The Inhibitory Effects of Arresten Protein on Tumor Formation△
Yi Lv and Jin-ping Zheng*
Department of Health Toxicology,School of Public Health in Shanxi Medical University,Taiyuan 030001,China
ANGIOGENESIS is a complex process that involves extracellular matrix (ECM) remodeling,endothelial cells (ECs) migration,proliferation,and functional maturation of new ECs into mature blood vessels.1,2The development of the ECM,a complex structure comprising of different proteins,is a crucial event in the evolution of multicellular organisms.2,3During angiogenesis,new capillaries sprout from preexisting blood vessels by an invasive process called“neovascularization”that occurs physiologically during embryonic development,reproduction,and wound healing,and pathologically during tumor growth,metastasis,arthritis onset,and development of age-related macular degeneration.4,5It has been proposed that tumor growth and metastasis are mediated by angiogenesis.6Prevention of angiogenesis has been found to inhibit the growth of tumors.7-10Solid tumors can not grow beyond 2-3 mm in diameter without recruitment of their own blood supply and nutrients.5,6Tumor growth thus depends on the balance between circulating endogenous pro-angiogenic factors and angiogenesis inhibitors.7,8Some non-collagenous C-terminal domains from various collagens have been identified to be anti-angiogenic and anti-tumorigenic.11,12ECM-derived endogenous anti-angiogenic peptides control tumor cell proliferation,apoptosis,and tumor angiogenesis,which are critical for the growth of primary tumors and metastases.Given their crucial role in anti-angiogenesis,those peptides have been the focus of much research work with the aim of finding potential novel therapies for tumors.12,13
Arresten,or human non-collagenous domain 1 of the α1 chain of type IV collagen,is derived from the carboxy terminal of type IV collagen.It is an effective endogenous angiogenesis inhibitor,which can specifically prevent the growth of solid tumor.12The administration of arresten can suppress the growth of primary and metastatic lesions in multiple murine tumor models and appears not to induce acquired drug resistance.Arresten protein has been applied as a primary agent in anti-angiogenic treatment,and the results in tumor treatment have been optimistic.12-16In one of our previous studies,a novel arresten gene was identified and deposited in the GenBank database (accession number:DQ414683).After expression from bacterial cells,the arresten protein (accession number:ABE73157)exhibited significant anti-angiogenic effect.15In this study,the arresten protein was purified and further examined for its inhibitory effects on tumor formation.
MATERIALS AND METHODS
Arresten protein expressed in prokaryotic system
The sequence encoding arresten was amplified by reverse transcription-polymerase chain reaction using total RNA isolated from human placental tissue that was provided by a healthy mother (age,27 years;weight,75 kg;first baby birth;Caesarean section in the Second Affiliated Hospital of Shanxi Medical University).The study was approved by Hospital Ethics Committee.The forward primer (5’-GCCGAATTCATGTCTGTTGATCACGGC-3’) and reverse primer(5’-GCGTCGACCTATTAGTGGTGGTGGTGGTGGTGTGTTCTTCTC-3’) were designed to amplify the cDNA encoding the arresten protein.The amplified product was digested withEcoRI andSalI,ligated into a pMD19-T transfer vector(TaKaRa Biotechnology Co.,Ltd.,Dalian,China) that was predigested with the same restriction enzymes,and subsequently ligated into a pBV221 expression vector,which was preserved in our laboratory,forming pBV221-arresten.The recombinant clones were sequenced using a high-throughput DNA sequencing facility.The sequences were further confirmed by a BLAST search.Clones expressing the 6×His tagged version of arresten were transformed inE.colicompetent JM109 cells.The expressed arresten protein was purified using a Ni2+-NTA Column (QIAGEN GmbH,Hilden,Germany) under denaturing conditions,and the purity was over 95% after purification.The purified products were dissolved in phosphate-buffered saline (PBS) and filtrated with a 0.22-μm micro-pore filter membrane to produce arresten protein solution.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Shanghai BioLeaf Biotech Co.,Ltd.The HeLa cell line was kindly donated by Dr.Wu (First Affiliated Hospital of Shanxi Medical University).The murine colon carcinoma CT26 cell line was kindly donated by Dr.Chen(Molecular Biology Research Institute of Shanxi Medical University).The cells were grown in high/low glucose DMEM medium (Gibco BRL,Grand Island,NY,USA) supplemented with 10% fetal bovine serum (FBS,Hangzhou Sijiqing Biological Engineering Materials,China),100 U/mL penicillin,and 100 U/mL streptomycin,and maintained at 37°C in a 95% humidified atmosphere with 5% CO2.The cells were subcultivated every 2 or 3 days.
Cell proliferation assay
When reaching 70%-80% confluence,the HUVECs and HeLa cells were harvested by trypsinization (Life Technologies,Inc.,Shanghai,China) at 37°C for 3 minutes.A suspension of cells (1×105/mL) in DMEM with 0.5% FBS was added to each well of a 96-well plate.After a 12-hour incubation,the cells were treated with arresten at 0,0.4,0.8,1.6,and 3.2 μg/mL for 24,48,and 72 hours.Twenty microliters of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,5 mg/mL,AMRESCO,Solon,OH,USA) was added to cultured cells 4 hours before ending the experiment to produce formazan with light purple and the formazan was subsequently dissolved with 100 μL dimethyl sulfoxide.The absorbance (A) was detected at 490 nm,and the proliferation inhibitory rate was calculated.
Cell apoptosis assay
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI),a calcium-dependent phospholipid-binding protein with a high affinity for phosphatidylserine,was used to detect cell apoptosis.HUVECs or HeLa cells were added to a 6-well plate in DMEM supplemented with 10%FBS and incubated for 36 hours.The media was then replaced by fresh DMEM containing arresten at different concentrations (0,0.4,0.8,1.6,and 3.2 μg/mL).Cells incubated in PBS at the same volume served as control.After 24 hours of treatment,the cells were digested with 0.25% trypsin,collected,and resuspended in 500 μL binding buffer.Annexin V-FITC and PI (5 μL of each,KeyGEN Biotech,Nanjing,China) were added,and the cells were incubated in darkness for 15 minutes.Annexin V-FITC/PI-labeled cells were counted using a Becton-Dickinson FACSan flow cytometer (Franklin Lakes,NJ,USA).These data were analyzed using Cell Quest software(Becton-Dickinson).
Migration assay
Migration of HUVECs and HeLa cells was assessed with Boyden chamber chemotaxis assay (Kylin-bell,Haimen,China).Chambers with 8-μm pore filters were used.The cells were cultured until reaching the exponential phase of growth,resuspended to a final concentration of 2×106/mL by trypsinizing in serum-free medium with 0.05% FBS,and seeded into the wells of the upper compartment of the Boyden chamber.Serum-free medium containing different concentrations of arresten was added to the wells of the bottom compartment of the chamber.The control group received an equal volume of PBS.The Boyden chamber was incubated at 37°C in a 10% CO2atmosphere for 6 hours.The filter was removed from the chamber,and the non-migratory cells on the upper side of the filter were scraped off with a rubber policeman.The migratory cells on the lower side of the filter were fixed and stained with hematoxylin and counted under microscope in high power fields of view (×400).Migration experiment was repeated at least three times unless otherwise indicated.
Animal study
A total of 20 C57BL/6 colon carcinoma-bearing mice (6-8 weeks,weighing about 20 g) were used.The procedures were conducted according to the National Institute of Healthy (USA) guidelines for the care and use of laboratory animals.In a sterile environment,after injection with 2×107/mL CT26 cells to establish a transplanted mice tumor model,the mice were divided randomly into arresten treatment group (n=10) and control group (n=10).Injection of arresten was performed every other day for the 10 mice in the arresten treatment group,while injection of physiological saline for the 10 mice in the control group.The mice were observed for viability,weighed,and the diameters of the tumors were measured.Ten days later,the tumor volume (TV),relative tumor volume (RTV),and relative tumor inhibition ratio (RTVR) were determined according to the following formulas:TV=1/2×a×b2[a,width (mm);b,length (mm)];RTV=Vt/V0(V0,initial tumor volume;Vt,final tumor volume);RTVR=T/C×100%=TRTV/CRTV×100% (T,arresten treatment group;C,control group;TRTV,RTV of arresten treatment group;CRTV,RTV of control group).
The blood vessel density in tumor tissues was measured by immunohistochemistry using a CD31 polyclonal antibody (Wuhan Boster Bio-engineering Limited Company,China).Microvessel density was calculated as previously reported.17After the area with the highest neovascularization was identified at 200× magnification under a light microscope,the number of blood vessels in 10 regions at 200× magnification was counted,and the average number of microvessels was recorded.
Statistical analysis
SPSS 13.0 software was employed for statistical analysis.The data were presented as means±SD.Comparisons were performed with analysis of variance (ANOVA) with onetailed Student’sttest.P<0.05 was considered statistically significant.
RESULTS
Expression of arresten protein
The sequence for arresten is diagrammatically represented in Figure 1.Sodium dodecyl sulfonate polyacrylamide gel electrophoresis detected a single protein with a molecular mass of 26 kDa and that the purity of the protein was at least 95% (Fig.2).
Impact of arresten on the growth of HUVECs and HeLa cells
Arresten (0.8 μg/mL) inhibited the proliferation of HUVECs(P<0.05) in a dose-dependent manner to some extent (Fig.3).However,arresten had no effect on the proliferation of HeLa cells (Fig.4).
Arresten significantly induced apoptosis in HUVECs,which was dose-dependent within the range of 0.4-3.2 μg/mL (P<0.01).No such effect of arresten was observed in HeLa cells (Fig.5).
Arresten protein inhibited HUVECs migration (P<0.01)in a dose-dependent manner within the range of 0.8-3.2 μg/mL (Fig.6).Arresten protein could also inhibit the migration of HeLa cells,yet the extent of inhibition was lower than that observed in HUVECs (Fig.6).
Figure 1.Nucleotide sequence of arresten.
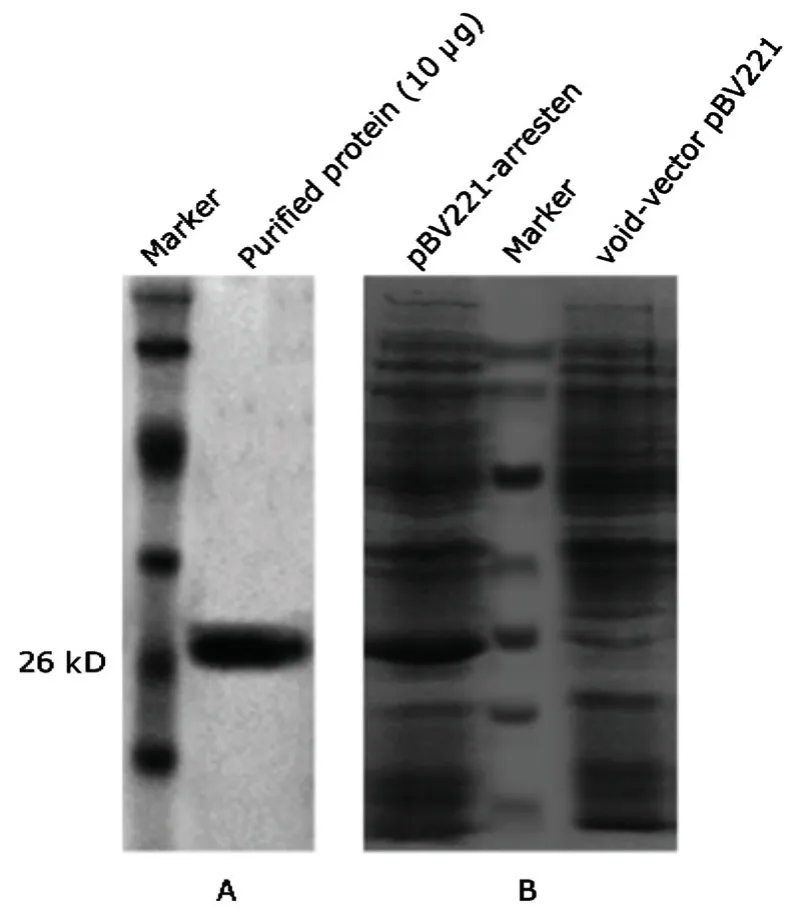
Figure 2.Expression and purification of recombinant human arresten.
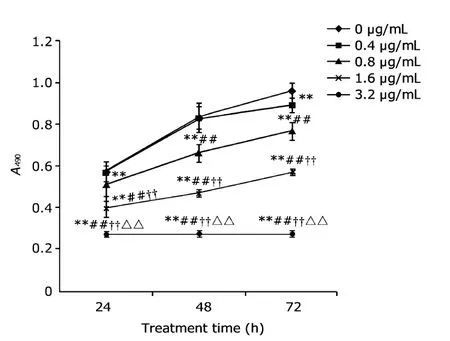
Figure 3.The effect of arresten on the proliferation of human umbilical vein endothelial cells (HUVECs).The absorbance (A) was detected at 490 nm.
**
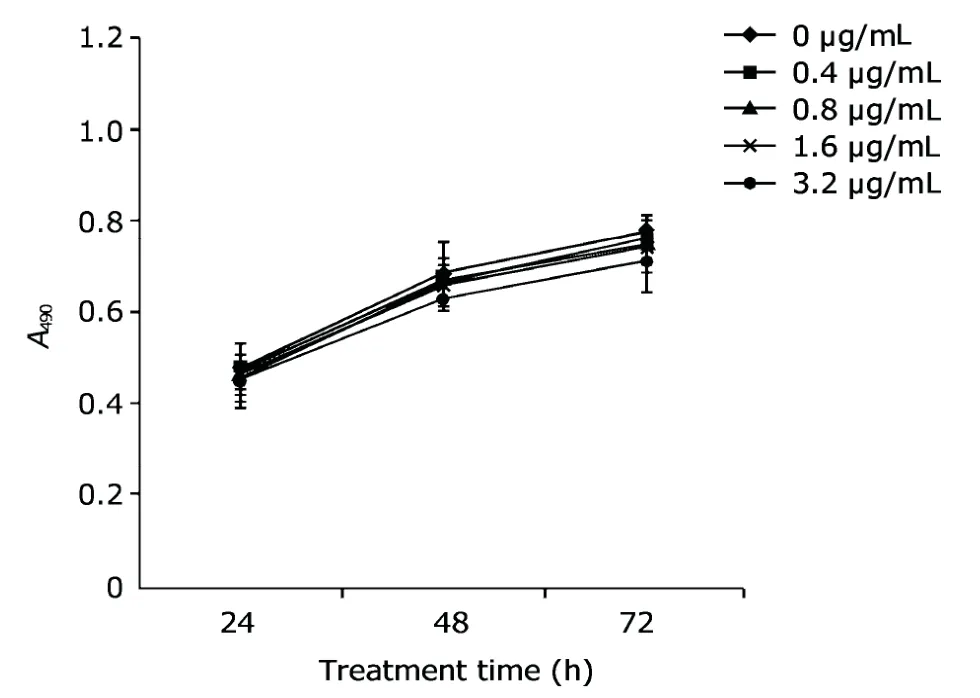
Figure 4.Arresten protein had no obvious effect on the growth of HeLa cells.
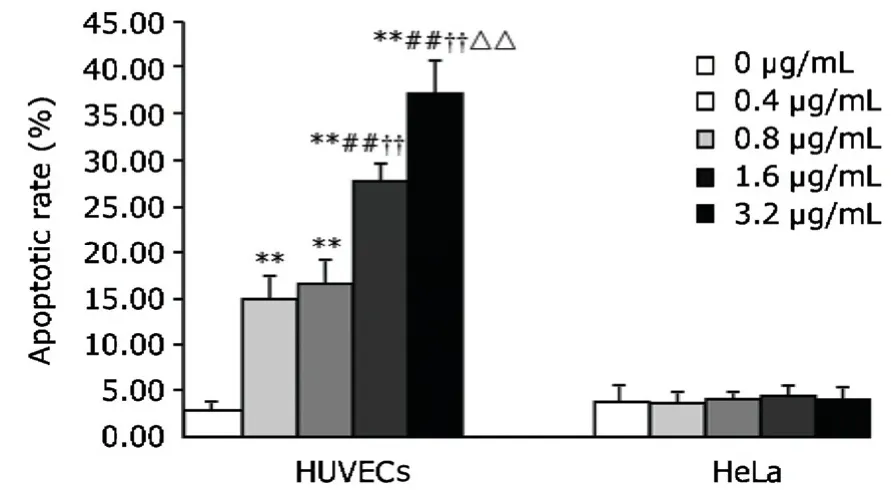
Figure 5.The effect of arresten on the apoptosis of HUVECs and HeLa cells.
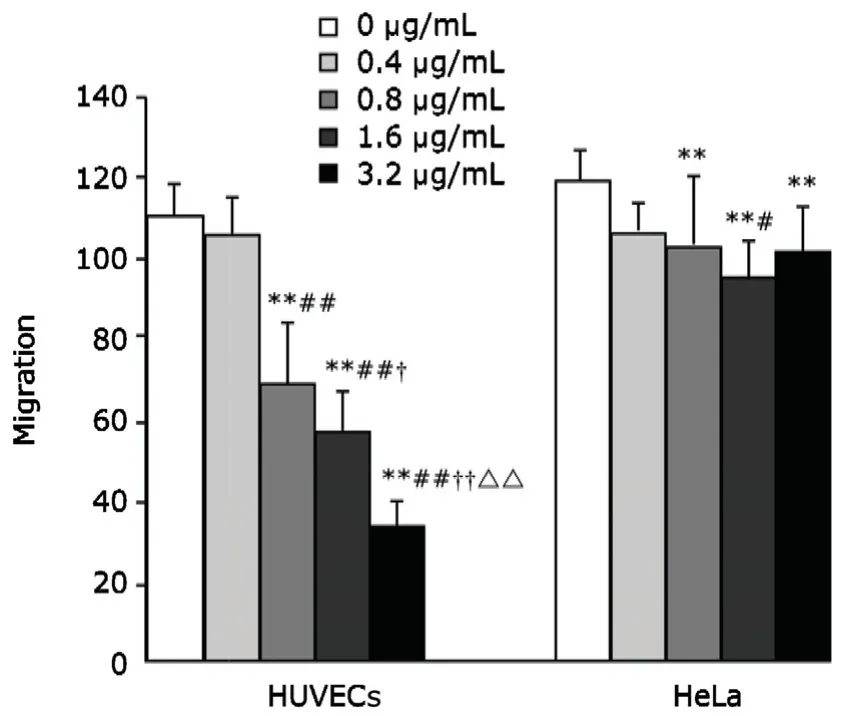
Figure 6.The effect of arresten on the migration of HUVECs and HeLa cells.Arresten effectively inhibited the migration of HUVECs,which was dose-dependent within the range of 0.8-3.2 μg/mL.It modestly inhibited the migration of HeLa cells,but to a lesser extent than that observed in HUVECs.
**
Therapeutic effect of arresten on the tumor-bearing mice
Mice in the control group exhibited progressive tumor growth with a decline in diet,activity,and response,which developed into cachexia and one mouse died eventually.In contrast,mice in the arresten treatment group exhibited normal diet behavior both before and after treatment.The tumor volume and weight in the arresten treatment group was significantly lower than those in control group(P<0.01).It was found that the growth of tumor was inhibited with a relative inhibition ratio of 80.81%.The therapeutic effect of arresten on the tumor-bearing mice is shown in Table 1.
The prepared tumor tissues were stained with CD31(1:100) immunohistochemistry by Strept-Avidin-Biotin-Complex (SABC) method.Tumor tissue in the control group demonstrated a wide distribution of vascular lumen formation,while in the arresten treatment group,significantly reduced angiogenesis was noted in the tumors (P<0.01,Fig.7).
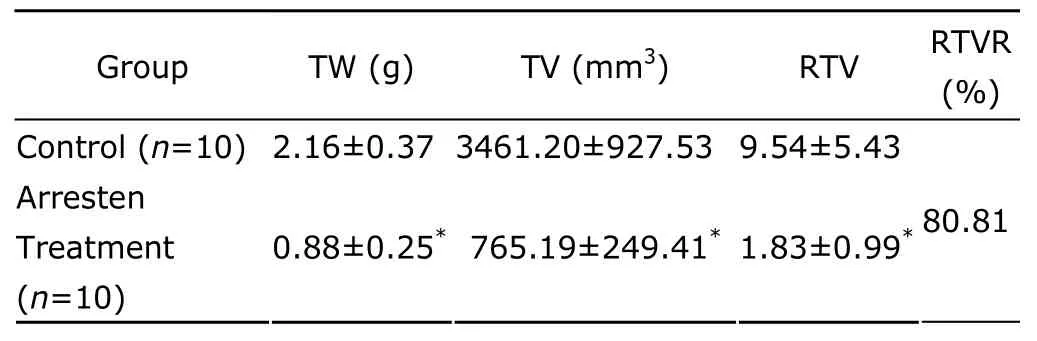
Table 1.Inhibitory effect of arresten on tumor in tumor-bearing mice§
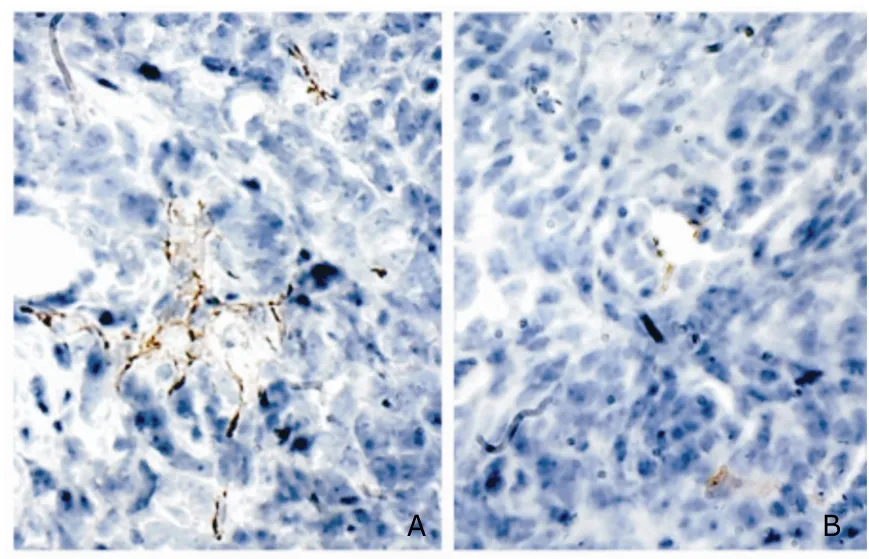
Figure 7.Inhibitory effect of arresten on tumor angiogenesis in tumor-bearing mice.hematoxylin ×200
DISCUSSION
The malignant growth and metastasis of tumors rely upon angiogenesis to receive an adequate supply of oxygen and nutrients.17Inhibiting neovascularization may efficiently inhibit tumor cell growth and lead to tumor dormancy.17,18Arresten,an endogenous angiogenesis inhibitory factor,has been found to have no toxic side effects and is more stable than endostatin,12,15thus may be a potential therapeutic agent in tumor therapy.Lu et al19have shown that transfected arresten could inhibit the growth of an SGC-7901 tumor xenograft model in nude mice and prevent hepatic metastasis of colorectal cancer.However,the gene transfection method in that study was difficult to perform clinically and might lead to mutation and carcinogenesis.In addition,the cost of gene transfection therapy restricts it application for tumor patients.Recombinant arresten production by prokaryotic expression is an attractive method for producing a large amount of the protein,and the dosage can be adjusted according to the different needs of individual patients.Additionally,bacterial production of recombinant arresten avoids the danger of the rapid growth of recombinant cells into a new tumor.Chemotherapy based on prokaryotic expression of arresten is a safe,highly efficient,and low-cost tumor therapy,which can be combined with surgery and radiotherapy.Thus,this method could be readily applied in clinical practice.15
In the animal study in this research,we found the dose of arresten we used safe for animals and effective in inhibiting tumor growth.These results show that the pure arresten prepared in our laboratory has a satisfactory therapeutic effect,thus could be further considered as a potential novel drug in the treatment for solid tumors.As shown by the results of the present study,arresten inhibited the proliferation and migration of HUVECs while promoting apoptosis of these cells;however,it had no such significant effect on the proliferation or viability of HeLa cells.These findings confirm that arresten plays the inhibitory role through suppressing angiogenesis.This conclusion is supported by the decrease in tumor vessel density in arresten-treated tumor-bearing mice.We hypothesize that the mechanism underlying the effects of arresten may be as follows:arresten binds to α1β1 integrin,competing with type IV collagen binding to α1β1 integrin.13Additionally,arresten pretreatment could inhibit the FAK/c-Raf/MEK/ERK1/2/p38 MAPK activation in ECs and inhibits the migration,proliferation,and vessel formation by ECs.14,20However,the present study demonstrates that the migration of tumor cells per se can also be inhibited by arresten,although to a lesser extent.The relevant mechanism is not clear;study in this area may shed some light on how to therapeutically inhibit the metastasis of cancer.
ACKNOWLEDGMENT
We sincerely thank Prof.Jun Xie,Prof.Xian-jiu Chen,Prof.Hai-ying Tang,and other administrative personnel forhelping in organizing and facilitating the research.We would also like to thank the Department of Biochemistry and Molecular Biology as well as the Second Affiliated Hospital of Shanxi Medical University for their support in the project.More importantly,we feel grateful for the mother who voluntarily donated the placental tissue for this study.
1.Honma K,Miyata T,Ochiya T.Type I collagen gene suppresses tumor growth and invasion of malignant human glioma cells.Cancer Cell Int 2007;7:12.
2.Clamp AR,Jayson GC.The clinical potential of antiangiogenic fragments of extracellular matrix proteins.Br J Cancer 2005;93:967-72.
3.Ortega N,Werb Z.New functional roles for non-collagenous domains of basement membrane collagens.J Cell Sci 2002;115:4201-14.
4.Boosani CS,Sudhakar A.Cloning,purification,and characterization of a non-collagenous anti-angiogenic protein domain from human alpha1 type IV collagen expressed in Sf9 cells.Protein Expr Purif 2006;49:211-8.
5.Folkman J.Angiogenesis:an organizing principle for drug discovery? Nat Rev Drug Discov 2007;6:273-86.
6.Folkman J.Anti-angiogenesis:new concept for therapy of solid tumors.Ann Surg 1972;175:409-16.
7.Luo YQ,Wang LH,Yi Q,et al.Expression of soluble,biologically active recombinant human tumstatin in Escherichia coli.Clin Exp Med 2008;8:37-42.
8.Carmeliet P,Jain RK.Angiogenesis in cancer and other diseases.Nature 2000;407:249-57.
9.Kieran MW,Folkman J,Heymach J.Angiogenesis inhibitors and hypoxia.Nat Med 2003;9:1104.
10.Petitclerc E,Boutaud A,Prestayko A,et al.New functions for non-collagenous domains of human collagen type IV.Novel integrin ligands inhibiting angiogenesis and tumor growthin vivo.J Biol Chem 2000;275:8051-61.
11.Sudhakar A,Boosani CS.Inhibition of tumor angiogenesis by tumstatin:insights into signaling mechanisms and implications in cancer regression.Pharm Res 2008;25:2731-9.
12.Colorado PC,Torre A,Kamphaus G,et al.Anti-angiogenic cues from vascular basement membrane collagen.Cancer Res 2000;60:2520-6.
13.Brown MC,Staniszewska I,Del Valle L,et al.Angiostatic activity of obtustatin as alpha1beta1 integrin inhibitor in experimental melanoma growth.Int J Cancer 2008;123:2195-203.
14.Sudhakar A,Nyberg P,Keshamouni VG,et al.Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin.J Clin Invest 2005;115:2801-10.
15.Zheng JP,Tang HY,Chen XJ,et al.Construction of recombinant plasmid and prokaryotic expression inE.coliand biological activity analysis of human placenta arresten gene.Hepatobiliary Pancreat Dis Int 2006;5:74-9.
16.Zhang Y,Wang W,Zhou J,et al.Tumor anti-angiogenic gene therapy with microencapsulated recombinant CHO cells.Ann Biomed Eng 2007;35:605-14.
17.Saaristo A,Karpanen T,Alitalo K.Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis.Oncogene 2000;19:6122-9.
18.Folkman J,Browder T,Palmblad J.Angiogenesis research:guidelines for translation to clinical application.Thromb Haemost 2001;86:23-33.
19.Lu CR,Chen L,Dou CQ,et al.Arresten expressedin vivosuppresses the growth of SGC-7901 tumor xenografts in nude mice.Zhonghua Wai Ke Za Zhi 2005;43:1391-4.
20.Nyberg P,Xie L,Kalluri R.Endogenous inhibitors of angiogenesis.Cancer Res 2005;65:3967-79.
 Chinese Medical Sciences Journal2012年1期
Chinese Medical Sciences Journal2012年1期
- Chinese Medical Sciences Journal的其它文章
- Management of Pregnancy with Ankylosing Spondylitis
- Applicability of Community Periodontal Index Teeth and Random Half-mouth Examination to Gingival Bleeding Assessment in Untreated Adult Population in Beijing
- Personalized Management of Anastomotic Leak after Surgery for Esophageal Carcinoma
- Spectral Domain Optical Coherence Tomography of Vogt-Koyanagi-Harada Disease:Novel Findings and New Insights into the Pathogenesis
- Radiofrequency Ablation of Hepatic Paragonimiasis:a Case Report
- Electrocorticography with Direct Cortical Stimulation for a Left Temporal Glioma with Intractable Epilepsy
