Electrocorticography with Direct Cortical Stimulation for a Left Temporal Glioma with Intractable Epilepsy
Resha Shrestha,Kuo Li,Wei Wang,Hai-ping Lian,and Mao-de Wang*
Department of Neurosurgery,First Affiliated Hospital of Medical College of Xi’an Jiaotong University,Xi’an 710061,China
ELECTROCORTICOGRAPHY (ECoG),the intraoperative recording of cortical potentials,has played an important role in the surgical management of patients with medically intractable epilepsy.This technique is useful in epilepsy surgery to delineate margins of epileptogenic zones,guide resection,and evaluate completeness of resection.1About one third of the patients with intractable epilepsy have obvious causes,such as tumor,sclerosis, or vascular malformation.However,the presence of tumor in the eloquent cortex like speech area makes it challenging to completely resect the tumor.ECoG-based surgery in conjunction with direct cortical stimulation in such cases helps surgical resection of tumor with no functional tissue affected,producing good surgical outcome.This report describes a case treated with ECoG-based surgery in combination with direct cortical stimulation.
CASE DESCRIPTION
A 54-year-old lady presented with paroxysmal loss of consciousness accompanied by abnormal movements of limbs for 40 years.The frequency of the seizure was low initially,till 30 years ago when a mass lesion was discovered in the left temporal region.The frequency gradually increased to about 1-2 seizures in two weeks.It was not associated with aura but was demonstrated as generalized tonic-clonic seizure in nature with head-turning to the left,which lasted for few minutes and was associated with brief loss of consciousness with no loss of memory.But self injury was inflicted many times. She has also gradually developed mild slurring of speech with no obvious aphasia since 10 years ago.She was taking carbamazepine,leviteracetam,and valproate since 10 years ago.Her medical history showed no previous head injury or family history of seizure.She is a non-smoker and does not drink.On examination,no obvious neurological deficits were observed,yet scars of self injury were visible over her body.Magnetic resonance imaging of her head showed hypointense lesion in the left temporal region on T1W image and hyperintense lesion on T2W image with mild peripheral contrast enhancing lesion (Fig.1).
She came to our hospital for further treatment and the possibility of ECoG-based surgery was explained to her.After admission to the hospital,video-electroencephalography monitoring was done to localize the focus of seizure.During 48 hours of monitoring,a single seizure was caught,which showed that the origin of the seizure was in the left temporal region,the same site of the tumor.Finally the therapy was decided upon,including ECoG-based surgical removal of the tumor with awake anesthesia to monitor her speech during the surgery.The possibility of aphasia after the surgery was fully explained to the patient.
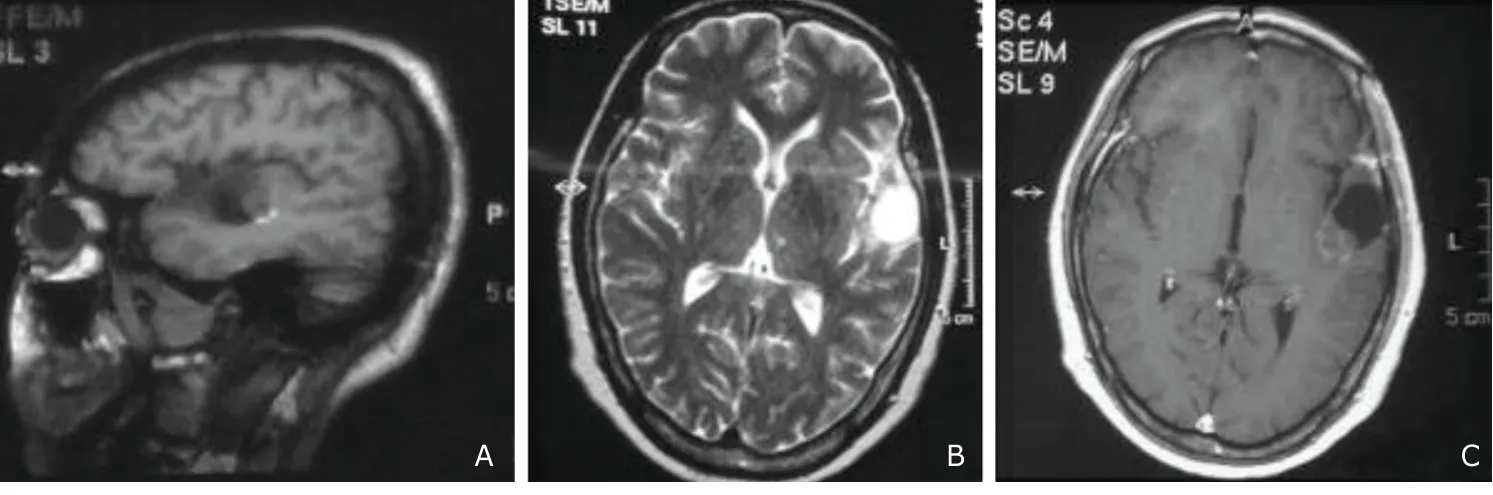
Figure 1.Preoperative magnetic resonance imaging (MRI) images of a 54-year-old female.
General anesthesia was given with propofol and isoflurane.A horse shoe shaped incision was made just above the left ear such that the anterior margin was at the pterion and the posterior margin just above the left ear.Craniotomy was performed with a size about 5 cm×5 cm.Dura was seen to be tight.In the meantime while dural tenting was performed,200 mL of 20% mannitol was administered.Then the dura was cut,and the cystic tumor capsule with slightly brownish discoloration was visible on the superior temporal gyrus with no clear-cut demarcation of the tumor.At this time cortical electrodes were placed to perform ECoG.The ECoG result was inconclusive and did not show seizure activities.With the help of anesthestists,the patient was waken up,and cortical stimulation was performed with bipolar stimulating electrode and a dialogue with the patient was started regarding her family.She even counted from one to ten.The cortical stimulation did not reveal any disruption in her language.The areas were marked,and she was re-intubated after neurophysiological monitoring.The total removal of the tumor was achieved.The tumor was both solid and cystic,with grayish red.Histopathological examination was conducted.Then hemostasis was achieved and the dura was sutured.
On the first postoperative day,the patient was fine with no neurological deficits but did not speak at all. She could neither understand nor answer our questions.Complete aphasia lasted for two days.From the third postoperative day on,she started to speak gradually and recovered to her previous state of speech.She had nominal aphasia at the time when the report was finished.She was shifted to neurosurgical general ward on the third postoperative day.Her postoperative image shows complete resection of tumor (Fig.2).Seizure did not develop after the operation and the histopathology report revealed oligodendroglioma at WHO grade II (Fig.3) with immunohistochemistry revealing olig2 (+),glial fibrillary acidic protein (+),and Ki-67(+10%).
DISCUSSION
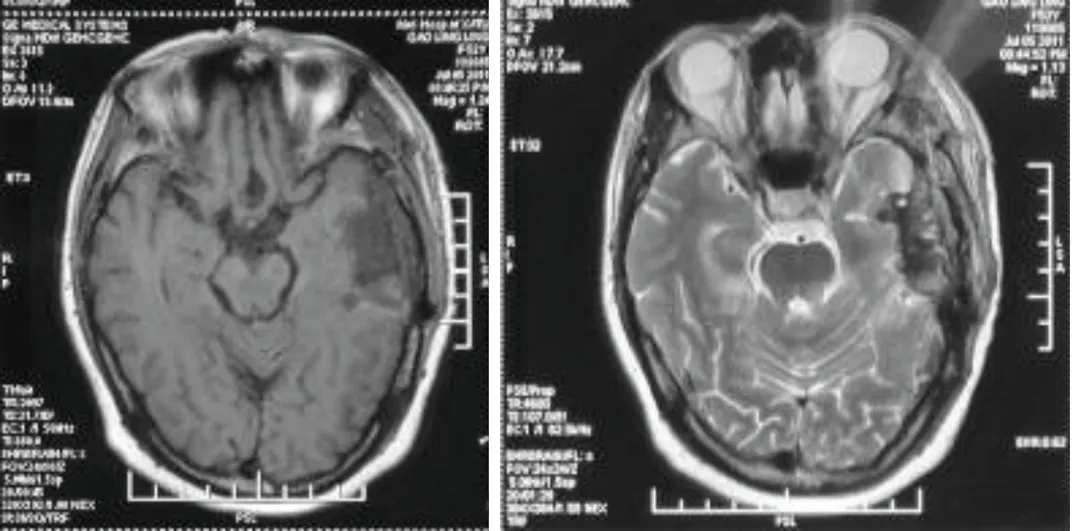
Figure 2.Transverse T1 weighted (A) and T2 weighted (B) MRI images show complete resection of tumor.
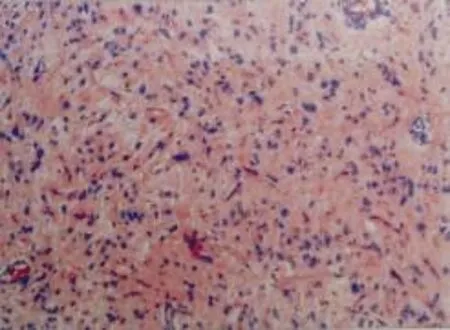
Figure 3.Histopathological examination of the resected tumor shows infiltration of tumor cells with oligodendrocytes(WHO grade II).HE ×10
The surgical indication for medically refractory epilepsies has already been well defined.It has been suggested that malformation of cortical development and mixed neuronal glial tumors are the most important cause of intractable epilepsy,especially in children and adolescents.Tumors specially represent the primary pathology in 10%-30% of the cases.2Chang et al3have reported that approximately half of their patients presenting with seizures with low grade glioma were pharmacoresistant before surgery.The causal factors in intractable epilepsy have been a favorable prognostic indicator to determine the good surgical outcome.4Furthermore,intracranial neoplasm involving the temporal lobe structures is more associated with intractable epilepsies than tumors in other locations.However,the surgical resection of eloquent areas is challenging due to high risk of postoperative neurological impairment.5It has been well accepted that epileptogenic zone is often located adjacent to intra-axial tumors but does not necessarily involve the tumor nidus.This can result in negative ECoG mapping in the intraoperative period as the surgical exposure will limit the extension to which ECoG can be performed.Additionally,both the background activity and epileptiform discharges may be altered by anesthetics,narcotic analgesics,and the surgery itself.6
Many glial tumors are located within or adjacent to the functional areas such as motor cortex,speech area,and sensory cortex.In such cases awake anesthesia with direct cortical stimulation can be considered as it allows intraoperative mapping to delineate any eloquent cortex and its relationship to the tumor to facilitate the safest tumor removal and the preservation of functional tissue.7Nowadays awake craniotomy with electrophysiological mapping can be further aided by intraoperative MRI for safer results.8The surgical resection of tumors in dominant temporal lobe are particularly difficult due to variation in the localization of the language area.9The traditional concept of cortical organization of language involves the anterior language region,the posterior portion of inferior frontal gyrus (Broca’s area),and perisylvian region in temporoparietal region (Wernicke’s area).However,some studies have suggested that language sites in temporal lobe can differ,stating that the patient without tumors had significantly more language sites in superior temporal gyrus than those with temporal lobe tumors.9,10
The immediate postoperative complete aphasia in this case could not be explained except for the possibility of physiological disruption of the arcuate fasciculus during the surgery,which improved after the third postoperative day.However,the nominal aphasia still persisted with gradual improvement.
In conclusion,ECoG-based surgery in conjunction with direct cortical stimulation of the cortex for language mapping in case of temporal gliomas in dominant hemisphere is useful for adequate surgical resection.The negative language mapping in the vicinity of the tumor could help the surgeons to perform complete resection of the tumor.
The immediate postoperative complete aphasia in this case could not be explained except for the possibility of physiological disruption of the arcuate fasciculus during the surgery,which improved after the third postoperative day.However,the nominal aphasia still persisted with gradual improvement.
In conclusion,ECoG-based surgery in conjunction with direct cortical stimulation of the cortex for language mapping in case of temporal gliomas in dominant hemisphere is useful for adequate surgical resection.The negative language mapping in the vicinity of the tumor could help the surgeons to perform complete resection of the tumor.
1.Tripathi M,Garg A,Gaikwad S,et al.Intra-operative electrocorticography in lesional epilepsy.Epilepsy Res 2010;89:133-41.
2.Piao YS,Lu DH,Chen L,et al.Neuropathological findings in intractable epilepsy:435 Chinese cases.Brain Pathol 2010;20:902-8.
3.Chang EF,Potts MB,Keles GE,et al.Seizure characteristics and control following resection in 332 patients with low grade gliomas. J Neurosurgery 2008;108:227-35.
4.Schaller B,Rüegg SJ:Brain tumor and seizures:pathophysiology and its implication for treatment revisited.Epilepsia 2003:44:1223-32.
5.Devaux B,Chassoux F,Landré E,et al.Surgical resections in functional areas:report on 89 cases.Neurochirugie 2008;54:409-17.
6.Zumsteg D,Weiser HG.Presurgical evaluation:current role of invasive EEG.Epilepsia 2000;41 Suppl 3:S55-60.
7.Berger MS.Lesions in functional (“eloquent”) cortex and subcortical white matter.Clin Neurosurg 1994;41:444-63.
8.Lu JF,Zhang J,Wu JS,et al.Awake craniotomy and intraoperative language cortical mapping for eloquent cerebral glioma resection:preliminary clinical practice in 3.0 T intraoperative magnetic resonance imaging integrated surgical suite.Zhonghua Wai Ke Za Zhi 2011;49:693-8.
9.Richardson MP.Epilepsy and surgical mapping.Br Med Bull 2003;65:179-92.
10.Haglund MM,Berger MS,Shamseldin M,et al.Cortical localization of temporal lobe language sites in patients with gliomas.Neurosurgery 1994;34:567-76.
 Chinese Medical Sciences Journal2012年1期
Chinese Medical Sciences Journal2012年1期
- Chinese Medical Sciences Journal的其它文章
- Management of Pregnancy with Ankylosing Spondylitis
- Applicability of Community Periodontal Index Teeth and Random Half-mouth Examination to Gingival Bleeding Assessment in Untreated Adult Population in Beijing
- Personalized Management of Anastomotic Leak after Surgery for Esophageal Carcinoma
- Spectral Domain Optical Coherence Tomography of Vogt-Koyanagi-Harada Disease:Novel Findings and New Insights into the Pathogenesis
- Radiofrequency Ablation of Hepatic Paragonimiasis:a Case Report
- Primary Sjögren's Syndrome Accompanied by Intestinal Obstruction:a Case Report and Literature Review
