Spectral Domain Optical Coherence Tomography of Vogt-Koyanagi-Harada Disease:Novel Findings and New Insights into the Pathogenesis
Chan Zhao,Mei-fen Zhang*,Fang-tian Dong,Xu-qian Wang,Xin Wen,Rong-ping Dai,Wei-hong Yu,Zhi-qiao Zhang,Zhi-kun Yang,and Fei Gao
Department of Ophthalmology,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100730,China
VOGT-KOVANAGI-HARADA disease (VKH) is a chronic,bilateral,granulomatous panuveitis with extraocular manifestations in the central nervous system,auditory system,and integumentary system.Most VKH patients follow progressive steps well recognized as prodromal,acute uveitic,convalescent,and chronic recurrent stages,each with characteristic clinical features.1-4With the aid of optical coherence tomography (OCT),a widely used imaging modality in retinal diseases,growing literature is enriching our knowledge on this entity.5-8One good example is the recognition of intraretinal cysts,which were interpreted differently in reports using earlier OCT systems.5,9-11With the most advanced OCT techniques,however,they were later confirmed to be a characteristic pathophysiological finding in acute VKH.7In the present study,we compare the findings of spectral domain OCT (SD OCT) and fluorescein angiography (FA) in acute VKH,aiming to provide new findings which can further expand our understanding of this disease.
PATIENTS AND METHODS
This observational case series included 18 consecutive VKH patients who were in the acute uveitic stage at presentation and were diagnosed between December 2007 and April 2009 in Department of Ophthalmology,Peking Union Medical College Hospital with satisfactory SD OCT results.All the cases were diagnosed according to the revised international criteria by American Uveitis Society.12Accurate staging (acute uveitic stage) was based on clinical manifestations (no signs of vitiligo,alopecia,or poliosis),ophthalmoscopic and FA findings (without diffuse and focal retinal pigment epithelium depigmentation),and OCT findings [retinal detachment (RD) in the macula in at least one eye].3D OCT 1000 (Topcon Inc.,Tokyo,Japan) was used in this study.For each patient,3D data sets centered on the macular fovea were obtained at the first visit;additional 3D,circular,or radial scans centered on areas of interest were performed in some patients.After the completion of each scan,a color fundus photograph was taken with an integrated camera.All the patients had been followed up for at least 6 month when data were collected.Reevaluations of SD OCT were performed in some patients during follow-up.
All the investigations adhered to the tenets of the Declaration of Helsinki.The study was approved by the Institutional Review Board (IRB) of Peking Union Medical College Hospital,Chinese Academy of Medical Sciences &Peking Union Medical College.Informed consent for all examinations and treatments was obtained from all the patients in accordance with the IRB’s requirements.
RESULTS
General information
The 18 patients included 11 women and 7 men,with mean age of 35.2±12.7 years (range,16-59 years).The mean duration at the first SD OCT was 34±28 days (range,5 days to 3 months).10 patients received reevaluation(s) of SD OCT during follow-up (Table 1).
Early retinal/choroidal pathologies of VKH revealed by the OCT system included:exudative RD and intraretinal cysts,choroidal fluctuations/folds,subretinal exudates and subretinal fibrinoid deposit.Demographic features,visual acuity and duration at the first visit,FA pattern,and various OCT findings of all the patients are presented in Table 1.
Exudative RD and intraretinal cysts
In this study,5 patients (28%) showed multilobular dye pooling at the first visit.Among these patients,3 had another special FA pattern that we named donut-shaped dye pooling,in which a small round hypofluorescent area was surrounded by a hyperfluorescent ring (Fig.1).In patient 8,SD OCT revealed a single dome-shaped elevation of the retina which was divided into several compartments by septa;on FA,the subretinal compartments corresponded well to lobes of hyperfluorescent dye pooling (Fig.1A).Around the central subretinal bulla,a normofluorescent ring was detected (the circular zone between f and h,d’ and f’,b’and c’,d and e in Fig.1A);on OCT,this ring was separated from the central subretinal bulla by a very thin,yet fluorescein-tight membrane (indicated by e,f,c’,and d’ in Fig.1A).The normofluorescent ring itself was further divided into two compartments (indicated by g and e’ in Fig.1A).In the outer compartment,the fluid accumulated beneath the outer plexiform layer;however,in the inner compartment,the fluid seemed to accumulate within the photoreceptor layer (Fig.1A).In patient 14,multiple bullae could be found on fundus photography and OCT (Fig.1B).Hypofluorescent nuclei of donut-shaped dye poolings corresponded well to intraretinal cysts (between d and e,c’ and d’ in Fig.1B) just beneath the outer plexiform layer on OCT.However,another intraretinal cyst (between a’’ and b’’ in Fig.1B) which was located within the photoreceptor layer was relatively hyperfluorescent on FA.Surrounding the central subretinal bulla,a hypofluorescent ring was detected on FA.On OCT,it appeared as a diffuse fluid accumulation within the photoreceptor layer,without formation of cysts (Fig.1B,indicatedby red arrows).In patient 6,intraretinal accumulation of fluid spanning the external limiting membrane could be found in areas without prominent RD (Fig.2).
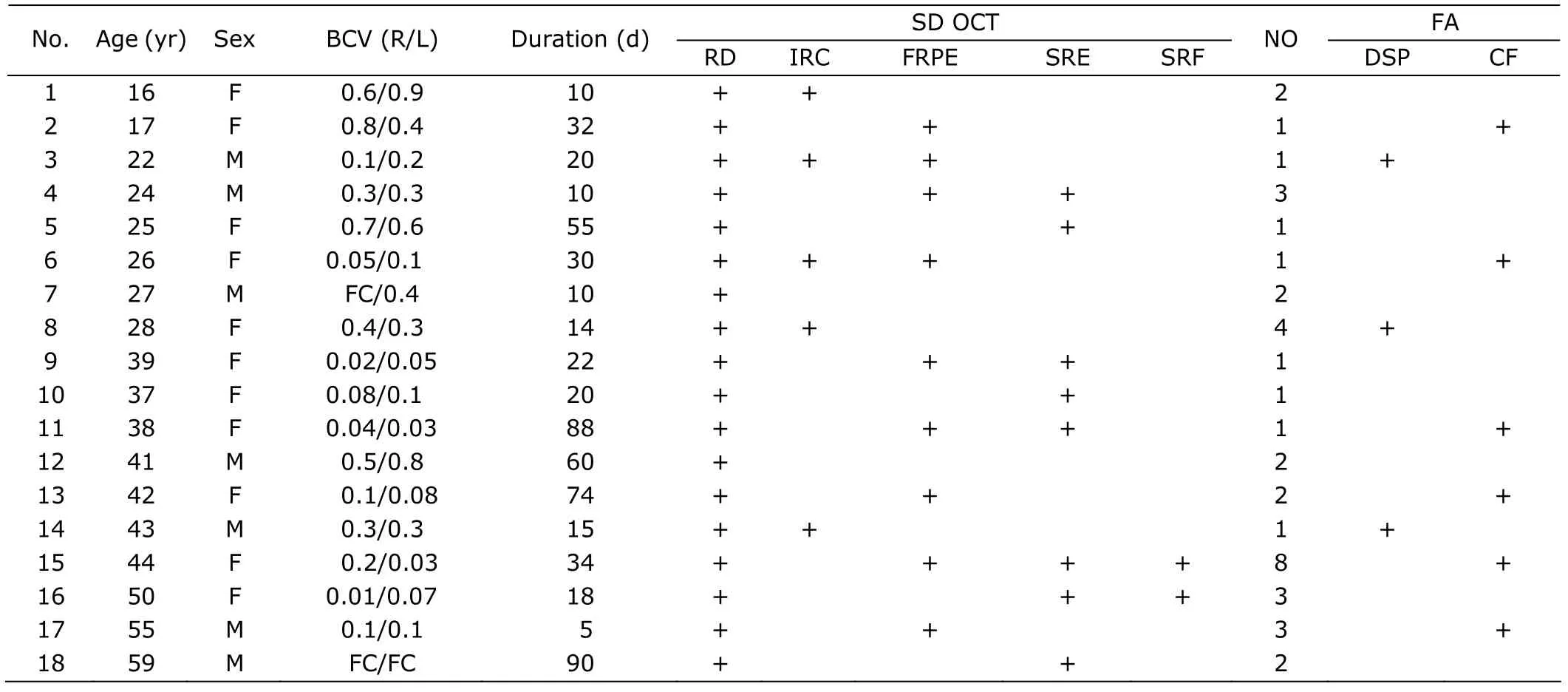
Table 1.Demographic features,duration,visual acuity,spectral domain optical coherence tomography (SD OCT)findings,and fluorescein angiography (FA) pattern of the 18 included patients
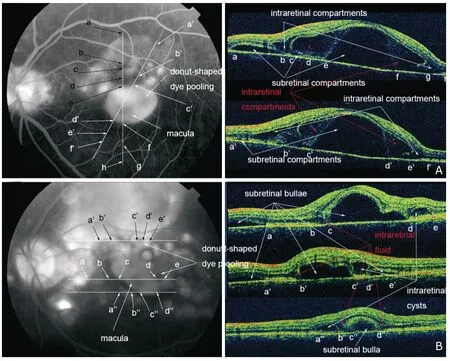
Figure 1.SD OCT and FA findings in patient 8 (A) and patient 14 (B).Late phase FA images show typical multilobular dye pooling and donut-shaped dye pooling in both patients.Representative scans of SD OCT are presented,with their scan lines indicated by white lines on corresponding FA.Each scan line is marked by a set of consecutive letters.Subretinal and intraretinal pathologies revealed by SD OCT are indicated.
Subretinal exudates and subretinal fibrinoid deposit/fibrosis
Subretinal exudates,which appeared as hyporeflective to normal-reflective subneuroepithelial substances on SD OCT (Fig.3G),were found in 8 patients (44%) (Table 1),among which 2 patients (4 eyes) not satisfactorily treated developed subretinal fibrinoid deposit early during follow-up.Specifically,in patient 15,corticosteroid therapy was tapered too rapidly despite excellent initial response.In patient 16,accurate diagnosis was delayed in local hospital.On OCT,subretinal fibrinoid deposit appeared to develop directly from the subretinal exudates in both patients and evolved into typical subretinal fibrosis (Fig.3).Gradual transfiguration/migration and progressive proliferation/pigmentation of the subretinal fibrinoid deposit/fibrosis were observed in both eyes of patient 15 (changes in the right eye are shown in Fig.3).
Longitudinal observation of retinal tomographic features
In this study,10 patients received SD OCT more than once(median,2.5 times;range,2-9 times) during follow-up,including the 2 patients who developed subretinal fibrosis(Table 1).Despite complete absorption of subretinal fluid,the ultramicrostructure of the outer retinal layers did not recover completely.The two hyperreflective lines,representing cone/rod outer segment tips (COSTs/ROSTs) and the inner segment/outer segment junction (IS/OS),were disrupted to various degrees in all the 10 patients:from almost completely missing to almost normal in reattached areas.In some patients,thinning of the neuroepithelium was obvious(Fig.4).Delayed and incomplete recovery of COSTs/ROSTs and IS/OS were observed in one (patient 17) of the few patients who received OCT more than twice (Fig.4).

Figure 2.Fundus photography (A) and SD OCT (B) of patient 6.Within the intraretinal cyst,the external limiting membrane is well preserved (B).Choroidal fluctuations/folds are also indicated.
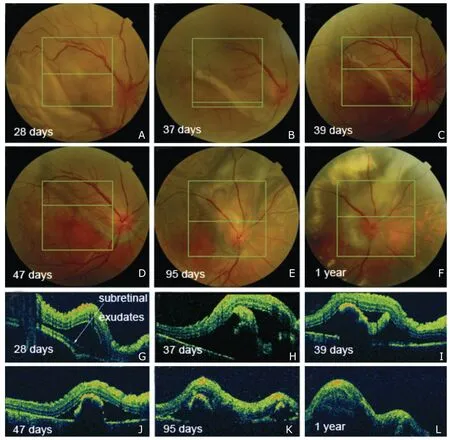
Figure 3.Consecutive fundus photography and SD OCT in the right eye of patient 15.Subretinal exudates is observed on fundus photography (A) and OCT on day 28 (G).Gradual transfiguration/migration and progressive proliferation/pigmentation of the subretinal fibrinoid deposit/fibrosis are observed in the follow-up visits.The transition from subretinal fibrinoid deposit into subretinal fibrosis demonstrates no clear-cut point.

Figure 4.Consecutive fundus photography and SD OCT scans of patient 17 in chronic phase.Although the thickness of the foveal neuroepithelium remains unchanged,delayed and incomplete recovery of cone/rod outer segment tips (COSTs/ROSTs) and the inner segment/outer segment junction (IS/OS) are observed.While the COSTs/ROSTs and IS/OS are almost completely missing at the earlier visit (C),both structures could be identified at the later visit (D).
DISCUSSION
Intraretinal cysts were found to be located within the photoreceptor layer (at the level of IS/OS) in a previous report.7In this study,we found that intraretinal cysts could form in various layers of the outer retina.They were located between the outer plexiform layer and the outer nuclear layer,spanned the external limiting membrane,and within the photoreceptor layer.We propose that both intraretinal and subretinal cysts form during the inflammatory process.As the disease progresses,enlargement and coalescing of subretinal cysts and intraretinal cysts may occur.On the edge of a large subretinal bulla,fluid may diffusely accumulate within the photoreceptor layer,forming a hypofluorescent or normofluorescent ring,or progressing to be more cystic-like.
Because intraretinal cysts are angiographically distinct from the subretinal bulla,we infer that fluids within these two compartments are independent in origin.Intraretinal vessels seem to be the source of the fluid in intraretinal cysts.The formation of intraretinal cysts may result from cytokines-mediated spillover of inflammation from the choroid,which is believed to be the major target of inflammation in VKH disease.5,13Polarized secretion of cytokines by the retinal pigment epithelium (RPE) cells may also contribute to the extended inflammation in the subretinal space and the outer retina.14,15
Subretinal fibrosis has been well recognized as a sequela of chronic ocular inflammation in VKH patients.
Subretinal fibrosis has been well recognized as a sequela of chronic ocular inflammation in VKH patients.4,16-21In one report,time from diagnosis of VKH to development of subretinal fibrosis ranged from 0 (present at the time of diagnosis) to 27 years,with a median of 10 months.18All the patients in that study were in the chronic recurrent phase of VKH disease and had experienced multiple recurrences of either anterior or posterior uveitis.18However,both patient 15 and patient 16 in the present study developed subretinal fibrinoid deposit within the second month of duration when exudative RD had not reattached completely and finally evolved into typical subretinal fibrosis.The transition from subretinal fibrinoid deposit into subretinal fibrosis was gradual,with no clear-cut point.Subretinal fibrinoid deposit/fibrosis seemed to develop directly from subretinal exudates.The continuing transfiguration/mi-gration associated with progressive proliferation/pig-mentation of the subretinal fibrinoid deposit/fibrosis were well documented (Fig.3).We believe this is a vivid demonstration of type 2 epithelial-mesenchymal transition previously reported,which is engaged in the context of inflammation.22Like in other fibrotic disor-In one report,time from diagnosis of VKH to development of subretinal fibrosis ranged from 0 (present at the time of diagnosis) to 27 years,with a median of 10 months.18All the patients in that study were in the chronic recurrent phase of VKH disease and had experienced multiple recurrences of either anterior or posterior uveitis.18However,both patient 15 and patient 16 in the present study developed subretinal fibrinoid deposit within the second month of duration when exudative RD had not reattached completely and finally evolved into typical subretinal fibrosis.The transition from subretinal fibrinoid deposit into subretinal fibrosis was gradual,with no clear-cut point.Subretinal fibrinoid deposit/fibrosis seemed to develop directly from subretinal exudates.The continuing transfiguration/mi-gration associated with progressive proliferation/pig-mentation of the subretinal fibrinoid deposit/fibrosis were well documented (Fig.3).We believe this is a vivid demonstration of type 2 epithelial-mesenchymal transition previously reported,which is engaged in the context of inflammation.22Like in other fibrotic disorders in the eye,inflammatory/fibrogenic growth factors and cytokines produced in the inflamed tissues may play a pivotal role23in the pathogenesis of subretinal fibrosis in VKH patients.Interactions of resident cells (RPE cells,Müller cells,choroidal fibrocytes,etc.) with infiltrated inflammatory cells and their products may also be involved.17
From another point of view,profound subretinal exudates secondary to uncontrolled acute inflammation,which is more easily detected with OCT than with fundus photography,might be a precursor of subretinal fibrinoid/ fibrosis,thus having prognostic and therapeutic values.
In this study,delayed and incomplete recovery of COSTs/ROSTs and IS/OS were observed in one patient during follow-up.This may be the anatomical basis for similarly delayed and incomplete recovery of macular function observed on multifocal electroretinography in VKH patients.24However,further studies are needed to prove that speculation.
1.Moorthy RS,Inomata H,Rao NA.Vogt-Koyanagi-Harada syndrome.Surv Ophthalmol 1995;39:265-92.
2.Rajendram R,Evans M,Rao NA.Vogt-Koyanagi-Harada disease.Int Ophthalmol Clin 2005;45:115-34.
3.Andreoli CM,Foster CS.Vogt-Koyanagi-Harada disease.Int Ophthalmol Clin 2006;46:111-22.
4.Yang P,Ren Y,Li B,et al.Clinical characteristics of Vogt-Koyanagi-Harada syndrome in Chinese patients.Ophthalmology 2007;114:606-14.
5.Yamaguchi Y,Otani T,Kishi S.Tomographic features of serous retinal detachment with multilobular dye pooling in acute Vogt-Koyanagi-Harada disease.Am J Ophthalmol 2007;144:260-5.
6.Gupta V,Gupta A,Gupta P,et al.Spectral-domain cirrus optical coherence tomography of choroidal striations seen in the acute stage of Vogt-Koyanagi-Harada disease.Am J Ophthalmol 2009;147:148-153.e2.
7.Ishihara K,Hangai M,Kita M,et al.Acute Vogt-Koyanagi-Harada disease in enhanced spectral-domain optical coherence tomography.Ophthalmology 2009;116:1799-807.
8.Zhao C,Zhang M,Wen X,et al.Choroidal folds in acute Vogt-Koyanagi-Harada disease.Ocul Immunol Inflamm 2009;17:282-8.
9.Maruyama Y,Kishi S.Tomographic features of serous retinal detachment in Vogt-Koyanagi-Harada syndrome.Ophthalmic Surg Lasers Imaging 2004;35:239-42.
10.de Smet MD,Rao NA.Retinal cystoid spaces in acute Vogt-Koyanagi-Harada syndrome.Am J Ophthalmol 2005;140:962.
11.Tsujikawa A,Yamashiro K,Yamamoto K,et al.Retinal cystoid spaces in acute Vogt-Koyanagi-Harada syndrome.Am J Ophthalmol 2005;139:670-7.
12.Read RW,Holland GN,Rao NA,et al.Revised diagnostic criteria for Vogt-Koyanagi-Harada disease:report of an international committee on nomenclature.Am J Ophthalmol 2001;131:647-52.
13.Bouchenaki N,Herbort CP.The contribution of indocyanine green angiography to the appraisal and management of Vogt-Koyanagi-Harada disease.Ophthalmology 2001;108:54-64.
14.Holtkamp GM,Kijlstra A,Peek R,et al.Retinal pigment epithelium-immune system interactions:cytokine production and cytokine-induced changes.Prog Retin Eye Res 2001;20:29-48.
15.Shi G,Maminishkis A,Banzon T,et al.Control of chemokine gradients by the retinal pigment epithelium.Invest Ophthalmol Vis Sci 2008;49:4620-30.
16.Damico FM,Kiss S,Young LH.Vogt-Koyanagi-Harada disease.Semin Ophthalmol 2005;20:183-90.
17.Lertsumitkul S,Whitcup SM,Nussenblatt RB,et al.Subretinal fibrosis and choroidal neovascularization in Vogt-Koyanagi-Harada syndrome.Graefes Arch Clin Exp Ophthalmol 1999;237:1039-45.
18.Kuo IC,Rechdouni A,Rao NA,et al.Subretinal fibrosis in patients with Vogt-Koyanagi-Harada disease.Ophthalmology 2000;107:1721-8.
19.Read RW,Rechodouni A,Butani N,et al.Complications and prognostic factors in Vogt-Koyanagi-Harada disease.Am J Ophthalmol 2001;131:599-606.
20.Bykhovskaya I,Thorne JE,Kempen JH,et al.Vogt-Koyanagi-Harada disease:clinical outcomes.Am J Ophthalmol 2005;140:674-8.
21.Tugal-Tutkun I,Ozyazgan Y,Akova YA,et al.The spectrum of Vogt-Koyanagi-Harada disease in Turkey:VKH in Turkey.Int Ophthalmol 2007;27:117-23.
22.Kalluri R,Weinberg RA.The basics of epithelial-mesenchymal transition.J Clin Invest 2009;119:1420-8.
23.Saika S,Yamanaka O,Sumioka T,et al.Fibrotic disorders in the eye:targets of gene therapy.Prog Retin Eye Res 2008;27:177-96.
24.Yang P,Fang W,Wang L,et al.Study of macular function by multifocal electroretinography in patients with Vogt-Koyanagi-Harada syndrome.Am J Ophthalmol 2008;146:767-71.
 Chinese Medical Sciences Journal2012年1期
Chinese Medical Sciences Journal2012年1期
- Chinese Medical Sciences Journal的其它文章
- Screening of Substrates of Protein Arginine Methyltransferase 1 in Glioma△
- Spatio-temporal Expression Study of Phosphorylated 70-kDa Ribosomal S6 Kinase (p70S6k) in Mesial Temporal Lobe Epilepsy△
- The Inhibitory Effects of Arresten Protein on Tumor Formation△
- A Combined Clinicopathologic Analysis of 658 Urothelial Carcinoma Cases of Urinary Bladder
- Primary Sjögren's Syndrome Accompanied by Intestinal Obstruction:a Case Report and Literature Review
- Electrocorticography with Direct Cortical Stimulation for a Left Temporal Glioma with Intractable Epilepsy
