenocyte Proliferation Assay
The MTT test was used for splenocyte proliferation assay following the method of Xu., (2009). Spleen was collected from sacrificed mice under aseptic conditions and was kept in Hank’s balanced salt solution. Then it was minced using a pair of scissors and passed through a fine steel mesh to obtain a homogeneous cell suspension, and the erythrocytes were lysed with ammonium chloride (0.8%). After centrifugation (380×at 4℃ for 10min), the pelleted cells were washed three times in PBS and resuspended in RPMI-1640 complete medium. Cell num- bers were counted with a haemocytometer by trypan blue dye exclusion technique. Cell viability exceeded 95%. Splenocytes were seeded into 4 wells of a 96-well flat- bottom microtiter plate at 5×10cellmLin 100µL complete medium; thereafter Con A (final concentration 5µgmL), LPS (final concentration 10µgmL), or RPMI- 1640 medium were added giving a final volume of 200µL. The plates were incubated at 37℃ in a humid atmosphere with 5% CO. After 44h, 50µL of MTT solution (2mgmL) were added to each well and incubated for 4h. The plates were centrifuged (1400×, 5min) and the untransformed MTT was carefully removed by pipetting. To each well 150µL of a DMSO working solution (180µL DMSO with 20µL 1molLHCl) was added, and the absorbance was evaluated in an ELISA reader after 15min. The results were expressed as a stimulation index (SI.), the ratio of absorbance values between mitogen treatment and blank control cells.
2.6 Macrophage Phagocytosis Assay
Phagocytosis of macrophages was detected by a procedure with minor modifications (Gan., 2004). The male mice were randomly divided into four groups: one control group and three tested groups (10 animals in each group). The control group (group I) received orally the same volume of 0.9% normal saline once per day as did the tested groups. The tested groups (group II–IV) received SCP orally once per day for 25d, at a dosage of 10, 20 and 40gkgbody weight, respectively. At the end of treatment, the mice were injected i.p. with 1mL 20% cock red blood cells (CRBC). After 30min, the macrophages were collected, fixed with methanol and stained with 4% Giemsa. The numbers of CRBC ingested by 100 macrophages were counted in an optical microscope and expressed as phagocytosis index (PI). The percentage of cells that had phagocytosed at least one CRBC was expressed as the phagocytosis percentage (PP).
2.7 Test for DPPH Radical Scavenging Activity
The antioxidant activity of the SCP extract, on the basis of the scavenging activity of the stable 1,1-diphenyl- 2-picrylhydrazyl (DPPH) free radical, was determined by the method described in a previous report (Yu., 2011). Different concentrations of SCP (1mL) were added to 0.004% methanol solution of DPPH (4mL). After a 30min incubation period in the dark at room temperature, the absorbance of the tested solutions was measured at 517nm with a spectrophotometer. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. The effect of DPPH radical scavenging activity was expressed as IC: the amount of the sample needed to inhibit DPPH radical concentration by 50%. BHT and BHA were used as positive controls.
2.8 Test for Hydroxyl Radical Scavenging Activity
The assay was based on the Fenton reaction method with modification (Smirnoff and Cumbes, 1989). Ortho- phenanthroline (5mmolL, 0.3mL), phosphate buffer (0.75molL, pH 7.4, 0.8mL) and FeSO(7.5mmolL, 0.3mL) were mixed. Then 1mL of different concentrations of SCP was added. Finally, 0.1mL of HOwas added. The reaction mixture was then incubated at 37℃ for 60min. The effect of hydroxyl radical scavenging activity was expressed as IC: the amount of the sample needed to inhibit hydroxyl radical concentration by 50%. BHT and BHA were used as positive controls.
2.9 Statistical Analysis
Data were expressed as mean ± standard deviation (SD) of three replicated determinations. Analysis of variance (ANOVA) was carried out by using the software Microcal Origin 6.0 (Microcal Software, Inc., Northampton, USA). Mean values were compared using the Tukey test.< 0.05 was considered to be statistically significant.
3 Results
3.1 Inhibition on the Growth of Transplanted Tumor in Mice
The inhibitory effect on the growth of tumor transplanted in mice was shown in Table 1. The growth of transplanted H22 hepatoma in mice was significantly inhibited by SCP extract compared with the control (<0.05), with an inhibitory rate of 44.93% at a dose of 40gkg. A dose-dependent relationship could be observed.
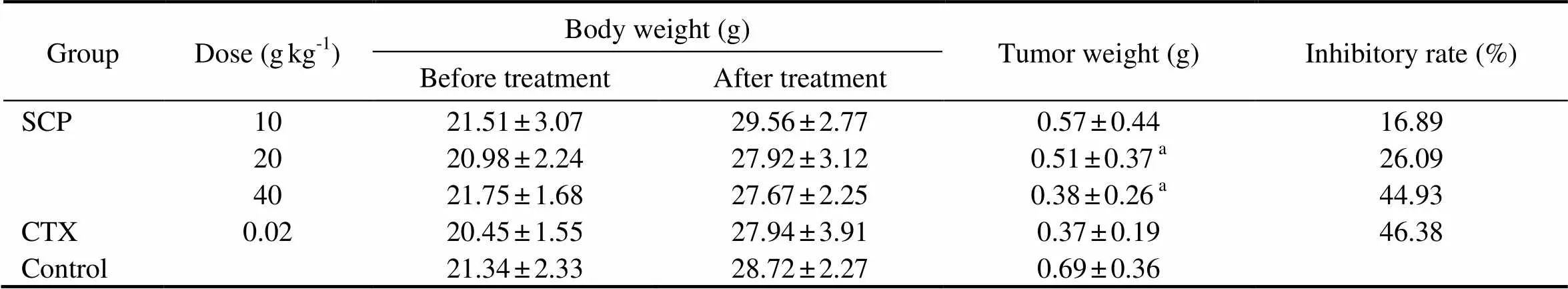
Table 1 Inhibitory effect of SCP on the growth of H22 hepatoma transplanted in mice
Notes: The values were expressed as means±S.D. (=12). Significant differences with control were designated as<0.05. CTX: cyclophosphamide (positive control).
3.2 Effect of SCP on Splenocyte Proliferation in Tumor-Bearing Mice
The effect on mitogen-stimulated splenocyte proliferation in H22-bearing mice is shown in Fig.1. The stimulation index (SI) was significantly enhanced in H22-bearing mice with SCP at the dose of 10, 20 and 40gkg, in a dose-dependent manner. The proliferation level in the presence of Con A at 40gkgwas significantly greater than that of control (<0.05). The cellular proliferation elicited by the B cell mitogen LPS was also significantly increased (<0.05).
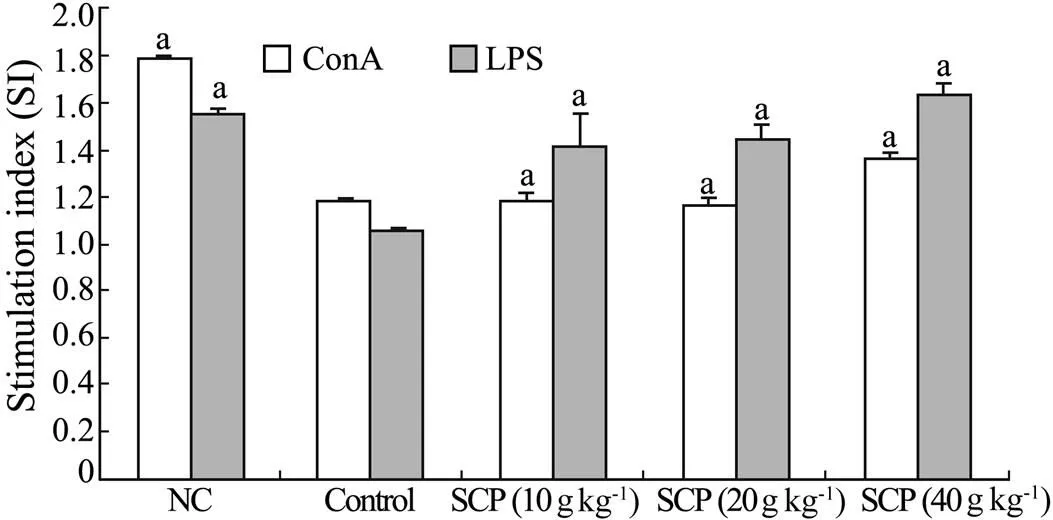
Fig.1 Effect of SCP on Con A- and LPS- stimulated splenocyte proliferation in H22-bearing mice. The values are presented as means±S.D. (=12). Significant differences from control group are designated as ‘a’,<0.05. NC stands for normal control without tumor inoculation and drug administration. Control group is with tumor inoculation but without drug administration.
3.3 Effect of SCP on the Phagocytosis of Macrophages
The phagocytosis of peritoneal macrophages isolated from tested mice is shown in Table 2. Compared with the control group, SCP significantly enhanced the phagocytic activity of macrophages at dose of 20 and 40gkg. At the end of the treatment, the PI was 0.55±0.18 and 0.55±0.17, respectively, accounting for 22.2% increase compared with the control group. Consistent with this result, PP (43.08%) and PP (37.58%) were also obtained in the high-dose and medium-dose groups, which were higher than those in the control group (<0.05).

Table 2 Effect of SCP on macrophage phagocytosis
Notes: The values were expressed as means±S.D. (=10). Significant differences with control were designated as a<0.05. PI, phagocytosis index; PP, phagocytosis percentage.
3.4 Effect of SCP on DPPH Radical Scavenging Activity
The model of scavenging the stable DPPH radical is a widely used method to evaluate the free radical scavenging activities of different materials. DPPH radical is a stable free radical with a characteristic absorption at 517 nm. As antioxidants donate protons to radicals, the absorbance decreases. The decrease in absorbance is taken as a measure of the extent of free radical scavenging. Free radical scavenging capacity of SCP measured by DPPH assay is shown in Fig.2. It was observed that an increase in DPPH free radical scavenging effect occurred in line with the increase of SCP concentration. The scavenging ability of SCP on DPPH radical was 32.02% at 0.2mgmL, and increased to 59.29% at 1.0mgmL. The ICvalue of SCP, BHT and BHA was 0.79mgmL, 0.36mgmLand 0.15mgmL, respectively. Although the activity of SCP was weaker than those of BHT and BHA, it would scavenge DPPH radical effectively in higher concentrations.
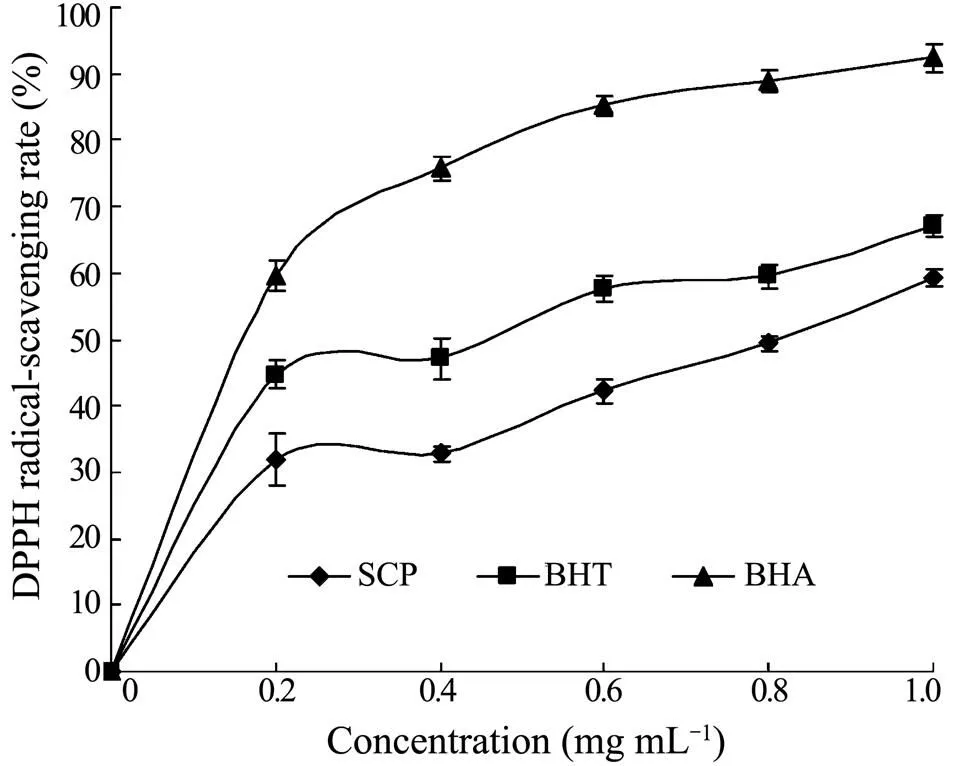
Fig.2 DPPH radical scavenging activity of SCP, BHT and BHA. Results are presented as means±standard deviation (n=3).
3.5 Effect of SCP on Hydroxyl Radical Scavenging Activity
Hydroxyl radical is the most harmful free radical, which is capable of attacking most biological substrates,carbohydrates, DNA, polyunsaturated fatty acids and proteins. The prevention of such deleterious reactions is important in terms of human health, cosmetics and pharmaceuticals (Sasaki., 1996). Therefore, the protective ability of SCP against hydroxyl radicals was assessed. It was found that SCP, BHT and BHA were capable of scavenging hydroxyl radical in a concentration-dependent manner (Fig.3). The hydroxyl radical scavenging rate of SCP was up to 50% at 0.4mgmL, and reached to 65.91% at 1.0mgmL. The ICvalue of SCP, BHT and BHA was 0.27, 0.21 and 0.24mgmL, respectively. The scavenging ability of SCP was comparable to those of BHA and BHT. Theseresults indicated that SCP had substantial effects on scavenging hydroxyl radicals.
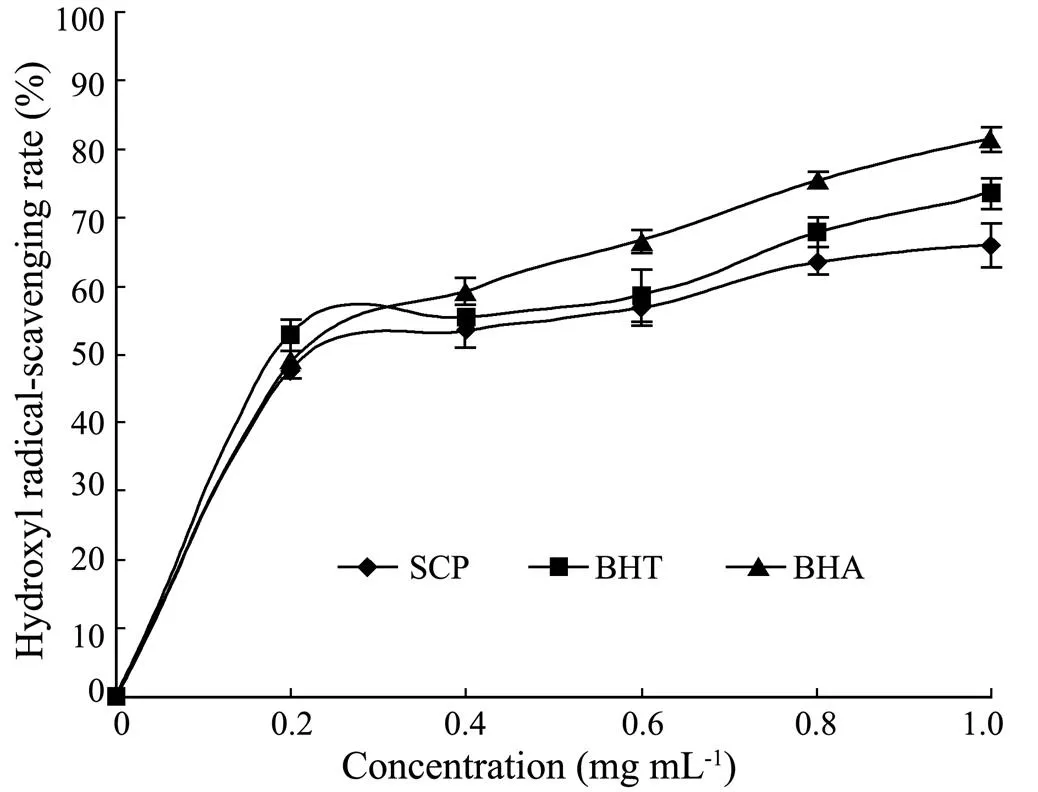
Fig.3 Hydroxyl radical scavenging activity of SCP, BHT and BHA. Results are presented as means±standard deviation (n=3).
4 Discussion
In the present study, antitumor and immunomodulatory effects of SCP were testedin hepatoma H22 tumor mice model, and its effect on free radical scavenging was evaluated. SCP showed inhibiting effect on the growth of murine transplanted tumor, and promoting effect on splenocyte proliferation and macrophage phagocytosis. Meanwhile, SCP exhibited potent scavenging activities on DPPH and hydroxyl radicals.
According to the traditional Chinese medicine theory, SCP could strengthen body resistance ability, soften hard lumps, and dispel nodes. Modern pharmacological research demonstrates that the herbs in SCP may possess immunomodulatory and antitumor effects..is classified as an herb that can soften hard lumps and dispel nodes. The polysaccharide from.can inhibit the growth of tumor and prolong the living time of the mice (Ji., 2001).has the actions of invigorating health and strengthening spleen. Thepolysaccharides can enhance both humoral and cellular immune responses via activating the TLR4 signaling pathway and inhibiting the expression of TGF-β and frequency of T cells (Du., 2011)..has the effects of tonifying liver and kidney and improving eyesight..extracts induce human glioma cell death through regulation of the Akt/mTOR/ survivin pathwayand inhibit glioma tumor growth(Jeong., 2003). Total saponin of.shows cytotoxic activities on EAC cells, S180A cells and HepA cells, with IC242, 193 and 130mgmL, respectively (Qiu., 1999). A carboxyme- thylated-sulfated derivative of (1-3)-β-D-glucan (PCS3-II) extracted fromcan significantly enhance immunological function in mice. There are significant increases in phagocyte and thymus indexes, spleen index, hemolytic activity as well as spleen antibody production and delayed type hypersensitivity (DTH)(Chen., 2010).
According to the compatibility theory and dialectical view, the strategy in treating cancer is to promote the body’s resistance ability and eliminate pathogenic factors. Successfully treating cancer not only depends on eliminating toxin and pathogenic factors, but also relies on the defense system and foundation of body. The immune system plays an important role in body defensive mechanisms against tumor. Protective immunity against tumor comprises cellular and humoral immunity. Immune response, especially cellular immunity, plays an important role in elimination of locally growing and circulating tumor cells, which results in the inhibition against growth and metastasis of tumors(Han., 1999). In this study, we found that SCP could not only promote the splenocyte proliferation, but also improve the phagocytosis of macrophages. The humoral immune response by B-cells is an antigen-specific antibody reaction. Cell-mediated immune defense is mediated specifically by T-cells. T- cells can produce lymphocyte factors consisting of macrophage mobile factor, lymphotoxin, transfer factor and interferon. These factors can promote proliferation and differentiation of immune cells, macrophage phagocytosis and the capacity of killing target cells, so they play an important role in preventing tumor (Han., 1999). It is generally known that Con-A stimulated T-cells and LPS stimulated B-cell proliferation. The increased cell number of splenocytes in mice after induction of Con A and LPS indicates that ingestion of SCP might enhance the sensitivity of T cell and B cell population on mitogen stimulation. This suggested that SCP could enhance the humoral immunity when the host suffers from foreign invaders. Macrophages have diverse functions, including phagocytosis, tumor cytotoxicity, cytokine secretion and antigen presentation, which represent a defense line against pathocytes and tumor cells, recognizing and destroying them. Macrophage phagocytosis has potential benefit because of its defensive ability. The enhancement of phagocytic function can be applied in therapy of cancer, because phagocytes act as regulator and effector cells in the immune system, and phagocytosis represents an indispensable barrier in the immunological defense system. Therefore, stimulation of macrophages might be another target for therapeutic application (Nie., 2006).
Free radicals and reactive oxygen species (ROS) are well known inducers of cellular and tissue pathogenesis leading to human diseases including cancer, inflammatory disorders as well as aging processes. They include a number of chemically reactive molecules, hydrogen peroxide (HO), superoxide (O) and hydroxyl radical (·OH). Removing ·OH is recognized as an effective way for protecting living systems(Fu., 2009). DPPH and hydroxyl radicals scavenging activities have been used to evaluate the capacity of antioxidant activity. Many antioxidant substances have been proved to be anticancer agents. For example, epigallicatechin-3-galate (EGCG) in green teahas been reported to scavenge free radicalsand found to inhibit carcinogen-inducedtumors in the skin, lung, forestomach and colon of rodents (Hannasaki., 1994; Stoner and Mukhtari, 1999).The methanol extract ofshows high DPPH radical scavenging activity and lipid peroxidation inhibitory activity. It also has protective effects against hydrogen peroxide in the Chinese hamster lung fibroblast (V79-4) cell line via enhancing cell viability, and induces apoptotic cell death in human promyelocytic leukemia (HL-60) cells (Ju., 2004). Based on the present study, we deduced that the significant scavenging activity on DPPH and hydroxyl radicals might be the mechanism of antitumor effects of SCP.
5 Conclusions
SCP is an organic unity with complicated chemical constituents exerting multiple antitumor effects. Considering that TCMPs can treat cancer by promoting the body resistance ability and eliminating pathogenic factors, the potent effects on splenocytes proliferation and macrophage phagocytosis, as well as scavenging activities on free radicals, might be effective ways in antitumor effects of SCP. This study provides evidence for clinical application of SCP. The investigation on the chemical constituents of SCP is under way.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30572314), the Basic Research Program of Science and Technology, Ministry of Science and Technology of China (No. 2007FY210500), the Program of Chinese Offshore Investigation and Assessment, State Oceanic Administration of China (Nos. 908-01-ST12, 908-02-05-04),and Science and Technology Program of Shandong Province, China (No. 03BS- 109).
Cao, Z, Y., Chen, X. Z., Liao, L. M., Peng, J., Hu, H. X., Liu, Z. Z., and Du, J., 2011. Fuzheng Yiliu Granule inhibits the growth of hepatocellular cancer by regulating immune function and inducing apoptosisand., 17: 691-697.
Chen, X., Zhang, L., and Cheung, P. C., 2010. Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated beta-(1-3)-D-glucan from., 10: 398-405.
Du, X., Chen, X., Zhao. B., Lv, Y., Zhang, H., Liu, H., Chen, Z., Chen, Y., and Zeng, X., 2011.polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting the expression of transforming growth factor β and the frequency of regulatory T cells., 63: 228-235.
Ehrke, M. J., 2003. Immunomodulation in cancer therapeutics., 3: 1105-1119.
Fang, Y. C., Gu, Q. Q, Xin, X. L., and Zhang, X. N., 2000. Studies on the pharmacodynamics of algae preparation., 19: 18-20.
Fu, X. J., Liu, H. B., Wang, P., and Guan, H. S., 2009. A study on the antioxidant activity and tissues selective inhibiton of lipid peroxidation by saponins from the roots of., 37: 1- 9.
Gan, L., Sheng, S. H., Yang, X. L., and Xu, H. B., 2004. Immunomodulation and antitumor activity by a polysaccharide- protein complex from., 4: 563-569.
Han, S. B., Lee, C. W., Jeon, Y. J., Hong, N. D., Yoo, I. D., Yang, K. H., and Kim, H. M., 1999. The inhibitory effect of polysaccharides isolated fromon tumor growth and metastasis., 41: 157-164.
Hannasaki, Y., Ogawa, S., and Fukui, S., 1994. The correlation of between active oxygen scavenging and antioxidative effects of flavonoids., 16: 845- 850.
Jeong, J. C., Kim, J. W., Kwon, C. H., Kim, T. H., and Kim, Y. K., 2003.extracts induce human glioma cell death through regulation of Akt/mTOR pathwayand reduce glioma tumor growth in U87MG xenograft mouse model., 25: 429-434.
Ji, Y. B., Gao, S. Y., and Cheng, B. C., 2001. Experiment study on antitumor function of Haimiding., 17: 11-12.
Ju, E. M., Lee, S. E., Hwang, H. J., and Kim, J. H., 2004. Antioxidant and anticancer activity of extract from., 74: 1013-1016.
Lee, S. J., Saiki, I., Hayakawa, Y., Nunome, S., Yamada, H., and Kim, S. H., 2003. Antimetastatic and immunomodulatory properties of a new herbal prescription, Bojung-bangam-tang., 3: 147-157.
Nie, X. H., Shi, B. J., Ding, Y. T., and Tao, W. Y., 2006. Antitumor and immunomodulatory effects of Weikangfu granule compound in tumor-bearing mice., 67: 138-150.
Qi, L. F., and Xu, Z. R., 2006.antitumor activity of chitosan nanoparticles., 16: 4243-4245.
Qiu, G., Zhang, M., and Yang, Y., 1999. The antitumour activity of total saponin of., 22: 351-353.
Sasaki, S., Ohta, T., and Decker, E. A., 1996. Antioxidant activity of water soluble fractions of salmon spermary tissue., 44: 1682-1686.
Smirnoff, N., and Cumbes, Q. J., 1989. Hydroxyl radical scavenging activity of compatible solutes., 28: 1057-1060.
Stoner G. D., and Mukhtar, H., 1999. Polyphenols as cancer chemopreventive agents.,22: 169-180.
Xu, H. S., Wu, Y. W., Xu, S. F., Sun, H. X., Chen, F. Y., and Yao, L., 2009. Antitumor and immunomodulatory activity of polysaccharides from the roots of., 125: 310-317.
Yu, Y. G., He, Q. T., Yuan, K., Xiao, X. L., Li, X. F., Liu, D. M., and Wu, H., 2011.antioxidant activity offlower extracts., 49: 569-576.
Zhang, J. C., Liu, L., Gong, Y. Q., Zheng, X. M., Yang, M. S., Cui, J. R., and Shen, S. G., 2009. Synthesis, characterization and antitumor activity of new type binuclear platinum (II) complexes., 44: 2322-2327.
Zhao, H. X., Li, Y. L., Wang, Y. Z., Zhang, J., Ouyang, X. M., Peng, R. X., and Yang, J., 2012. Antitumor and immunostimulatory activity of a polysaccharide–protein complex fromin tumor-bearing mice., 50: 2648- 2655.
(Edited by Qiu Yantao)
10.1007/s11802-013-2017-z
ISSN 1672-5182, 2013 12 (3): 515-520
. Tel: 0086-532-82031536 E-mail: changyun@ouc.edu.cn
(April 24, 2012; revised June 25, 2012; accepted August 20, 2012)
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2013
 Journal of Ocean University of China2013年3期
Journal of Ocean University of China2013年3期
- Journal of Ocean University of China的其它文章
- Effects of Exposure to Four Endocrine Disrupting-Chemicals on Fertilization and Embryonic Development of Barbel Chub (Squaliobarbus curriculus)
- Shallow Water Body Data Processing Based on the Seismic Oceanography
- Prediction of China’s Submerged Coastal Areas by Sea Level Rise due to Climate Change
- A Homogeneous Linear Estimation Method for System Error in Data Assimilation
- The Suspended Sediment Concentration Distribution in the Bohai Sea, Yellow Sea and East China Sea
- Role of Ekman Transport Versus Ekman Pumping in Driving Summer Upwelling in the South China Sea
