Personalized Management of Anastomotic Leak after Surgery for Esophageal Carcinoma
Hong-yu Ye,Wei-zhao Huang*,Yin-meng Wu,Yi Liang,Jun-meng Zheng,and Hai-ming Jiang
Department of Cardio-thoracic Surgery,Zhongshan Hospital of Sun Yat-sen University (Zhongshan People’s Hospital),Zhongshan 528403,China
ANASTOMOTIC leak following surgery for esophageal carcinoma is a severe and refractory complication.With improvement in anastomotic techniques,its incidence has been significantly reduced;however, due to a good number of reasons including physiology and anatomy of esophagus,the possibility of developing leak cannot be totally eliminated.1Therefore the appropriate management of this condition is crucial for surgical patients.In the present study,we performed a retrospective analysis to summarize the experience in treating anastomotic leak after surgery for esophageal carcinoma.
PATIENTS AND METHODS
Patients
From January 2003 to March 2011,554 patients with esophageal carcinoma were treated with surgery in the Department of Cardio-thoracic Surgery in our hospital,among which a total of 36 developed anastomotic leak,with an incidence of 6.5%.General items in medical records of those patients were reviewed,including demographic characteristics,tumor features,concomitant conditions,pathological staging (based on Union Internationale Contre le Cancer 2007 staging system),and surgical approaches.None of them underwent neoadjuvant therapy.
Management of anastomotic leak
Systemic treatment:Once anastomotic leak was confirmed,fasting was started.Gastric tube was placed and continued gastrointestinal decompression introduced.Nutrition support treatment was instituted.Sensitive antibiotics were given to control the infection based on the results of bacterial culture.
Local management:Three types of local management were adopted,namely surgical treatment (resurgey),stent implantation,and conservative treatment.In surgical treatment,operative re-exploration was performed.Necrotic gastric tissue was resected and leak closed by stapler.Pleural cavity was lavaged repeatedly and drainage tube inserted.In stent implantation,self-expanding metallic coated esophageal stent was insertedviaendoscopy to seal the leak.The conservative treatment consisted mainly of drainage.
Statistical analysis
SPSS 13.0 software was used for statistical analysis.Continuous data are presented as means±SD.Categorical data were compared using chi-squared test or Fisher’s exact test.P<0.05 was considered statistically significant.
RESULTS
General information of the patients
All the 36 patients were male,with a mean age of 64.1±11.8 years (range,44-77 years).All these 36 patients had pathologically confirmed esophageal squamous cell carcinoma,of which 11 had tumors located in upper thoracic segment,19 in middle thoracic segment,and 6 in lower thoracic segment.Before surgery,concomitant hepatic cirrhosis was seen in 1 patient,primary hypertension in 6,and obsolete pulmonary tuberculosis in 5.Twenty-one patients were long-term smokers.With pathological staging after surgery,the disease was rated as stage IIa in 9 patients,stage IIb in 13,and stage III in 14.
Surgical approaches
In 13 patients,the surgery started with the right thoracotomy.Affected esophagus was resected and left-cervical circular gastro-esophageal anastomosis performed (Mc-Keown modification).Four of them were anastomosed by staplers and 9 by manual suture.Drainage tube was placedviacervical incision.During surgery,gastric tube was inserted nasally and jejunostomy performed.The other 23 patients received left transthoracic approach,and intra-thoracic circular gastro-esophageal anastomosis was performed by staplers.During surgery,gastric tube and jejunal feeding tube were inserted nasally.In one of these patients,wedge resection of left lung was performed concomitantly.Following surgery,8 patients were given parenteral nutrition,18 were given enteral nutrition,and the other 10 both parenteral and enteral nutrition.
Postoperative leakage
Patients with fever,chest pain,dyspnea,or increased white blood cell count took oral methylene blue,and those without any of these symptoms were given follow-up contrast esophagogram on day 7 following surgery.All the patients were thereby confirmed with digestive tract leak.Among these 36 patients,13 had cervical anastomotic leak,18 had intra-thoracic anastomotic leak,and 5 had intra-thoracic gastric necrosis.The leak developed within 1-3 days after esophageal carcinoma resection in 6 patients,within 4-7 days in 11 patients,and within 8-14 days in 19 patients.
Treatment and outcome
Treatment lasted for 47.0±31.9 days (range,5-181 days).Complications mainly included pulmonary infection (50%),respiratory failure (19.4%),sepsis (11.1%),multiple organ failure (2.8%),and hepatic failure (2.8%).Nine patients deceased (mortality,25.0%).The cure rate of conservative treatment was significantly higher than that of resurgery [75.0% (18/24)vs.28.6% (2/7),P=0.037],but not higher than that of stent implantation [75.0%(18/24)vs.100% (6/6),P=0.302,Table 1].
Three patients with early onset anastomotic leak(within 3 days after surgery) and 4 patients with confirmed intra-thoracic gastric necrosis were given operative reexploration.The patient with early onset cervical anastomotic leak died of multiple organ failure on day 5 after surgery despite surgical repair.In the 2 patient with intra-thoracic anastomotic leak receiving resurgery,1 was cured.In the 4 patients with intra-thoracic gastric necrosis,necrosis of the gastric stump was found ruptured.Necrotic tissue was resected and sutured by stapler.Only one of the 4 was cured;while in the other 3 patients,leak developed again after surgical repair,causing 2 deaths because of severe infection and respiratory failure on day 21 and day 25,respectively.The remaining patient was switched to conservative treatment,receiving placement of pleural cavity drainage tube and repeated lavage.On day 90 after surgery,follow-up contrast swallow still revealed leak of contrast agent,which,however,was limited within thoracic apex,indicating small leak (Fig.1A,B).The patient was told to keep to semi-fluid diet and was free from significant fever and chest pain.On day 120,follow-up esophagogram suggested that the leak was healed,and residual abscess cavity in thoracic apex markedly reduced (Fig.1C).
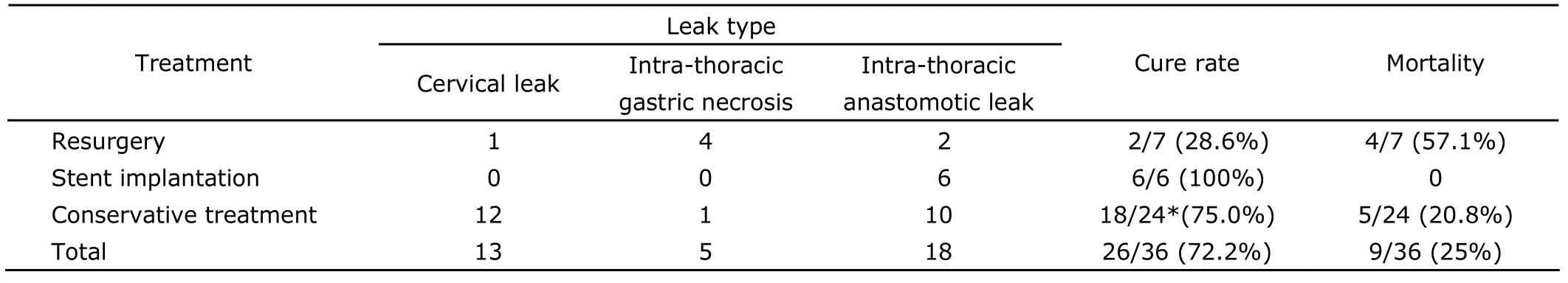
Table 1.Treatment profile in 36 patients with anastomotic leak following surgery for esophageal carcinoma
In 6 patients who underwent stent implantation,drainage from thoracic cavity was significantly reduced 3-5 days after surgery,and fever and symptoms of pulmonary infection remarkably relieved.One week after surgery,they started oral intake.But one patient’s stent migrated on day 8 after implantation and was replaced by a longer stent.The stent was removed under endoscopy in the 7th week,and the procedure was successful (Fig.2).
Of 12 patients with cervical anastomotic leak,7 with smaller leak were managed by local drainage and daily dressings.For the other 5 with larger cervical leak whose divergence of anastomosis was up to 50%,drainage tube was placed inviathe fistula;then fresh dressing was maintained for the wound within 5-7 days.When granulation was formed locally,latex drainage tube was placed into the leakviathe fistula and cervical incision,which was then sutured.The drainage tube was then connected to a negative pressure bottle for continued drainage.In 10 patients with intra-thoracic anastomotic leak,1 asymptomatic patient had localized leak as indicated by radiology,and was only given gastric tube and drainage,rather than thoracic cavity drainage.The other 9 patients were treated with traditional“three-tube”therapy,that is,gastric tube,feeding tube,and thoracic drainage tube,together with thoracic cavity lavage therapy.In the patients receiving conservative treatment,1 with cervical leak,1 with intra-thoracic gastric necrosis and 3 with thoracic leak deceased in day 6-71 due to severe infection.After being treated for 181 days,a patient with intra-thoracic leak was not completely cured,but abscess cavity was limited,so the patient was discharged with abscess cavity drainage tube and jejunal feeding tube.The remaining patients were cured and discharged after being treated for 46.3±14.9 days (range,20-73 days).Among them,one patient with intra-thoracic leak had limited outflow and was thus only treated with placement of gastric tube and drainage;follow-up esophagogram after fasting for 2 weeks showed minimal outflow (Fig.3),the patient started on semi-fluid diet and experienced no discomfort.In 5 patients managed with placement of drainage tubeviafistula and cervical incision,the drainage tubes were removed on day 14 after placement.On day 21,the leak was cured and the patients started eating.
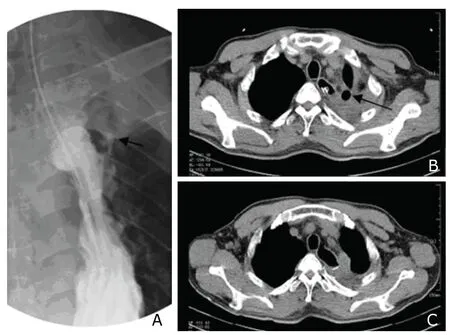
Figure 1.A follow-up contrast X-ray on day 90 after surgery (A)demonstrates an intra-thoracic anastomotic leak limited within thoracic apex (arrow).Computed tomography (CT) scan at the same time (B) shows the abscess cavity (arrow).The abscess cavity disappeared after 30 days in CT scan on day 120 (C).
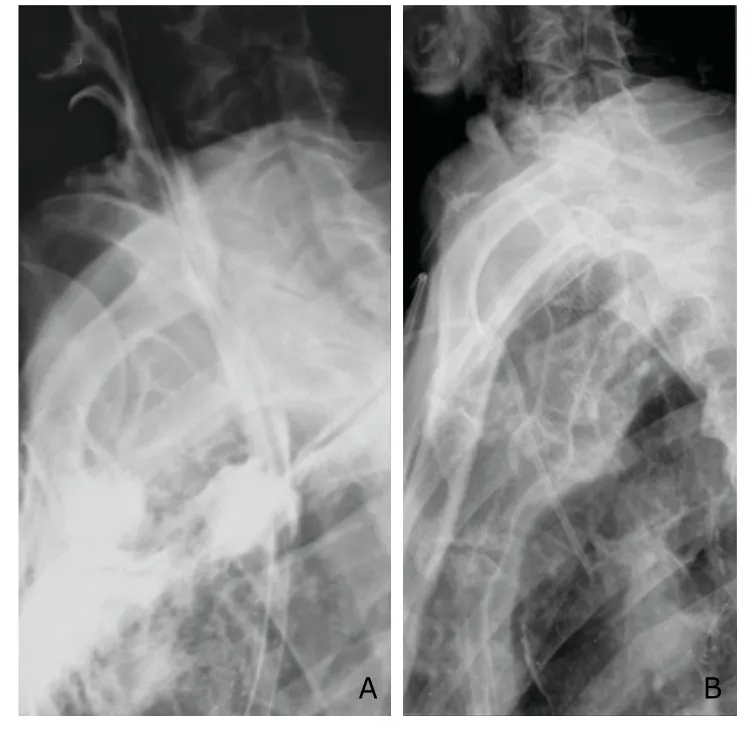
Figure 2.Intra-thoracic anastomotic leak (A) and implanted esophageal stent (B).
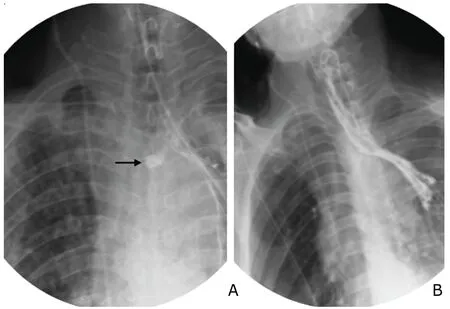
Figure 3.X-ray (A) shows a relatively small area of contrast extravasation contained by mediastinal structures(arrow).Esophagogram after fasting for 2 weeks (B)displays markedly reduced outflow.
DISCUSSION
Anastomotic leak is a serious complication of thoracic surgery,associated with high mortality and tends to necessitate long-term hospitalization.2,3In addition to systemic treatments including basic nutrition support and the use of broad-spectrum antibiotics,the most critical treatment is local management of the leak.Currently there is not standardized treatment.Turkyilmaz et al4suggested detailed treatment processes,which was of certain reference significance.
Drainage is a basic surgical method in local management for anastomotic leak.Keeping an effective drainage is key to conservative treatment and can cure most anastomotic leak.Based on that principle,new methods of drai-nage have been introduced and achieved good results.5,6We presume that the closure of anastomotic leak is often the results of tissue adhesion around the leak,enclosure of the leak by granulation tissue,and finally coverage by digestive tract mucosa,rather than the healing of both ends of the leak.Effective drainageviagastric tube and thoracic cavity tube can ensure adequate drainage of digestive fluid from both inside and outside of gastric cavity,so as to allow tissue adhesion and healing around the leak.In 5 patients with cervical leak in this study,drainage tube was insertedviacervical fistula and connected to negative pressure device to collect outflowing digestive fluid.Thereby,tissue around the incision was free from contamination,so that granulation tissue could form around the drainage tube;the tube could be removed once the sinus tract was established.The rationale is similar to that behind the“T-tube”drainage for biliary duct.It is easy to take care of,takes shorter time compared with routine dressing changing and drainage,and is particularly applicable to larger cervical anastomotic leak.This method can also be used for refractory intra-thoracic anastomotic leak.Wang et al7reported the resurgery in 9 patients and the plac ement of T-tube into the leak,of whom 8 were cur ed.
Resurgery for leak repair is a dilemma.Previous experience suggests that resurgery is associated with frequent complications and high mortality,but low rate of successful repair.8Current literature presents controversial findings about the efficacy of resurgery.Zhang et al9reported a cohort with intra-thoracic anastomotic leak;among 5 patients who underwent resurgery and repair or resection of anastomosis and re-anastomosis,outflow of digestive fluid was still seen after surgery at various degree.Crestanello et al10reported a group of 20 patients receiving resurgery,in which 3 died after surgery,but no further leak was seen among these patients.We suggest that rigorous compliance to the indications of resurgery should be practiced,which include:early onset anastomotic leak;thoracic gastric necrosis;encapsulated empyema,with poor drainage by routine methods and significant concomitant toxic symptoms.Resurgery for these scenarios can produce better efficacy than conservative treatment.The objective of resurgery is to:transform an intra-thoracic anastomosis into a cervical anastomosis,so as to facilitate management;repair the leak as much as possible,which can be achieved by using omentum and muscle flap;clear digestive fluid and purulent secretion in thoracic cavity,so as to ensure good drainage,reduce absorption of toxic substances,and relieve toxic symptoms.In the present study,resurgery resulted in poor efficacy.The high mortality associated with resurgery in this study might be partly due to the conservative surgical procedure.The choice of specific surgical procedures has yet to be further investigated.
Esophageal stent implantationviaendoscopy has been gradually accepted and widely adopted as a treatment for anastomotic leak associated with cancers.11-13The application of stent is somehow limited,for instance,expansion of coated stent may cause avulsion of the tissue around anastomosis,stents can not seal a leak caused by gastric perforation,the anatomic angle of aorta does not allow for linear journey of the stent,the stent may injure vessel wall,and the stent may be displaced.However,the mortality of stent therapy for anastomotic leak was lower than that of traditional surgical treatment.14-17For the present,it might be an optimal treatment strategy for intra-thoracic anastomotic leak.When the leak is sealed by the stent,thoracic exudation will be reduced.As solid adhesion forms along the pleura and the leak can be sealed from the outside,the stent can be removed.According to the method proposed by Leers et al,12the stent was removed in 6th to 8th week to avoid difficulty in removing resulting from adhesion of granulation tissue following prolonged placement of the stent.
In cases of localized intra-thoracic anastomotic leak,tube placement or resurgery is necessary to achieve adequate drainage for those with significant concomitant symptoms of infection,such as fever and chest pain.For asymptomatic localized anastomotic leak,capsulated residual cavity may exist for a long time,whereas complete healing of abscess or leak is not necessarily a prerequisite for restoration of normal diet.In management of localized anastomotic leak,patients should fast under observation for 1-2 weeks;if residual cavity is not expanding and infection not spreading,the patients can try to start semi-fluid diet.In a patient with intra-thoracic gastric necrosis who underwent resurgery in this study,localized leak still existed after the surgery,but the patient had no significant symptoms of infection,and follow-up esophagogram showed no increase in residual cavity,so the patient was allowed to start semi-fluid diet.In the follow-up visit one month later,residual cavity had already disappeared.
In conclusion,anatomotic leak after surgery for esophageal carcinoma can vary in location,onset time,size,and extent.For local management,personalized treatment should be decided based on patient’s particular situations.Conservative treatment is still a safe and effective method and resurgery should be considered carefully.Stent implantation might be a prospective therapeutic option but needs further verification.
1.Reavis KM.The esophageal anastomosis:how improving blood supply affects leak rate.J Gastrointest Surg 2009;13:1558-60.
2.Rizk NP,Bach PB,Schrag D,et al.The impact of complications on outcomes after resection for esophageal cancer and gastroeseophageal junction carcinoma.J Am Coll Surg 2004;198:42-50.
3.Whooley BP,Law S,Alexandrou A,et al.Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer.Am J Surg 2001;181:198-203.
4.Turkyilmaz A,Eroglu A,Aydin Y,et al.The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma.Dis Esophagus 2009;22:119-26.
5.Hu Z,Yin R,Fan X,et al.Treatment of intrathoracic anastomotic leak by nose fistula tube drainage after esophagectomy for cancer.Dis Esophagus 2011;24:100-7.
6.Qin J,Li Y,Zhang R,et al.Treatment of esophagogastric anastomotic leak with perianastomotic drain.J Thorac Oncol 2010;5:251-3.
7.Wang CG,Ye YX,Lou XB,et al.Experience of management of intrathoracic anastomotic leak after surgery for esophageal carcinoma (9 cases report).Chin J Gastrointest Surg 2007;10:318.
8.Alanezi K,Urschel JD.Mortality secondary to esophageal anastomotic leak.Ann Thorac Cardiovasc Surg 2004;10:71-5.
9.Zhang ZY,Yang YP.Treatment outcome analysis of intrathoracic anastomotic leak after surgery for esophageal carcinoma.Chin Clin Oncol 2007;12:454-5.
10.Crestanello JA,Deschamps C,Cassivi SD,et al.Selective management of intrathoracic anastomotic leak after esophagectomy.J Thorac Cardiovasc Surg 2005;129:254-60.
11.Freeman RK,Ascioti AJ,Wozniak TC.Postoperative esophageal leak management with the Polyflex esophageal stent.J Thorac Cardiovasc Surg 2007;133:333-8.
12.Leers JM,Vivaldi C,Schäfer H,et al.Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent.Surg Endosc 2009;23:2258-62.
13.Han XW,Li YD,Wu G,et al.New covered mushroom-shaped metallic stent for managing anastomotic leak after esophagogastrostomy with a wide gastric tube.Ann Thorac Surg 2006;82:702-6.
14.Freeman RK,Van Woerkom JM,Ascioti AJ.Esophageal stent placement for the treatment of iatrogenic intrathoracic esophageal perforation.Ann Thorac Surg 2007;83:2003-7.
15.Hünerbein M,Stroszczynski C,Moesta KT,et al.Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents.Ann Surg 2004;240:801-7.
16.Hofstetter W,Swisher SG,Correa AM,et al.Treatment outcomes of resected esophageal cancer.Ann Surg 2002;236:376-84.
17.DeMeester TR.Perforation of the esophagus.Ann Thorac Surg 1986;42:231-2.
 Chinese Medical Sciences Journal2012年1期
Chinese Medical Sciences Journal2012年1期
- Chinese Medical Sciences Journal的其它文章
- Management of Pregnancy with Ankylosing Spondylitis
- Applicability of Community Periodontal Index Teeth and Random Half-mouth Examination to Gingival Bleeding Assessment in Untreated Adult Population in Beijing
- Spectral Domain Optical Coherence Tomography of Vogt-Koyanagi-Harada Disease:Novel Findings and New Insights into the Pathogenesis
- Radiofrequency Ablation of Hepatic Paragonimiasis:a Case Report
- Electrocorticography with Direct Cortical Stimulation for a Left Temporal Glioma with Intractable Epilepsy
- Primary Sjögren's Syndrome Accompanied by Intestinal Obstruction:a Case Report and Literature Review
