A Combined Clinicopathologic Analysis of 658 Urothelial Carcinoma Cases of Urinary Bladder
Hui-zhi Zhang,Chao-fu Wang*,Juan-juan Sun,and Bao-hua Yu
Department of Pathology,Fudan University Shanghai Cancer Center;Department of Oncology,Shanghai Medical College,Fudan University,Shanghai 200032,China
BLADDER cancer is the second most common malignancy encountered by urologists.1It is a significant cause of cancer morbidity and mortality.The incidence and pattern of this disease has geographical variation.In addition,age,gender,and racial factors all affect the survival and prognosis of patients with bladder carcinoma.2However,the prognostic importance of various factors varies from one publication to another,3including age,gender,tumor size,number of tumor,grade,pathological stage,carcinoma in situ,surgical margin,vascular invasion,and lymph node status.1-7
The accurate prediction of clinical outcome is necessary for appropriate management of patients with urothelial carcinoma of the bladder (UCB).However,there were little relevant reports from China,especially with large samples.In the present study,we reviewed the clinicopathological features of 658 patients with UCB to detect the possible prognostic factors associated with recurrence and progression of bladder cancer.
PATIENTS AND METHODS
Patients
658 UCB patients who underwent bladder tumor surgery in Fudan University Shanghai Cancer Center from January 2006 to December 2010 were included.The clinical information and pathological data of the patients were acquired from the hospital database involving age (≤55 years or >55 years),gender,pathological stage,grade,and tumor growth pattern (solid or papillary).To minimize interobserver variability,all histopathologic materials from the tumors were reviewed by 2 senior pathologists.Staging and grading of each tumor were performed according to the international tumor-node-metastasis grading system and the World Health Organization 2004 grading system,respectively.While analyzing the recurrence rate and the progression rate,25 patients lacking follow-up information were excluded.Recurrence was defined as the reappearance of histopathologically confirmed UCB.Relapse within 3 months of primary diagnosis were excluded,considering the possibility of residual tumor.Progression was defined as an advance in stage or metastasis.
Statistical analysis
SPSS 17.0 software was used for statistical anaylsis.Univariate and multivariate analyses were adopted to assess the risk factors for tumor recurrence and progression.P<0.05 was considered statistically significant.
RESULTS
Clinicopathological characteristics
In terms of gender feature,83.3% (548/658) of the patients were male,with male to female ratio being about 5∶1.The mean age was 61.97±12.97 years (range,20-90 years).Most patients were between 50-70 years of age (Fig.1).The most frequent complaint was hematuria,recorded in 76.0% (500/658) of the patients,mainly painless hematuria.At initial diagnosis,49.6% (326/658) of the patients had stage Ta tumors,and 29.0% (191/658) had stage T1 tumors.Muscle invasive urothelial carcinomas were presented in 84 patients (12.7%),including T2 in 56 patients (8.5%),T3 in 12 patients (1.8%),and T4 in 16 patients (2.4%).Based on the differentiation of neoplasm cells,the tumors were divided into two categories:low and high grade tumors.The percentages of low-grade and high-grade tumors were 49.9% and 50.1%,respectively.
Microscopically,based on the tumor growth pattern,the tumors were classified into papillary and non-papillary(solid) (Fig.2).The papillary growth pattern was demonstrated as an exophytic tumor with a papillary stalk lined by dysplasia urothelium,while the solid growth pattern as an endophytic tumor composed of diffusely growing sheets of tumor cells without fibrovascular core formation.69.6%(458/658) of the tumors in this study had papillary growth pattern.Most (77.6%) of the superficial (Ta/T1) UCB were papillary,and more than half of them (56.3%) were low grade,while muscle invasive UCB were mostly solid(82.1%) and high grade (95.2%).The clinicopathological characteristics of 658 patients with UCB at diagnosis are presented in Table 1.
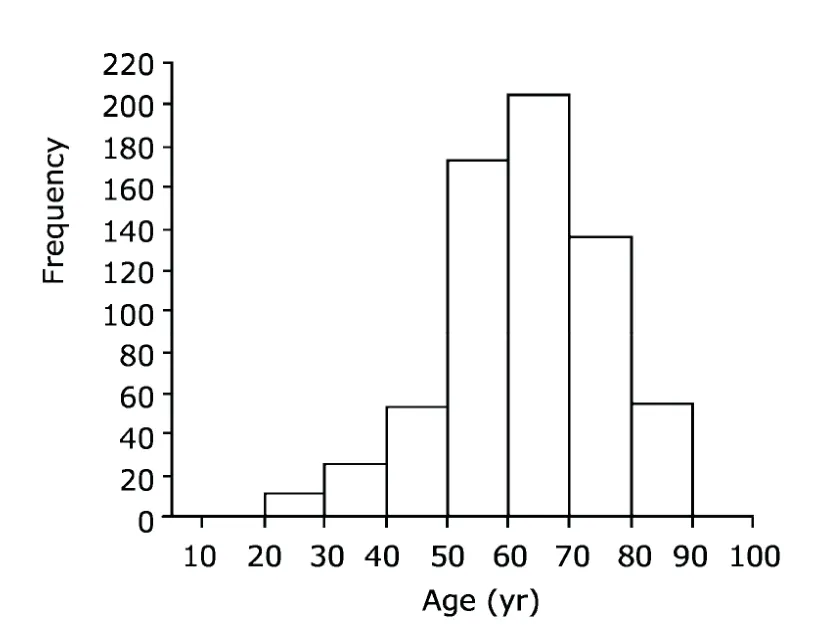
Figure 1.The age distribution of the 658 patients with urothelial carcinoma of the bladder (UCB).
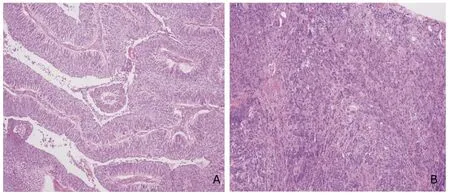
Figure 2.High-grade invasive papillary UCB (A) and high-grade invasive solid UCB (B).HE ×100.
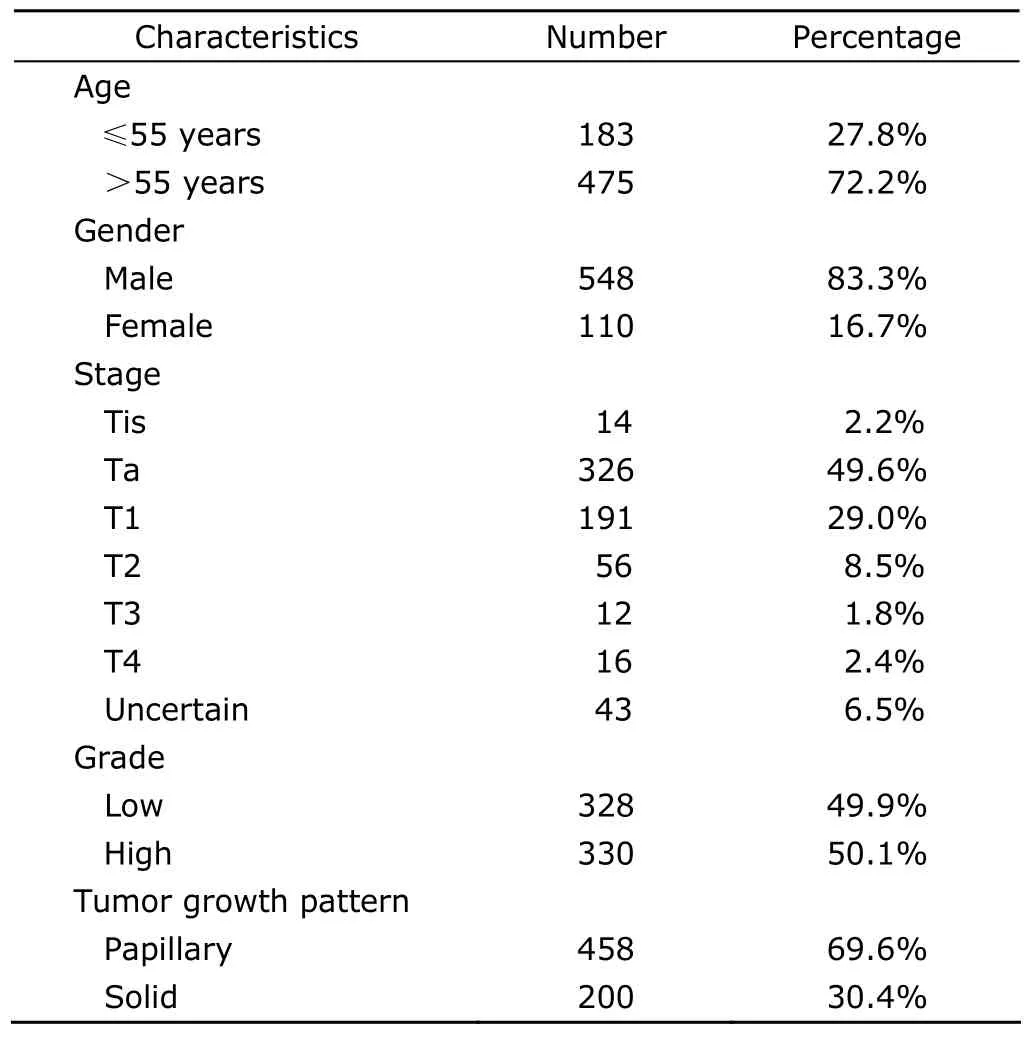
Table 1.Clinicopathological characteristics of 658 patients with UCB
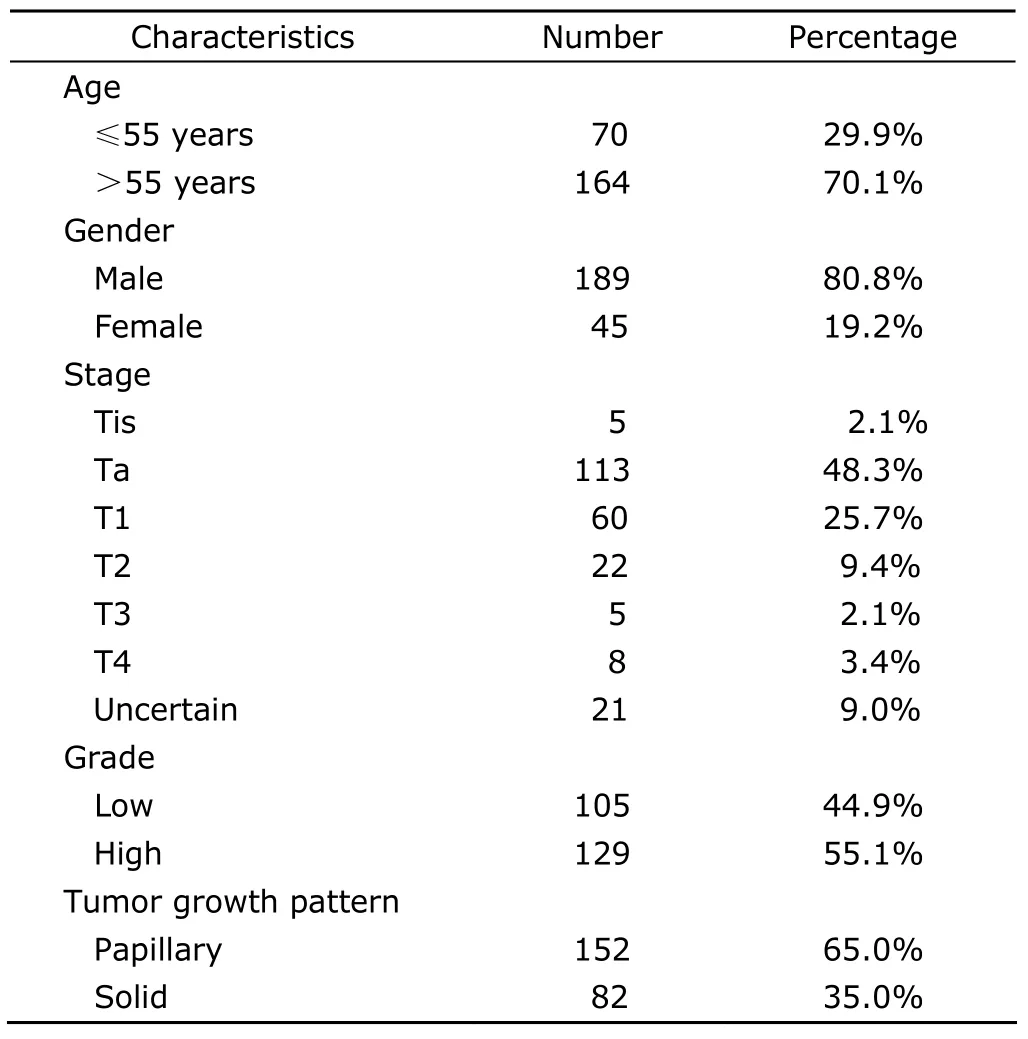
Table 2.Clinicopathological characteristics of 234 recurrent patients
Recurrence and progression
After a follow-up period of 22.36±24.92 months,recurrence was observed in 234 patients (37.0%,Table 2).Progression was seen in 39 patients (6.2%,Table 3).The most common extranodal site of metastases was bones(56.4%),followed by lungs (38.5%),livers (23.1%),pelvics (10.3%),and penis (7.7%).Nine patients had multiple metastases.
Association with recurrence and progression
According to univariate analysis,gender,age,grade,tumor growth pattern,and pathological stage were not associated with tumor recurrence,but multivariate analysis showed association between stage and recurrence(P<0.05,Table 4).Gender,grade,tumor growth pattern,and pathological stage were associated with tumor progression in univariate analysis (P<0.05);but in multivariate analysis,gender was not associated with progression (Table 4).
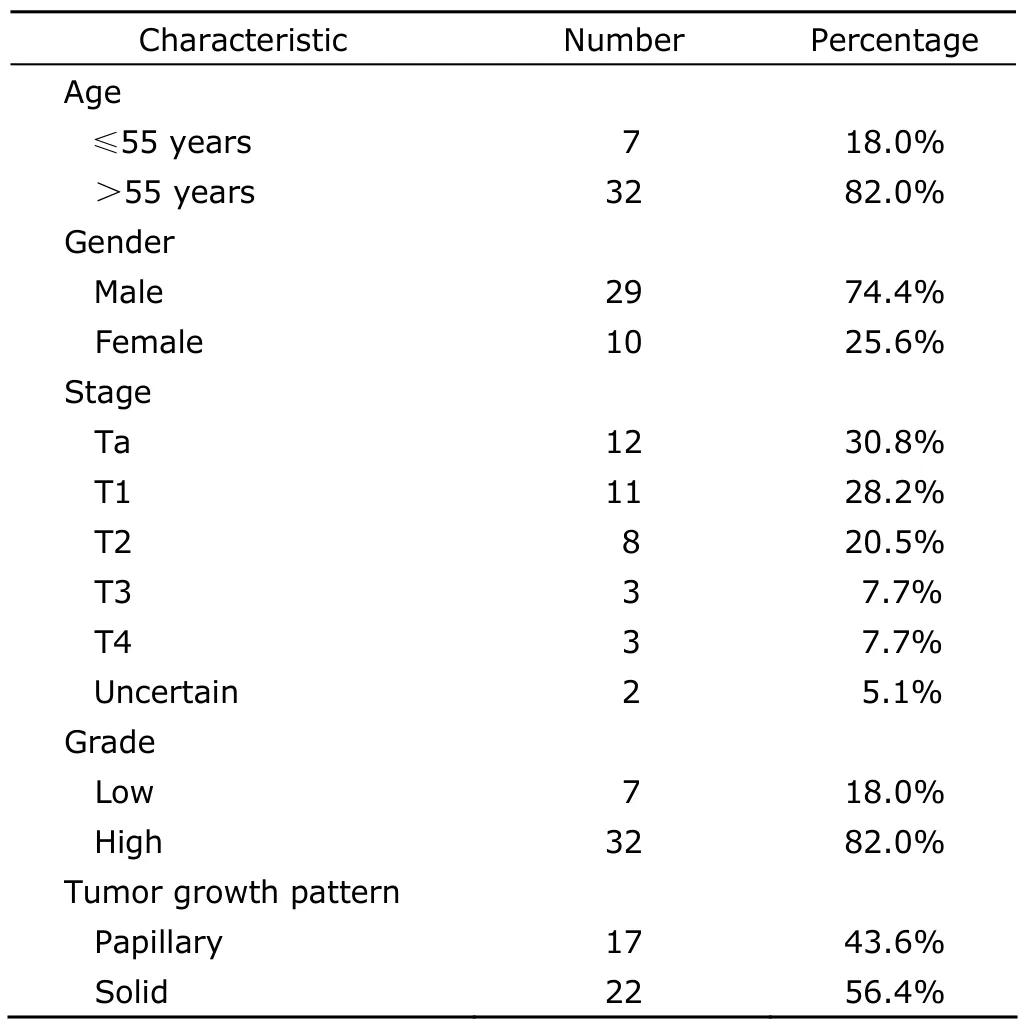
Table 3.Clinicopathological features of 39 progressive patients
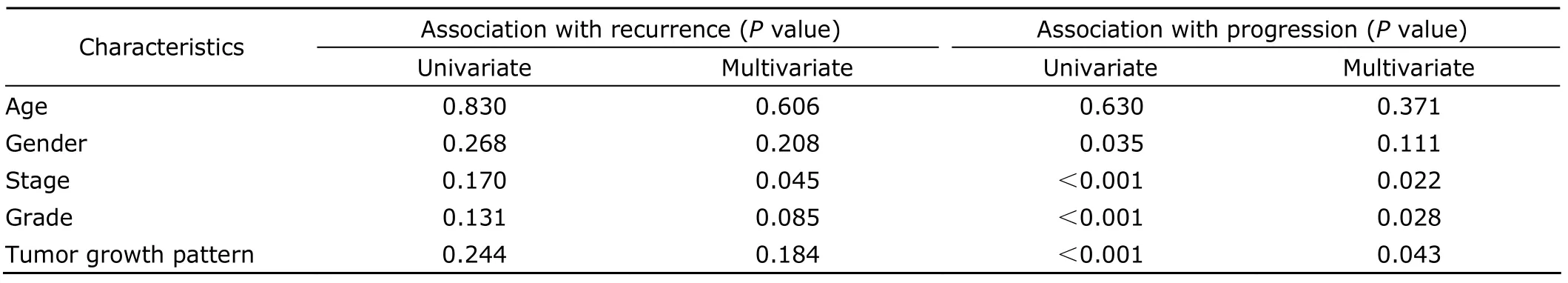
Table 4.The association of clinicopathological characteristics with tumor recurrence and progression detected by univariate and multivariate analyses
DISCUSSION
Some risk factors that are possibly associated with the recurrence and progression of UCB have been studied worldwide.In general,high grade,solid tumor growth pattern,and late pT stage were found to be risk factors closely associated with the unfavorable prognosis of UCB.6,8-10In the present study,we analyzed the possible risk factors associated with the recurrence and progression of UCB based on the clinicopathological features of 658 UCB patients,and compared our findings with previous reports.
de Vere White et al8reported that approximately 80%of the patients with newly diagnosed bladder carcinomas presented with superficial diseases (Ta/T1),about 20% of which were stage T1.Matalka et al9conducted a clinicopathological study with 115 UCB patients,the results of which revealed that 54.5% of the cases were stage pTa,17.3% were pT1,20% were pT2,7.3% were pT3,and 0.9% were pT4.The percentages of Ta/T1 patients in the above two reports were similar with those in the present study.However,in the study by Rafique et al10on the clinicopathological features of 221 bladder carcinoma patients in Pakistan,the majority (98%) showed invasion of lamina propria,and over 60% of the patients had muscle invasive diseases at the time of diagnosis.The difference might be a result of the patients not seeing a doctor in time.
Messing et al11reported that about 55%-60% of the newly diagnosed superficial UCB are of low histological grade,but majority of the patients with muscle invasive diseases have high histological grade.Pan et al12studied 1515 cases of non-muscle invasive urothelial tumors,concluding that 60.4% of the cases were low grade.Similarly,in the present study,56.3% of the superficial tumors were of low grade while 95.2% of the muscle invasive tumors were of high grade.
Andius et al6found that tumor growth pattern had independent prognostic value for predicting progression.They reported that solid tumor growth pattern was associated with an increased risk of progression in stage T1 patients in univariate and multivariate analyses.Moreover,Kikuchi et al13found that the presence or absence of tumor stalk was a significant predictor for tumor recurrence.Gofrit et al14showed that endophytic growth pattern was an independent prognostic factor of stage progression and disease-specific mortality.The finding of the present study also confirmed that the solid tumor growth pattern was a risk factor for tumor progression.
Research about the metastatic pattern of bladder cancer is relatively rare.Kishi et al15investgated 87 autopsy cases of bladder carcinoma,and metastasis was noted in 58 cases (66.7%),frequently in lymph nodes(37.9%),livers (29.9%),lungs (29.9%),and bones(24.1%).Shinaqare et al16found that the most common metastatic site was lymph nodes (69%),followed by bones(47%),lungs (37%),livers (26%),and peritoneums(16%).Metastatic sites in the present study are similar to the report by Shinaqare et al,but different from Kishi et al’s,possibly due to racial difference.
The prognostic significance of gender in bladder cancer is controversial.A retrospective study with 1131 bladder caner patients in Japan showed that gender was an independent risk factor for survival,in which female patients had a worse prognosis.17Thieblemont et al18reached a similar result after a study with 158 invasive urothelial bladder carcinoma patients.Mungan et al19evaluated gender differences in bladder cancer prognosis,finding that the survival in women remained worse than in men after adjusting for disease stage at presentation.Nevertheless,some studies did not find a significant association between gender and disease recurrence.20,21In the present study,gender was associated with progression in univariate analysis,with female patients having a worse prognosis;but not associated in multivariate analysis.The prognostic significance of gender would require analysis with larger samples,and the possible reasons underlying the worse outcome in women require further study.
Other factors may also be associated with prognosis of bladder cancer.One study showed that tumors greater than 5 cm had a 35% progression rate,compared with a 9% rate for smaller tumors.22In another study,tumor size greater than 3 cm was found as an independent risk factor for tumor recurrence in multivariate analysis.13The number of tumors is also important in predicting recurrence.In one study by Pan et al,12patients with multiple tumors showed significantly higher recurrence rate compared with those with single tumors (47.1%vs.26.0%,P<0.0001).Recurrence rate in patients with single tumors range from 18% to 60%,while the rate in patients with multiple tumors is 40%-90%.23
The prognostic factors studied in different publications vary.Kikuchi et al13concluded that the presence or absence of tumor stalk,tumor multiplicity,tumor size,pathological stage,and grade were significant predictors of tumor recurrence.Pan et al12concluded that tumor grade,pT stage,patient age,and multiplicity were significant risk factors for recurrence in univariate analysis;tumor grade,pT,and patient age were significant prognostic factors for progression in univariate analysis and stepwise multivariate regression analysis.Lipponen et al24showed that T category,N category,and M category were the most important clinical prognostic factors,followed by the age of the patient.In the present study,the pT stage was associated with tumor recurrence on multivariate analysis.Grade,tumor growth pattern,and pathological stage were significant prognostic factors for progression in univariate analysis and multivariate analysis;gender was associated with tumor progression in univariate analysis.
This study has its limitations.Foremost is the retrospective nature of the study.Another limitation is that this study did not cover some possibly important factors,such as the number and size of tumor,due to the lack of information in the reviewed medical records.
1.Yan Y,Andriole GL,Humphrey PA,et al.Patterns of multiple recurrences of superficial (Ta/T1) transitional cell carcinoma of bladder and effects of clinicopathologic and biochemical factors.Cancer 2002;95:1239-46.
2.Madeb R,Messing EM.Gender,racial and age differences in bladder cancer incidence and mortality.Urol Oncol 2004;22:86-92.
3.Millán-Rodríguez F,Chéchile-Toniolo G,Salvador-Bayarri J,et al.Multivariate analysis of the prognostic factors of primary superficial bladder cancer.J Urol 2000;163:73-8.
4.Cheng L,Weaver AL,Leibovich BC,et al.Predicting the survival of bladder carcinoma patients treated with radical cystectomy.Cancer 2000;88:2326-32.
5.Proctor I,Stoeber K,Williams GH.Biomarkers in bladder cancer.Histopathology 2010;57:1-13.
6.Andius P,Johansson SL,Holmäng S.Prognostic factors in stage T1 bladder cancer:tumor pattern (solid or papillary)and vascular invasion more important than depth of invasion.Urology 2007;70:758-62.
7.van Rhijn BW,Burqer M,Lotan Y,et al.Recurrence and progression of disease in non-muscle-invasive bladder cancer:from epidemiology to treatment strategy.Eur Urol 2009;56:430-42.
8.de Vere White RW,Stapp E.Predicting prognosis in patients with superficial bladder cancer.Oncology (Williston Park) 1998;12:1717-23.
9.Matalka I,Bani-Hani K,Shotar A,et al.Transitional cell carcinoma of the urinary bladder:a clinicopathological study.Singapore Med J 2008;49:790-4.
10.Rafique M,Javed AA.Clinico-pathological features of bladder carcinoma:experience from a tertiary care hospital of Pakistan.Int Urol Nephrol 2006;38:247-50.
11.Messing EM,Young TB,Hunt VB,et al.Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations.Urology 1995;45:387-96.
12.Pan CC,Chang YH,Chen KK,et al.Prognostic significance of the 2004 WHO/ISUP classification for prediction of recurrence,progression,and cancer-specific mortality of non-muscle-invasive urothelial tumors of the urinary bladder:a clinicopathologic study of 1,515 cases.Am J Clin Pathol 2010;133:788-95.
13.Kikuchi E,Fujimoto H,Mizutani Y,et al.Clinical outcome of tumor recurrence for Ta,T1 non-muscle invasive bladder cancer from the data on registered bladder cancer patients in Japan:1999-2001 report from the Japanese Urological Association.Int J Urol 2009;16:279-86.
14.Gofrit ON,Shapiro A,Pode D,et al.Subepithelial growth patterns in urothelial carcinoma-frequency and prognostic significance.Urol Oncol 2012;30:49-54.
15.Kishi K,Hirota T,Matsumoto K,et al.Carcinoma of the bladder:a clinical and pathological analysis of 87 autopsy cases.J Urol 1981;125:36-9.
16.Shinagare AB,Ramaiya NH,Jaqannathan JP,et al.Metastatic pattern of bladder cancer:correlation with the characteristics of the primary tumor.AJR Am J Roentgenol 2011;196:117-22.
17.Nishiyama H,Habuchi T,Watanabe J,et al.Clinical outcome of a large-scale multi-institutional retrospective study for locally advanced bladder cancer:a survey including 1131 patients treated during 1990-2000 in Japan.Eur Urol 2004;45:176-81.
18.Thieblemont C,Fendler JP,Trillet-Lenoir V,et al.Prognostic factors of survival in infiltrating urothelial bladder carcinoma.a retrospective study of 158 patients treated by radical cystectomy.Bull Cancer 1996;83:139-46.
19.Mungan NA,Aben KK,Schoenberg MP,et al.Gender differences in stage-adjusted bladder cancer survival.Urology 2000;55:876-80.
20.Konety BR,Joslyn SA.Factors influencing aggressive therapy for bladder cancer:an analysis of data from the SEER program.J Urol 2003;170:1765-71.
21.Honma I,Masumori N,Sato E,et al.Local recurrence after radical cystectomy for invasive bladder cancer:an analysis of predictive factors.Urology 2004;64:744-8.
22.Heney NM,Ahmed S,Flanagan MJ,et al.Superficial bladder cancer:progression and recurrence.J Urol 1983;130:1083-6.
23.Herr HW.Natural history of superficial bladder tumors:10-to 20-year follow-up of treated patients.World J Urol 1997;15:84-8.
24.Lipponen PK,Eskelinen M,Jauhiainen K,et al.Clinical prognostic factors in transitional cell cancer of the bladder.Uro Int 1993;50:192-7.
 Chinese Medical Sciences Journal2012年1期
Chinese Medical Sciences Journal2012年1期
- Chinese Medical Sciences Journal的其它文章
- Management of Pregnancy with Ankylosing Spondylitis
- Applicability of Community Periodontal Index Teeth and Random Half-mouth Examination to Gingival Bleeding Assessment in Untreated Adult Population in Beijing
- Personalized Management of Anastomotic Leak after Surgery for Esophageal Carcinoma
- Spectral Domain Optical Coherence Tomography of Vogt-Koyanagi-Harada Disease:Novel Findings and New Insights into the Pathogenesis
- Radiofrequency Ablation of Hepatic Paragonimiasis:a Case Report
- Electrocorticography with Direct Cortical Stimulation for a Left Temporal Glioma with Intractable Epilepsy
