新型双子表面活性剂在H2S和CO2盐水溶液中对碳钢的缓蚀性能
赵景茂 李 俊
(北京化工大学材料科学与工程学院,北京100029)
新型双子表面活性剂在H2S和CO2盐水溶液中对碳钢的缓蚀性能
赵景茂*李 俊
(北京化工大学材料科学与工程学院,北京100029)
合成了一系列含有羟基的双子表面活性剂:1,3-双(十二烷基二甲基氯化铵)-2-丙醇(12-3OH-12),1,3-双(十四烷基二甲基氯化铵)-2-丙醇(14-3OH-14),1,3-双(十六烷基二甲基氯化铵)-2-丙醇(16-3OH-16)和1,3-双(十八烷基二甲基氯化铵)-2-丙醇(18-3OH-18).采用静态失重法、极化曲线和交流阻抗技术研究了其在H2S/ CO2腐蚀环境中对L360钢的缓蚀作用.结果表明,三种研究方法取得的结论是一致的,缓蚀效果为14-3OH-14>12-3OH-12>16-3OH-16>18-3OH-18.其中,12-3OH-12和14-3OH-14都表现出很好的缓蚀效果,在35 mg·L-1的较低浓度下缓蚀率就达95%以上.极化曲线测试结果表明n-3OH-n(n=12,14,16,18)型双子表面活性剂是一种以阳极抑制为主的混合型缓蚀剂.除n=18外,其它三种双子表面活性剂n-3OH-n(n=12, 14,16)在L360钢表面的吸附服从朗缪尔等温线,并且属于物理和化学混合吸附.提出了一个用来解释双子表面活性剂在H2S/CO2溶液中缓蚀机理的吸附模型.
缓蚀剂;双子表面活性剂;腐蚀; 硫化氢;二氧化碳
1 Introduction
The combined effects of the acid gases CO2and H2S on carbon steel corrosion have always been a major concern to the natural gas and crude oil industries,due to the potential threat to equipments(e.g.,pumps,valves,pipes).1,2Inhibitors are compounds that control,reduce or prevent reactions between a metal and its surroundings.The use of inhibitors is in general a more cost-effective method for protection against corrosion and hence,is the most widely used method.3,4Different from conventional surfactants,gemini surfactants are composed of two hydrophilic head groups and two hydrophobic chains,separated by rigid and flexible aromatic or aliphatic spacers.Due to the special chemical structure of these components,it has been demonstrated that the novel surfactants are more efficient at reducing surface tension and forming micelles than conventional surfactants and have a low critical micelle concentration (CMC).5
In recent years,gemini surfactants become the focus of study.6-19In most of the available literature,gemini surfactants have been studied as corrosion inhibitors for carbon steel in hydrochloric acid and sulphuric acid solutions.13-19The studied gemini surfactants were based on triazole,16imidazoline quaternary ammonium salt,17Schiff base18,19etc.These works showed that they were excellent corrosion inhibitors.However the high cost of reactants and long production cycles limit their use in practical.
The aim of this work was to prepare a novel series of gemini surfactants containing hydroxyl group which have good water solubility,and also have the advantages of easy production and inexpensive cost.And their corrosion inhibition performance for carbon steel in brine solution containing H2S and CO2gases were studied by gravimetric,electrochemical polarization and electrochemical impedance spectroscopy(EIS).In addition, the adsorption model of these compounds on carbon steel surface was also investigated.
2 Experimental
2.1 Material preparation
The compositions of the aggressive solution were 58.86 g· L-1NaCl,2.19 g·L-1KCl,6.33 g·L-1CaCl2,0.43 g·L-1MgCl2· 6H2O,1.24 g·L-1NaSO4,and 0.04 g·L-1NaF,which is a simulated solution of produced water in a gas field in China.Analytical grade chemicals and distilled water were used in solution preparation.
L360 steel specimen has the chemical compositions(mass fraction):C 0.16%,Si 0.45%,Mn 1.60%,P 0.025%,S 0.015%, V 0.06%,Nb 0.05%,Ti 0.04%,and Fe blance.
2.2 Synthesis of gemini surfactants
The gemini surfactants were synthesized in three steps.20Firstly,one of the intermediates was prepared by reaction between tertiary amines which have different carbon numbers of long-chain alkyl(10,12,14,16)with epichlorohydrin at a molar ratio of 1:5 for 4 h at 35-40°C.Secondly,the other intermediate was synthesized by reaction of hydrochloric and tertiary amines which also have different carbon chain lengths(10, 12,14,16)at a molar ratio of 1:1 using acetone as solvent.Finally,the two intermediates were mixed with a molar ratio 1:1 and then reacted at 40-45°C for 5 h.All the used chemicals were analytically pure except tertiary amines which were industrial products from Feixiang Chemicals,Ltd.The chemical structures of the prepared gemini surfactants n-3OH-n(n=12, 14,16,18)are shown in Table 1.Their chemical structures were confirmed by infrared spectroscopy.
2.3 Weight loss method
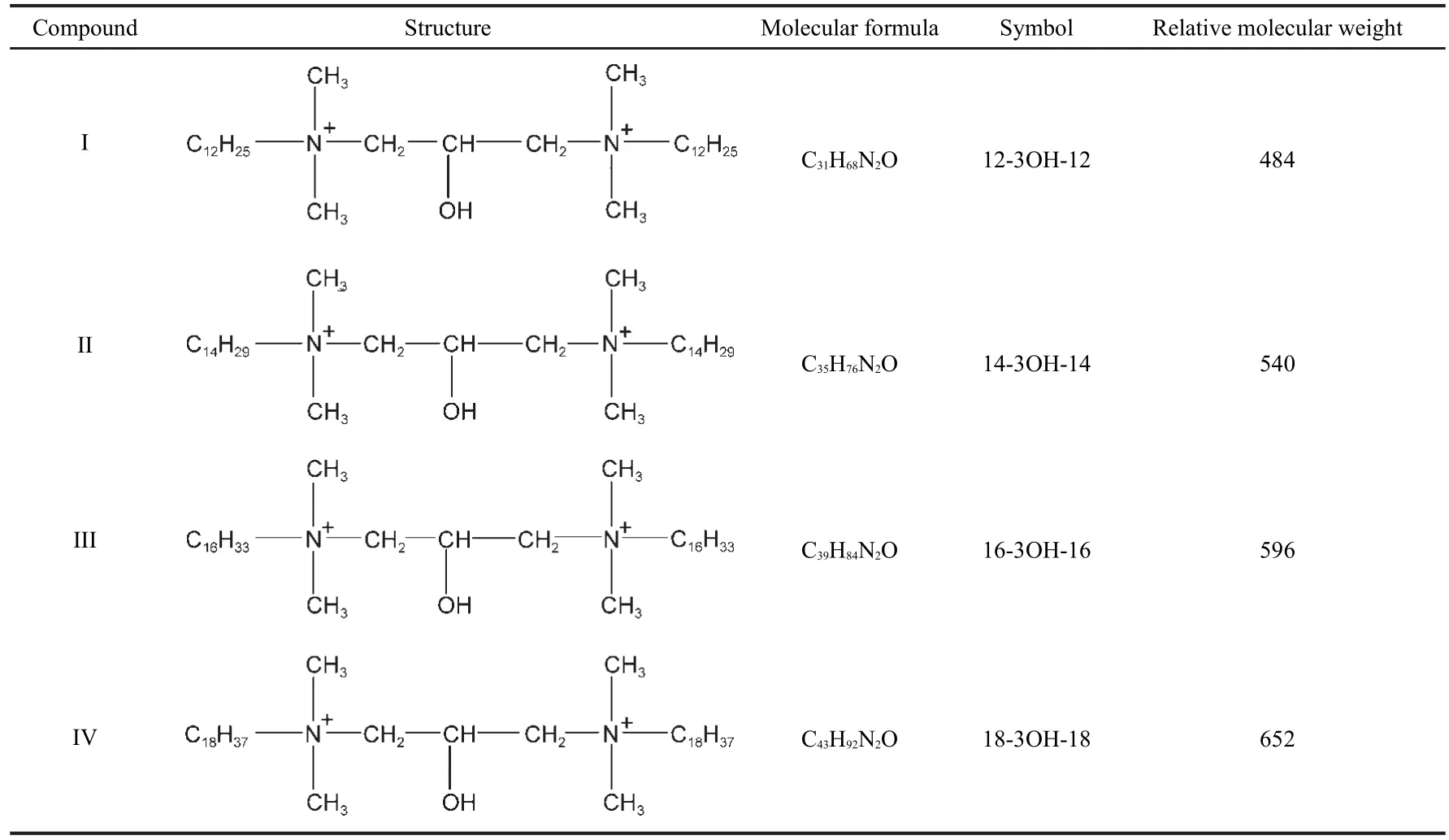
Table 1 Structures,molecular formulae,symbols,and molecular weights of the gemini surfactants
For weight loss measurements,rectangular L360 steel specimens(50 mm×10 mm×3 mm)were mechanically polished with 240,400,600,and 1000 grades of emery paper,cleaned with absolute ethanol,degreased with acetone,and finally dried in dry air.After weighing accurately,three parallel specimens were immersed in the test solution with or without addition of different concentrations of the gemini surfactants.After that,the solution was purged with high purity N2for 1 h in order to deoxygenate first.Then H2S and CO2were introduced into the glass bottle for 1 h according to a flow rate of 20 and 10 mL·min-1respectively.pH value of the solution was about 4.5 at room temperature.The test duration of gravimetric measurements was 72 h.The experiments were carried out at(60± 1)°C.
Corrosion rate(k)was calculated from the following equation:21

where Δw is the average mass loss(in mg)of three specimens, S is the total area(in cm2)of the specimen,and t is the immersion time(in h).
With the calculated corrosion rate,inhibition efficiency(ηw) of a corrosion inhibitor was defined by the following expression:

where k and kʹare,respectively,the corrosion rates of steel with and without the addition of surfactants in the solution.
2.4 Electrochemical technique
For electrochemical measurements,a conventional glass cell of 250 mL with three electrodes was used.The working electrode in the form of disc cut from L360 steel with an area of 0.35 cm2was embedded with epoxy except the working surface.The sample was polished with different grit emery paper up to 1000 grades,cleaned with absolute ethyl alcohol,acetone,and finally dried in dry air.Aplatinum electrode and a saturated calomel electrode(SCE)were used,respectively,as auxiliary and reference electrodes.Potentiodynamic polarization curves were obtained by changing the potential automatically from-200 to+200 mV versus open circuit potential(OCP) with a scan rate of 0.5 mV·s-1.
Electrochemical impedance spectroscopy(EIS)was measured in a frequency range from 64 kHz to 10 mHz with a 10 mV sine wave as the excitation signal at open circuit potential. Before each run,a time interval of about 15 min was given for the system to attain a steady state.The measurements were automatically recorded by using computer programs.EIS data were analyzed by means of ZSimpwin software.Potentiodynamic polarization and EIS were conducted using CS350(Wuhan Corrtest Instruments Company,China).
3 Results and discussion
3.1 Weight loss measurements
Corrosion rates of carbon steel in the absence and presence of different concentrations of gemini surfactants in brine solution containing H2S and CO2were determined by weight loss method at 60°C,respectively.The results are listed in Table 2.
It can be clearly noticed from Table 2 that gemini surfactants 12-3OH-12 and 14-3OH-14 can prevent carbon steel from corrosion efficiently in brine solution containing H2S and CO2. Their inhibition efficiencies(IEs)were higher than 95%at the concentration of 35 mg·L-1.The ranking of IEs for the four gemini surfactants is as follows:14-3OH-14>12-3OH-12>16-3OH-16>18-3OH-18 according to inhibition efficiency.There are many factors affecting the corrosion inhibition performance of the gemini surfactants.One of these factors is the length of alkyl chain.When the length of alkyl is relatively shorter,the compound will have a better water solubility which makes the adsorption sooner;However,if the carbon chain of alkyl is overlength,the space baffle effect will be predominant and poor water solubility will hinder the adsorption process on the metals,consequently the corrosion inhibition of surfactant is decreased.Hegazy19thought that the adsorption efficiency of gemini surfactants increased with the increase of the chain length due to the increase of electronic effect of alkyl chain. The corrosion inhibition of three Schiff-base cationic gemini surfactants(10-S-10,12-S-12,and 14-S-14)on the carbon steel corrosion in hydrochloric acid was ranked as following: 14-S-14>12-S-12>10-S-10.19The corrosion inhibition performance of gemini surfactants(10-2-10,12-2-12,14-2-14)in hydrochloric acid were also measured and similar results were obtained byAchouri et al.13
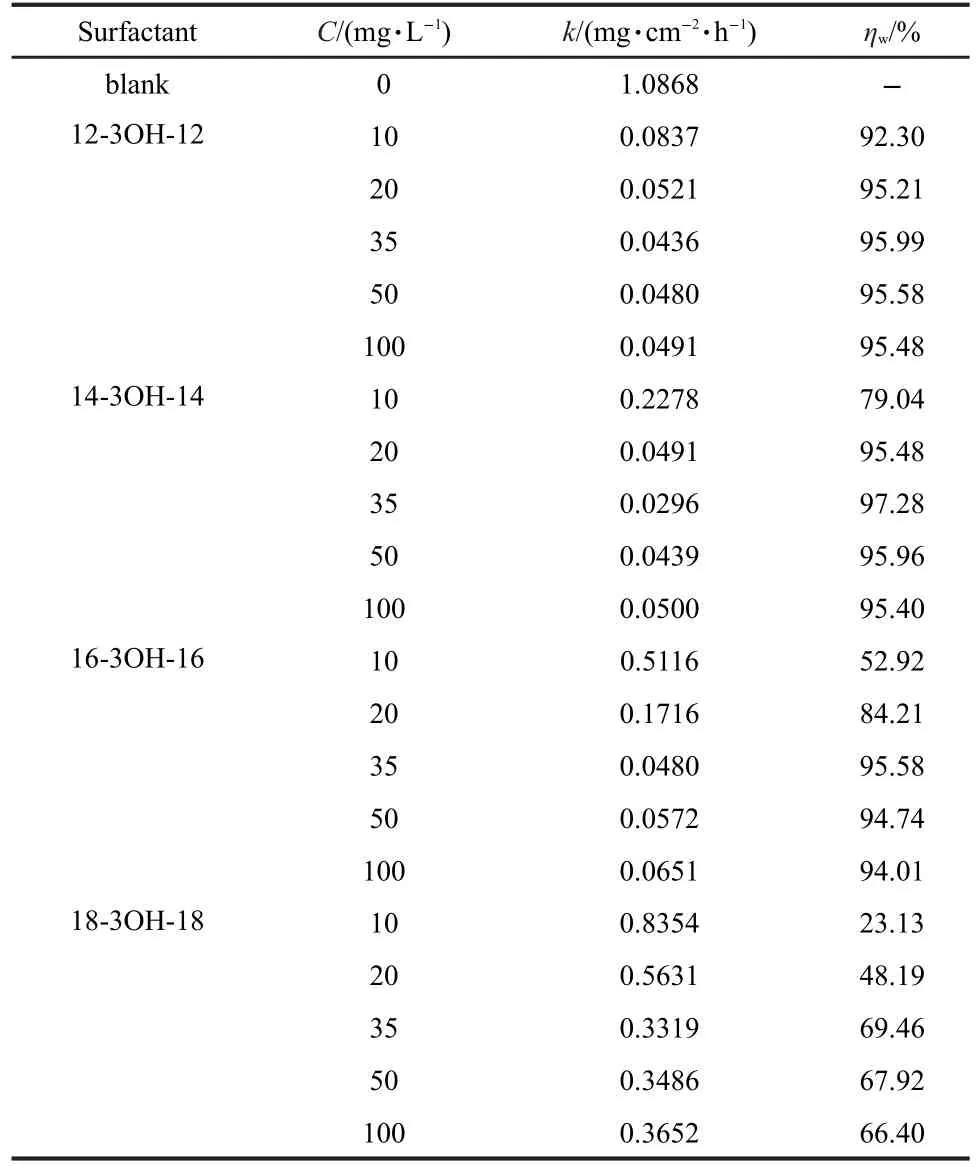
Table 2 Corrosion rates of mild steel and IE values for various concentrations of gemini surfactants at 60°C obtained from weight loss measurements
The results in Table 2 also show that inhibition efficiency increases with an increase in the additive concentration of gemini surfactants when the gemini surfactants concentration is lower than 35 mg·L-1.Above this concentration,however,increasing concentration does not affect inhibition efficiency significantly,suggesting that a saturated adsorption of surfactant molecules on carbon steel surface is achieved.
3.2 Potentiodynamic polarization measurements
Fig.1 shows the polarization curves of carbon steel in brine solution containing H2S and CO2at 60°C without or with the synthesized gemini surfactants at the optimum concentration of 35 mg·L-1.Values of associated electrochemical parameters such as,corrosion potential(Ecorr),apparent Tafel slopes(βcand βa),corrosion current density(icorr),and inhibition efficiency (ηp)for all surfactants were calculated and listed in Table 3. The inhibition efficiencies are defined as:

where i and iʹare corrosion current densities in the solution with and without surfactants,respectively,determined by Gauss-Newton fitting in weak polarization zone of the curves.22
Inspection of Table 3 and Fig.1 reveals that:(i)the values of Ecorrshift toward more positive potential and the values of icorrdecreases sharply after adding the gemini surfactants indicating the inhibiting effect of the gemini surfactants,(ii)compared with the blank(without inhibitor),the values of Tafel slopes(βcand βa)become larger,which means that these gemini surfactants are mixed type inhibitors which preferentially retard the anodic process of corrosion,and(iii)it is apparent from the results that the IEs of the gemini surfactants tested by potentiodynamic polarization method decrease in the following order:14-3OH-14>12-3OH-12>16-3OH-16>18-3OH-18,which is consistent with the result obtained by weight loss method.
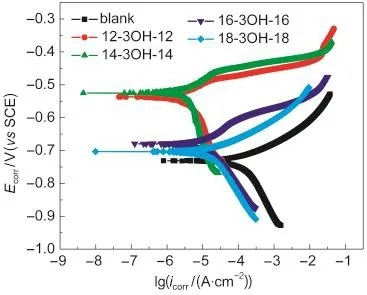
Fig.1 Polarization curves of carbon steel in brine solution containing H2S and CO2at 60°C with or without different gemini surfactants at the concentration of 35 mg·L-1

Table 3 Electrochemical parameters obtained from polarization measurements for carbon steel in brine solution containing H2S and CO2at 60°C with or without different gemini surfactants at the concentration of 35 mg·L-1
3.3 Electrochemical impedance spectroscopy measurements
Corrosion behavior of carbon steel,in the solution in the absence or presence of the gemini surfactants at the optimum concentration of 35 mg·L-1were investigated by EIS measurements.The corresponding Nyquist diagrams and Bode plots are shown in Fig.2.The Nyquist plots can be regarded as one part of a semicircle.The slightly depressed capacitive semicircle whose centre is below the x-axis is the characteristic for solid electrodes and has been attributed to roughness and other inhomogeneties of the electrode.23,24
Inspection of the Fig.2 reveal that the diameter of the semicircle increases significantly in the presence of the different gemini surfactants.It is clear that,the corrosion of carbon steel in brine solution containing H2S and CO2is obviously inhibited.IE(ηz)is estimated from the charge transfer resistance values according to the following equation:25

where Rctand Rʹctare the charge transfer resistance values with-out and with gemini surfactants,respectively.

Fig.2 Nyquist(a)and Bode(b)plots for carbon steel in brine solution saturated by H2S and CO2at 60°C with or without different gemini surfactants at the concentration of 35 mg·L-1
It can be seen from the Bode plots(Fig.2b)that there is one time constant,so the impedance data can be analyzed using the simple equivalent circuit shown in Fig.3,where Rsand Rctare the solution resistance and the charge transfer resistance,respectively.The constant phase element,CPE,is introduced in the circuit instead of a pure double layer capacitor to give a more accurate fit.26The impedance of the CPE(ZCPE)is defined as:

where Y0and n are the CPE constant and exponent,respetively, ω is the angular frequency(f)in rad·s-1(ω=2πf),and j2=-1,is an imaginary number.
The double layer capacitances(Cdl)are calculated from the following equation:27

where fmaxis the frequency at which the imaginary component of the impedance is maximal.
The impedance quantitative results are listed in Table 4,it can be seen that Rctvalue in blank solution is 104.4 Ω·cm2and increases to 2185 Ω·cm2in presence of 12-3OH-12,increases to 3766 Ω·cm2in presence of 14-3OH-14,and then decreases slightly to 1295 and 521.3 Ω·cm2in presence of 16-3OH-16, 18-3OH-18,respectively.Cdlvalues decreases in the presence of gemini surfactants,which can result from a decrease in local dielectric constant and/or an increase in the thickness of the electrical double layer,suggesting that the surfactant molecules act by adsorption at the metal/solution interface.28
This behavior reveals that the changes in Rctand Cdlvalues are caused by the gradual replacement of water molecules by adsorption of the gemini surfactant molecules on the metal surface.The gemini surfactant molecules may form a protective layer on the carbon steel surface,reducing the extent of corrosive dissolution for the carbon steel.29
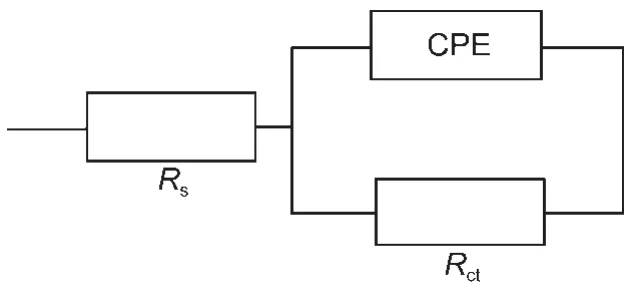
Fig.3 Equivalent circuit model for the studied gemini surfactants
It also can be interpreted that the adsorption degree increases with the alkyl chain length from 12 to 14 and decreases slightly with the alkyl chain length from 16 to 18.The inhibiting efficiency follows the order:14-3OH-14>12-3OH-12>16-3OH-16>18-3OH-18.Results obtained from EIS measurements are in good agreement with that obtained from both weight loss and potentiodynamic polarization measurements.
3.4 Adsorption isotherm
It has been considered that organic inhibitor molecules establish their corrosion inhibition performances via adsorption of the inhibitor onto the metal surface,and the inhibition efficiency is directly proportional to surfactant coverage(θ).30Adsorption isotherms are very important in understanding the mechanism of corrosion inhibition.In previous studies,6,9,15,31it was found that the most frequently used adsorption isotherms of the gemini surfactants are Langmuir adsorption model.
Assuming that the adsorption of the prepared gemini surfactants on carbon steel surface obey Langmuir adsorption isotherm given by the following equation:

where C is the concentration of gemini surfactants,θ is the surface coverage which can be calculated from weight loss experimental results,and K is the standard adsorption equilibrium constant,related to the standard free energy of adsorption (ΔGads)by the following equation:31

where the value of 55.5 is the molar concentration of water in solution in mol·L-1,R is the gas constant,T is absolute temperature,and ΔGadsis the standard free energy of adsorption process.The plots of C/θ versus C yield a straight line as shown in Fig.4.
Inspection of the Fig.4 reveal that the obtained plots of the gemini surfactants are linear with both correlation coefficient and the slope near 1 except 18-3OH-18.So the adsorption of the gemini surfactants n-3OH-n(n=12,14,16)on the carbon steel surface obeys the Langmuir adsorption isotherms.
The values of K and ΔGadswere calculated using Eqs.(7)and (8);the slope(α)and the intercept(β)were derived from the plots of C/θ versus C.The results were summarized in Table 5.
The negative value of ΔGadsindicates a spontaneous adsorption of the prepared gemini surfactants on carbon steel surface.32,33Generally,ΔGadsvalues more negative than-40 kJ· mol-1involve charge sharing or transfer from the inhibitor molecules to the metal surface to form a coordinate type of bond (chemisorption),while ΔGadsvalues around-20 kJ·mol-1or lower mean physisorption.34,35The calculated ΔGadsvalues indicate that the adsorption mechanism of the prepared gemini surfactants n-3OH-n(n=12,14,16)on carbon steel in brine solu-tion containing H2S and CO2is a mixed physical and chemical adsorption.
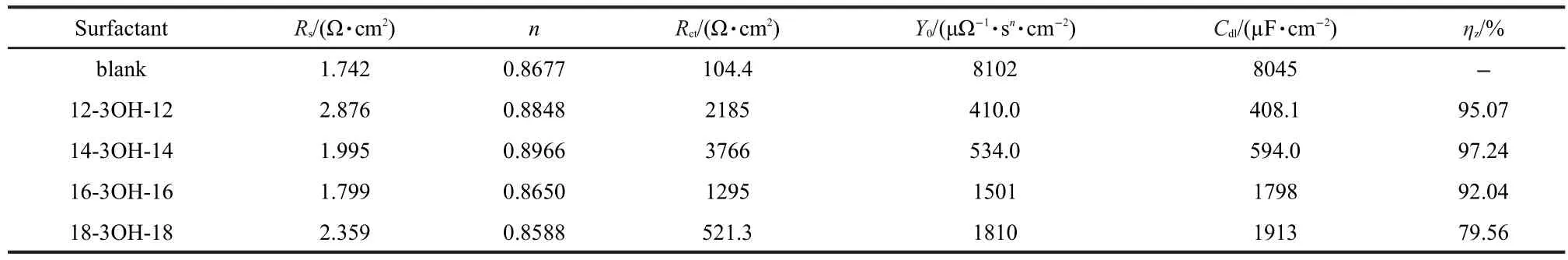
Table 4 Impedance parameters for carbon steel in brine solution containing H2S and CO2at 60°C with or without different gemini surfactants at the concentration of 35 mg·L-1
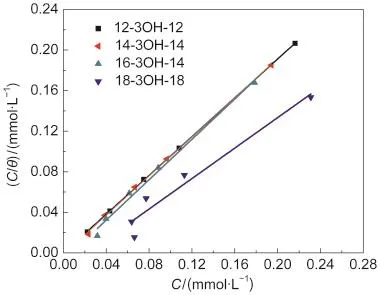
Fig.4 Langmuir adsorption plots of carbon steel in brine solution containing H2S and CO2with the gemini surfactants

Table 5 Adsorption parameters of the gemini surfactants on the carbon steel surface
3.5 Inhibition mechanism
Although the adsorption of gemini surfactants on the steel surface is complicated because the gemini surfactants contain two hydrophilic groups and two hydrophobic groups,the above results enable the authors to propose a relatively simple inhibition mechanism.
In H2S solution,HS-ions may be preferentially adsorbed on carbon steel surface thereby,making the surface negatively charged.36-39Then,the two quaternary ammonium head groups (N+)of gemini surfactant molecules could be absorbed on the layer of adsorbed anions by an electrostatic interaction18.Subsequently,chemisorption may take place.As a result,the tight adsorbed layer of gemini surfactants on the carbon steel surface can isolate the corrosive media and retard the corrosion reaction.40The gemini surfactant molecules may be lying on metal surface at lower concentrations,and at higher concentrations a perpendicular adsorption takes place as a result of an inter-hydrophobic chain interaction.The gemini surfactant molecules are also adsorbed onto the metal surface through the two ammonium head groups(N+).
4 Conclusions
(1)The inhibiting efficiency of the synthesized gemini surfactants for carbon steel in brine solution containing H2S and CO2follows the order:14-3OH-14>12-3OH-12>16-3OH-16>18-3OH-18.
(2)The obtained results show that 12-3OH-12 and 14-3OH-14 act as excellent corrosion inhibitors with inhibition efficiencies higher than 95%at 35 mg·L-1additive concentration.
(3)The polarization curves indicate that the gemini surfactants are mixed type inhibitors which preferentially restrain the anodic process of corrosion.
(4)Adsorption of gemini surfactants n-3OH-n(n=12,14, 16)on carbon steel surface obeys Langmuir isotherm and is a mixed physical and chemical adsorption.
(1) Choi,Y.S.;Nesic,S.;Ling,S.Electrochim.Acta 2011,56,1752.
(2) Yin,Z.F.;Zhao,W.Z.Electrochim.Acta 2008,53,3690.
(3) Hu,S.Q.;Hu,J.C.;Shi,X.;Zhang,J.;Guo,W.Y.Acta Phys.-Chim.Sin.2009,25(12),2524. [胡松青,胡建春,石 鑫,张 军,郭文跃.物理化学学报,2009,25(12),2524.]
(4) Trabanelli,G.Corrosion 1991,47,410.
(5)Asefi,D.;Arami,M.;Mahmoodi,N.M.Corrosion Sci.2010, 52,794.
(6) Qiu,L.G.;Xie,A.J.;Shen,Y.H.Appl.Surf.Sci.2005,246,1.
(7) Chen,Q.;Zhang,D.;Li,R.;Liu,H.;Hu,Y.Thin Solid Films 2006,496,42.
(8) Menger,F.M.;Keiper,J.S.Angew.Chem.Int.Edit.2000,39, 1906.
(9) Bagha,A.R.T.;Bahrami,H.;Movassagh,B.;Arami,M.; Arirshahi,S.H.;Menger,F.M.Colloid Surf.A 2007,307,121.
(10) Yao,S.Z.;Jiang,X.H.;Zhou,L.M.;Lv,Y.J.;Hu,X.Q.Mater. Chem.Phys.2007,104,301.
(11) Bagha,A.R.T.;Bahrami,H.;Movassagh,B.;Arami,M.; Menger,F.M.Dyes and Pigments 2007,72,331.
(12) Qiu,L.G.;Wang,Y.M.;Jiang,X.Corrosion Sci.2008,50,576.
(13) Achouri,M.E.;Infante,M.R.;Izquierdo,F.;Kertit,S.; Gouttaya,H.M.;Nciri,B.Corrosion Sci.2001,43,19.
(14) Qiu,L.G.;Xie,A.J.;Shen,Y.H.Corrosion Sci.2005,47,273.
(15) Huang,W.;Zhao,J.Colloid Surf.A 2006,278,246.
(16) Qiu,L.G.;Xie,A.J.;Shen,Y.H.Mater.Chem.Phys.2005,91, 269.
(17) Wang,X.;Yang,H.;Wang,F.Corrosion Sci.2010,52,1268.
(18) Hegazy,M.A.;Abdallah,M.;Ahmed,H.Corrosion Sci.2010, 52,2897.
(19) Hegazy,M.A.Corrosion Sci.2009,51,2610.
(20) Yang,J.Z.;Miao,Z.C.;Xu,L.Fine Chemicals 2005,22,49. [杨建洲,苗宗成,徐 亮.精细化工,2005,22,49.]
(21) Tang,L.B.;Mu,G.N.;Liu,G.H.Corrosion Sci.2003,45, 2251.
(22) Cao,C.N.Journal of Chinese Society of Corrosion and Protection 1985,5,155. [曹楚南.中国腐蚀与防护学报, 1985,5,155.]
(23) Jüttner,K.Electrochim.Acta 1990,35,1501.
(24) Paskossy,T.J.Electroanal.Chem.1994,364,111.
(25) Saliyan,V.R.;Adhikari,A.V.Corrosion Sci.2008,50,55.
(26) Benedetti,A.V.;Sumodjo,P.T.A.;Nobe,K.;Cabot,P.L.; Proud,W.G.Electrochim.Acta 1995,40,2657.
(27) Hassan,H.H.Electrochim.Acta 2007,53,1722.
(28) Quraishi,M.A.;Rawat,J.Mater.Chem.Phys.2001,70,95.
(29) Muralidharan,S.;Phani,K.L.N.;Pitchumani,S.; Ravichandran,S.;Iyer,S.V.K.J.Electrochem.Soc.1995,142, 1478.
(30) Christov,M.;Popova,A.Corrosion Sci.2004,46,1613.
(31) Qiu,L.G.;Wu,Y.Progress in Corrosion Research;Bettini,E. L.Ed.;Nova Science Publishers,Inc.:New York,2007;p 159.
(32) Elachouri,M.;Hajji,M.S.;Salem,M.;Kertit,S.;Aride,J.; Coudert,R.;Essassi,E.Corrosion 1996,52,103.
(33) Savitri,B.V.;Mayanna,S.Indian J.Chem.Technol.1996,3, 103.
(34) Hu,S.Q.;Hu,J.C.;Fan,C.C.;Mi,S.Q.;Zhang,J.;Guo,W.Y. Acta Phys.-Chim.Sin.2010,26,2163.[胡松青,胡建春,范成成,米思奇,张 军,郭文跃.物理化学学报,2010,26,2163.]
(35) Okafor,P.C.;Zheng,Y.G.Corrosion Sci.2009,51,850.
(36) Ma,H.Y.;Cheng,X.L.;Chen,S.H.;Wang,C.;Zhang,J.P.; Yang,H.Q.J.Electroanal.Chem.1998,451,11.
(37) Cheng,X.L.;Ma,H.Y.;Zhang,J.P.;Chen,X.;Chen,S.H.; Yang,H.Q.Corrosion 1998,54,369.
(38) Ma,H.Y.;Cheng,X.L.;Chen,S.H.;Li,G.Q;Chen,X.;Lei,S. B.;Yang,H.Q.Corrosion 1998,54,634.
(39) Gao,Y.M.;Chen,J.J.;Lei,L.C.;Yang,H.Y.;Cao,D.Z.;Wu, W.T.Journal of Chinese Society of Corrosion and Protection 2000,20,142.[高延敏,陈家坚,雷良才,杨怀玉,曹殿珍,吴维弢.中国腐蚀与防护学报,2000,20,142.]
(40) Zhang,J.;Yu,W.Z.;Yan,Y.G.;Yu,L.J.;Ren,Z.J.Acta Phys.-Chim.Sin.2010,26,1386.[张 军,于维钊,燕友果,于立军,任振甲.物理化学学报,2010,26,1386.]
October 17,2011;Revised:December 26,2011;Published on Web:December 29,2011.
Corrosion Inhibition Performance of Carbon Steel in Brine Solution Containing H2S and CO2by Novel Gemini Surfactants
ZHAO Jing-Mao*LI Jun
(College of Materials Science and Engineering,Beijing University of Chemical Technology,Beijing 100029,P.R.China)
A series of novel gemini surfactants containing hydroxyl group have been synthesized including 1,3-bis(dodecyl dimethyl ammonium chloride)-2-propanol,1,3-bis(myristyl dimethyl ammonium chloride)-2-propanol,1,3-bis(hexadecyl dimethyl ammonium chloride)-2-propanol,and 1,3-bis(octadecyl dimethyl ammonium chloride)-2-propanol,designated as n-3OH-n(n=12,14,16,18,respectively).The corrosion inhibition for carbon steel in brine solution containing H2S and CO2was investigated using weight loss method,potentiodynamic polarization,and electrochemical impedance spectroscopy(EIS).The results showed that the inhibition efficiencies(IEs)obtained from all of the methods employed demonstrated a clear trend,with the IEs of the gemini surfactants ranked as 14-3OH-14>12-3OH-12>16-3OH-16>18-3OH-18.Among them,14-3OH-14 and 12-3OH-12 acted as excellent corrosion inhibitors with IE values greater than 95%at an additive concentration of 35 mg·L-1.Potentiodynamic polarization curves clearly revealed that the gemini surfactants are mixed-type inhibitors which preferentially inhibit the anodic corrosion process.Adsorption of the synthesized gemini surfactants n-3OH-n(n=12,14,16)onto a carbon steel surface obeys the Langmuir adsorption isotherm and they exhibit a mixed physical and chemical adsorption.An adsorption model was proposed to elucidate the inhibition mechanism of gemini surfactants.
Corrosion inhibitor;Gemini surfactant;Corrosion;Hydrogen sulfide;Carbon dioxide
10.3866/PKU.WHXB201112293
O647
∗Corresponding author.Email:jingmaozhao@126.com;Tel:+86-10-64442286;Fax:+86-10-64434908. The project was supported by the National Natural Science Foundation of China(51171013).
国家自然科学基金(51171013)资助项目

