Spatio-temporal Expression Study of Phosphorylated 70-kDa Ribosomal S6 Kinase (p70S6k) in Mesial Temporal Lobe Epilepsy△
Xiao-liang Xing,Long-ze Sha,Yuan Yao,Yan Shen,Li-wen Wu*,and Qi Xu*
1National Laboratory of Medical Molecular Biology,Institute of Basic Medical Sciences,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100005,China
2Department of Neurology,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100730,China
EPILEPSY affects almost 1% people around the world,and mesial temporal lobe epilepsy (mTLE)is the most common form in adult.1Majority of these patients have seizures that can not be well controlled with classical antiepilepsy drugs,2except by the surgical resection of sclerous hippocampus.3Thus,it is very important to investigate the process of epileptogenesis,and develop an effective strategy for treatment.In recent years,the focus of related researches has been shifted from the ion channel to intracellular molecules that may contribute to mTLE.4
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that regulates cell growth,proliferation,survival,differentiation,and homeostasis.5-7In nervous system,mTOR can regulate the neuron development,neurite outgrowth,and synaptic plasticity.8,9Recent studies using the model of mTLE suggest that overactivition of mTOR signaling pathway may contribute to epileptogenesis.10,11In the model mice,inhibition of mTOR suppresses dentate granule cell axon sprouting.10Rapamycin administered prior to kainite can decease kainite-induced neuron death,neurogenesis,mossy fiber sprouting,development of spontaneous epilepsy,and epilepsy-related hypertrophy.12The mTOR pathway is certainly involved in temporal lobe epileptogenesis.Obtaining insight into the downstream pathway of mTOR,and its spatio-temporal pattern in the hippocampus will increase our understanding of the mechanism and the process of mTLE.The present study addressed this topic by evaluating the expression and distribution of phosphorylated 70-kDa ribosomal S6 kinase (p70S6k),a downstream molecule of mTOR,with immunohistochemical assays.
MATERALS AND METHODS
Experimental animals and kainite acid (KA)-induced mouse model of mTLE
Adult male C57BL/6 mice (n=18,Beijing Vitalrive Co.,Ltd)of 8-week and weighing 20-24 g were treated in accordance with the institutional guidelines of the Beijing Administration Office of Laboratory Animals and acclimated for 2 weeks before experiments.The use of mice in the present study was approved by Ethics Committee of Chinese Academy of Medical Sciences and Peking Union Medical College.
The mice were first anesthetized by intraperitioneal injection of 10% chloral hydrate solution (5 mL/kg),and stereotaxically unilateral injection of 200 ng KA (Sigma,Shanghai,China) in 50 nL saline into the right hippocampus (anteroposterior=-2.0 mm,mediolateral=-1.8 mm,dorsoventral=-2.3 mm).The scalps were sutured up.All efforts were made to minimize animal suffering and reduce the mortality of model mice.We selected two time points after the KA injection (5 days,n=5;5 weeks,n=8)to represent latent and chronic phases of epileptogenesis,respectively.At least 5 KA-injected mice were sacrificed at each time point for immunohistochemical evaluation.Control mice (n=5) were injected with saline alone prior to sacrifice.
Immunohistochemical assays
Mouse hippocampus tissues were incubated for at least 48 hours in 4% paraformaldehyde (Sigma) and embedded in paraffin.Paraffin-embedded tissues were sectioned into 2-μm slices for immunohistochemical study.Sections with stereotypical hippocampus structure were used for immunohistochemistry.p70S6k antibody (1∶200 in mouse section,Cell Signaling Technology,Danvers,MA,USA,#2708) was used.After incubation with the primary antibody at 4°C overnight,the tissues were treated with polymer helper (Golden Bridge,Beijing,China) for 20 minutes,and then treated with poly peroxidase-anti-goat IgG (Golden Bridge) for 20 minutes.Subsequently,the tissues were incubated with diaminobenzidine Horseradish Peroxidase Color Development Kit (Golden Bridge) for 2 minutes.Counterstaining was conducted with hematoxylin(Golden Bridge).After each incubation step,the sections were washed with Tris-buffered saline with Tween for 3 times,5 minutes for each time.
RESULTS
Two mice died in the chronic phase after KA injection.The mortality of model mice was 11%.
Control mouse hippocampus (n=5) displayed p70S6k immunoreactivity (IR) in neuron of dentate gyrus (DG) and Cornu Ammonis (CA) (Fig.1A-C).In the 5-day model mice(n=5),increased p70S6k IR was observed in the ipsilateral DG compared with control mice (Fig.1D).But p70S6k IR was absent in CA,and neuron death is indicated by nuclear pyknosis (Fig.1E,F)
In the 5-week model mice (n=6),intensified p70S6k IR was observed in both ipsilateral DG and contralateral DG (Fig.2A,B).In addition,p70S6k IR in ipsilateral DG was higher in 5-week model mice than in 5-day model mice (Fig.2A).Granule cells in the contralateral hippocampus displayed dispersion to a certain extent (Fig.2C).In 5-week model mice,the granule cells in the ipsilateral hippocampus were found larger than those in the contralateral hippocampus,and the shape turned to spindle-like (Fig.2C).

Figure 1.Distribution of p70S6k immunoreactivity (IR) in the hippocampus of saline-injected control mice (n=5) and 5-day kainite acid(KA)-induced mesial temporal lobe epilepsy (mTLE) model mice (n=5).hematoxylin ×40
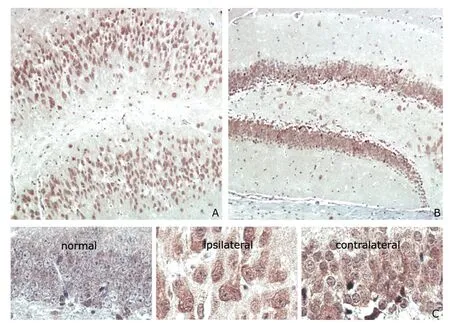
Figure 2.Distribution of p70S6k IR in the hippocampus of 5-week mTLE model mice (n=6).hematoxylin ×400
DISCUSSION
Increasing evidence suggested that mTOR signaling pathway may be involved in the pathogenesis of temporal lobe epilepsy.13Hippocampus sclerosis,such as neuron loss,granule cells dispersion,astroglia hyperplasia,is the hallmark pathological feature of mTLE.14In this study,we investigated the spatio-temporal distribution of p70S6k in the mouse model of KA-induced mTLE.The activation of p70S6k was found intensified in the dispersed granule cells of DG in the chronic phase model mice.And the dispersed granule cells with intensified p70S6k IR in the ipsilateral hippocampus of 5-week model mice are larger than the granule cells in the control mice.Progressive p70S6k IR in granule cells of DG in model mice was demonstrated by the immunohistochemical assays in the control,latent phase mice (5 days),and chronic phase mice (5 weeks).Ribosomal protein S6 (S6),a downstream molecule of p70S6k,can regulate cell growth.15It has been reported that rapamycin suppresses DG hypertrophy,16which is a hallmark of mTLE.17,18According to the results,p70S6k activation may be associated with cell size.In the model mice,the granule cells hypertrophy maybe caused by excessive phosphorylation of S6 controlled by p70S6k IR.Further investigations are needed to confirm whether the observed up-regulation of p70S6k IR in the mTLE mouse model also occurs in mTLE patients with hippocampal sclerosis (HS),whether the observed change in cell size and morphology in the model mice could be found in mTLE patients with HS as well,and whether the over-activation of p70S6k in granule cells contributes to other aspects,such as the excitatory neural circuitry.
1.Schuele SU,Lüders HO.Intractable epilepsy:management and therapeutic alternatives.Lancet Neurol 2008;7:514-24.
2.Kwan P,Schachter SC,Brodie MJ.Drug-resistant epilepsy.N Engl J Med 2011;365:919-26.
3.Abou-Khalil B,Andermann E,Andermann F,et al.Temporal lobe epilepsy after prolonged febrile convulsions:excellent outcome after surgical treatment.Epilepsia 1993;34:878-83.
4.Okamoto OK,Janjoppi L,Bonone FM,et al.Whole transcriptome analysis of the hippocampus:toward a molecular portrait of epileptogenesis.BMC Genomics 2010;11:230.
5.Hay N,Sonenberg N.Upstream and downstream of mTOR.Genes Dev 2004;18:1926-45.
6.Beevers CS,Li F,Liu L,et al.Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells.Int J Cancer 2006;119:757-64.
7.Laplante M,Sabatini DM.mTOR signaling at a glance.J Cell Sci 2009;122:3589-94.
8.Choi Y J,Di Nardo A,Kramvis I,et al.Tuberous sclerosis complex proteins control axon formation.Genes Dev 2008;22:2485-95.
9.Morita T,Sobue K.Specification of neuronal polarity regulated by local translation of CRMP2 and Tauviathe mTOR-p70S6K pathway.J Biol Chem 2009;284:27734-45.
10.Buckmaster PS,Ingram EA,Wen X.Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy.J Neurosci 2009;29:8259-69.
11.Zeng LH,Rensing NR,Wong M.Developing antiepileptogenic drugs for acquired epilepsy:targeting the mammalian target of rapamycin (mTOR) pathway.Mol Cell Pharmacol 2009;1:124-9.
12.Zeng LH,Rensing NR,Wong M.The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy.J Neurosci 2009;29:6964-72.
13.McDaniel SS,Rensing NR,Thio LL,et al.The ketogenic diet inhibits the mammalian target of rapamycin (mTOR)pathway.Epilepsia 2011;52:e7-11.
14.Van Paesschen W.Qualitative and quantitative imaging of the hippocampus in mesial temporal lobe epilepsy with hippocampal sclerosis.Neuroimaging Clin N Am 2004;14:373-400,vii.
15.Volarević S,Thomas G.Role of S6 phosphorylation and S6 kinase in cell growth.Prog Nucleic Acid Res Mol Biol 2001;65:101-27.
16.Buckmaster PS,Wen X.Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy.Epilepsia 2011;52:2057-64.
17.Houser CR.Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy.Brain Res 1990;535:195-204.
18.Lurton D,El Bahh B,Sundstrom L,et al.Granule cell dispersion is correlated with early epileptic events in human temporal lobe epilepsy.J Neurol Sci 1998;154:133-6.
 Chinese Medical Sciences Journal2012年1期
Chinese Medical Sciences Journal2012年1期
- Chinese Medical Sciences Journal的其它文章
- Screening of Substrates of Protein Arginine Methyltransferase 1 in Glioma△
- The Inhibitory Effects of Arresten Protein on Tumor Formation△
- A Combined Clinicopathologic Analysis of 658 Urothelial Carcinoma Cases of Urinary Bladder
- Primary Sjögren's Syndrome Accompanied by Intestinal Obstruction:a Case Report and Literature Review
- Electrocorticography with Direct Cortical Stimulation for a Left Temporal Glioma with Intractable Epilepsy
- Radiofrequency Ablation of Hepatic Paragonimiasis:a Case Report
