PHBHHx膜表面亲水改性及其与神经干细胞生物相容性
吕海侠,杨志倩,卢晓云,李明川,焦倩,陈新林,王媛媛,张雅利
1 西安交通大学医学院神经生物学研究所,陕西 西安 710061
2 西安交通大学生命科学与技术学院 生物医学信息工程教育部重点实验室,陕西 西安 710049
组织工程与细胞培养
PHBHHx膜表面亲水改性及其与神经干细胞生物相容性
吕海侠1,杨志倩2,卢晓云2,李明川2,焦倩1,陈新林1,王媛媛1,张雅利2
1 西安交通大学医学院神经生物学研究所,陕西 西安 710061
2 西安交通大学生命科学与技术学院 生物医学信息工程教育部重点实验室,陕西 西安 710049
对羟基丁酸-羟基己酸共聚酯 (PHBHHx) 膜进行表面改性,研究神经干细胞 (NSCs) 在改性后的PHBHHx膜表面的贴附、增殖及分化情况,为开发新型脑组织工程支架材料奠定基础。采用溶剂挥发法制备PHBHHx膜,扫描电镜观察其表面性状;分别通过脂肪酶处理,NaOH处理的方法对PHBHHx膜进行表面改性,测量接触角以检测膜表面亲水性。分离培养孕 14.5 d大鼠胚胎大脑皮质 NSCs,接种在表面改性后的PHBHHx膜表面进行体外培养,扫描电镜观察膜表面细胞形态,MTT法检测细胞活力,免疫细胞化学染色观察NSCs存活和分化情况。结果显示,与未处理的PHBHHx膜相比,脂肪酶、NaOH处理能够显著提高PHBHHx膜表面亲水性,增加NSCs在PHBHHx膜表面贴附数量;NSCs在改性后的PHBHHx膜表面能够良好地存活并分化为神经元和胶质细胞。结果提示PHBHHx膜表面碱处理通过提高材料表面亲水性和粗糙程度,增加其与NSCs的生物相容性,改性后的PHBHHx材料是一种非常有潜力的新型脑组织工程支架材料,有望在NSCs移植修复脑损伤中发挥作用。
羟基丁酸-羟基己酸共聚酯,神经干细胞,表面改性,生物相容性
Abstract:To study the attachment, proliferation and differenciation of neural stem cells (NSCs) on surface modified PHBHHx films and to establish the theory of PHBHHx application in NSCs-based brain tissue engineering. PHBHHx film was fabricated by a solution-casting method, and the morphology of the fi lm was observed under scanning electron microscopy(SEM). The films were treated by NaOH or lipase, then the surface hydrophilic property was characterized using water contact angle measurement. NSCs were isolated from the cerebral cortex of rat embryos on embryonic day 14.5, and cultured on surface treated PHBHHx films. The morphology of NSCs attached on the film was visualized under SEM, and the survival and differentiation of NSCs were observed through immunocytochemical staining. Compared with the untreated PHBHHx films, the water contact angle of NaOH or lipase treated PHBHHx films decreased dramatically, and the number of NSCs attached significantly increased. NSCs survived well on treated PHBHHx films and differentiated into neurons and glial cells. The amelioration of hydrophilic property of PHBHHx film improved its biocompatibility with NSCs. PHBHHx can serve as a novel CNS tissue engineering biomaterial applied for NSCs transplantation, brain repairing and regeneration.
Keywords:PHBHHx, neural stem cells, surface treatment, biocompatibility
神经干细胞 (NSCs) 是具有自我更新和多向分化潜能的细胞,它能不断增殖,并分化为神经系统主要的细胞类型 (神经元、星形胶质细胞和少突胶质细胞)[1]。NSCs移植已成为目前促进神经损伤修复最有潜力的手段[2-4]。然而,单纯的NSCs移植效果并不理想[5-7]。因此,有需要、有必要在深入了解NSCs特性以及对于脑组织的修复作用的同时,积极寻求能够促进NSCs在移植部位更好地存活并向损伤修复所需的细胞分化的策略。目前基于NSCs修饰[8-10]、NSCs体外定向诱导分化后移植[11]、混合细胞共移植[12]以及干细胞-生物材料共移植[13-14]等的一些尝试取得了比较明显的效果,在一定程度上提高了移植细胞在移植部位存活及分化能力。特别是随着组织工程技术的快速发展,以生物可降解材料为支架进行神经细胞培养的脑组织工程及其在脑组织损伤修复和神经系统重建中的应用成为一新兴的前沿领域[15-17]。新型脑组织工程支架材料的开发及生物相容性研究成为这一领域的研究热点之一。
聚羟基脂肪酸酯 (PHA) 是一类可由微生物合成的具有良好生物相容性的天然生物可降解高分子材料[18-19],羟基丁酸-羟基己酸共聚酯(poly (3-hydroxybutyrate-co-3-hydroxhexanoate),PHBHHx) 是PHA家族中的一员[20-21],在生物相容性、生物活性及机械性能方面的性能优良,与多种细胞都具有良好的生物相容性[22-25],是近年来在组织工程中被广泛关注的新型生物材料[19,26]。PHBHHx作为外周神经修复支架材料和NSCs体外培养支架材料的研究已有报道[27-28]。但由于PHBHHx的聚酯性质,其表面疏水性强,阻碍了细胞在其表面的贴附生长,因此也局限了其应用范围[29]。有学者通过采用紫外辐照、丝素蛋白修饰等方法对 PHBHHx材料进行改性处理[30-32],提高了 PHBHHx材料与成纤维细胞、血管内皮细胞等的生物相容性。本研究进行了 PHBHHx膜材料表面改性条件的摸索,并研究了改性后PHBHHx膜与NSCs的生物相容性,为优化基于 PHA材料的新型脑组织工程支架奠定实验基础。
1 材料与方法
1.1 PHBHHx膜的制备及表面改性
1.1.1 PHBHHx膜的制备
溶剂挥发法制备PHBHHx膜[33]。15 mL氯仿溶解0.5 g PHBHHx,60 ℃水浴助溶1 h。通风橱中,将液体倾入9 cm玻璃培养皿中,室温下使氯仿挥发1~2 d,待膜形成后置于4 ℃老化2周。
1.1.2 PHBHHx膜的表面改性及处理
经老化后的PHBHHx膜用于表面改性处理,各组的处理方式分别为:对照组 (Control) 不做任何处理;碱处理组 (A),用2 mol/L NaOH室温处理1 h[29];酶处理组 (L),脂肪酶 (L3126,sigma) 37 ℃处理 24 h[34];酶+碱处理组 (A+L),脂肪酶37 ℃处理24 h后2 mol/L NaOH室温处理1 h;碱+酶处理组 (L+A),2 mol/L NaOH室温1 h后脂肪酶37 ℃处理24 h。
细胞接种之前,PHBHHx膜经 75%乙醇浸泡2 h,紫外线照射正、反两面各1 h进行消毒。PBS浸泡过夜,将膜裁剪成合适大小,放入 24孔板内备用。
1.2 NSCs的分离和培养
采用本实验室常规方法[35-36]分离孕14.5 d大鼠 (购自西安交通大学实验动物中心) 胚胎大脑皮质,细胞接种密度为1×105~2×105个细胞/mL,以完全培养基进行常规培养,每5 d传代1次,第3~8代的NSCs用于实验。完全培养基成分:无血清的 DMDEM/F12 (1:1) 培养基添加N2 (100×,GIBCO,USA)、B27 (50×,GIBCO,USA)、20 µg/L hEGF (GIBCO,USA) 和 10 µg/L bFGF (Sigma,USA)。NSCs分化培养基成分:去除完全培养基中hEGF和bFGF,并添加1%的胎牛血清 (Fetal bovine serum,FBS)。
1.3 在PHBHHx膜培养NSCs及细胞活力测定
传代时,将细胞重悬于分化培养基中,并以4×104个细胞/mL的密度接种于预先放入培养板中的PHBHHx膜,每孔加培养基500 μL,细胞培养箱内常规培养7 d,每隔3 d半量换液1次。MTT法测定NSCs细胞活力。
1.4 免疫细胞化学染色
细胞在膜表面贴附生长7 d以后,经PBS洗涤3次,0.3% Triton室温处理30 min,再用PBS洗涤3次后以5%的正常山羊血清室温封闭1 h,与一定比例稀释的一抗 (Nestin 1:200,β tubulinⅢ 1:200,GFAP 1:500) 室温孵育2 h后4 ℃过夜,次日以PBS洗涤3次后与FITC标记二抗于暗环境室温孵育2 h,以DAPI 标记细胞核 (室温,5 min),以50%的甘油缓冲液封片,荧光显微镜下观察。
1.5 扫描电镜样品制备
细胞在膜表面贴附生长7 d,经PBS洗涤2次后用2.5%的戊二醛4 ℃固定;再经0.1 mol/L磷酸缓冲液浸洗10 min后用1%四氧化锇固定液4 ℃固定 2 h;再用 0.1 mol/L磷酸缓冲液浸洗10 min后用乙醇梯度脱水并经乙酸异戊酯浸泡10 min;临界点干燥;喷金后扫描电镜 (TM-1000)下观察拍照。
2 结果
2.1 表面改性处理增加PHBHHx膜亲水性
分别对NaOH处理,脂肪酶处理以及NaOH联合脂肪酶处理后 PHBHHx膜表面接触角进行测量,结果提示:各种处理方式处理后膜表面接触角与未处理的对照组相比都有明显降低(图1,P<0.01),其中单纯NaOH处理PHBHHx膜表面接触角降低程度较单纯脂肪酶处理组更为明显 (P<0.05),与NaOH与脂肪酶联合处理组间没有显著性差异 (P>0.05)。
2.2 NSCs的培养与鉴定
由胚胎大鼠大脑皮质分离的原代细胞在完全培养基中呈悬浮状态生长,培养3~5 d后可形成大小不等的神经球 (图 2A),并随培养时间延长,神经球直径不断增大;在含1% FBS的分化培养基连续培养7 d后,部分细胞仍然保持nestin阳性的未分化NSCs,另有部分细胞分化为GFAP阳性星形胶质细胞和 β-tubulin Ⅲ阳性的神经元(图 2B,C,D)。
2.3 PHBHHx膜表面改性对 NSCs贴附的影响
24孔培养板中,将 NSCs接种在碱处理的PHBHHx膜表面,以分化培养基培养7 d,DAPI染色后进行荧光显微镜观察 (图3),结果显示,与未处理组 (3A) 相比,碱处理显著增加了NSCs在 PHBHHx膜表面的贴附生长 (3B),扫描电镜观察膜表面细胞保持原有的神经细胞形态特点 (3C,D)。
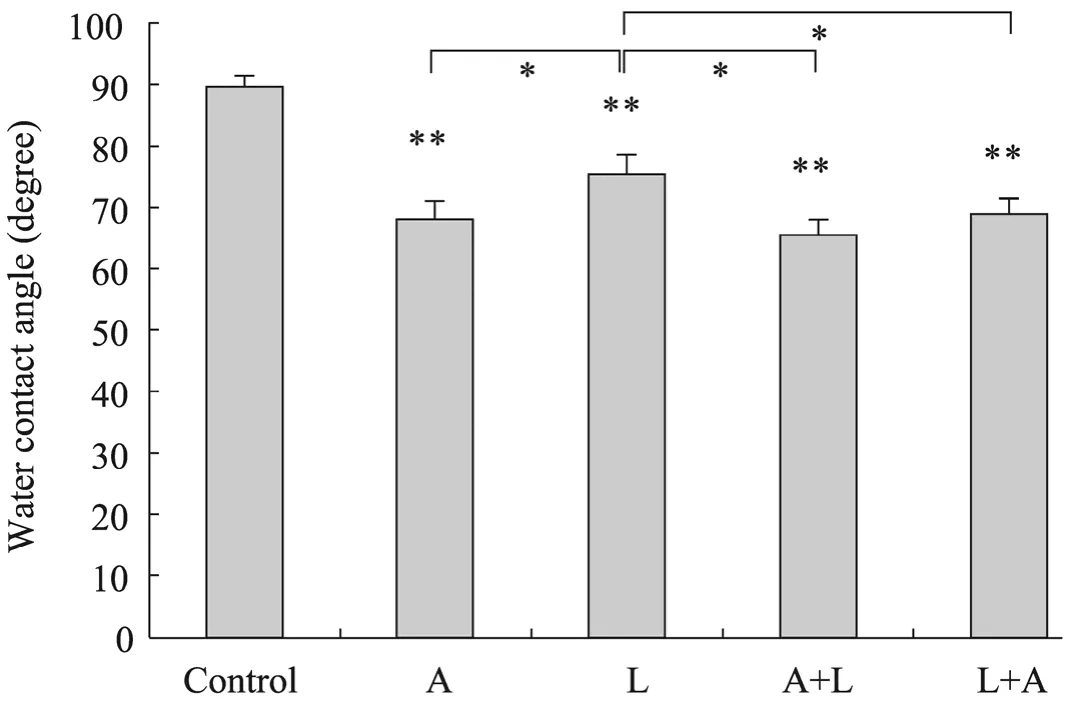
图1 表面改性对PHBHHx膜表面接触角的影响Fig.1 Water contact angle after surface treatment. A: NaOH treatment; L: lipase treatment; A+L: NaOH treatment followed by lipase treatment; L+A: lipase treatmemt followed by NaOH treatment. *P<0.05; **P<0.01.
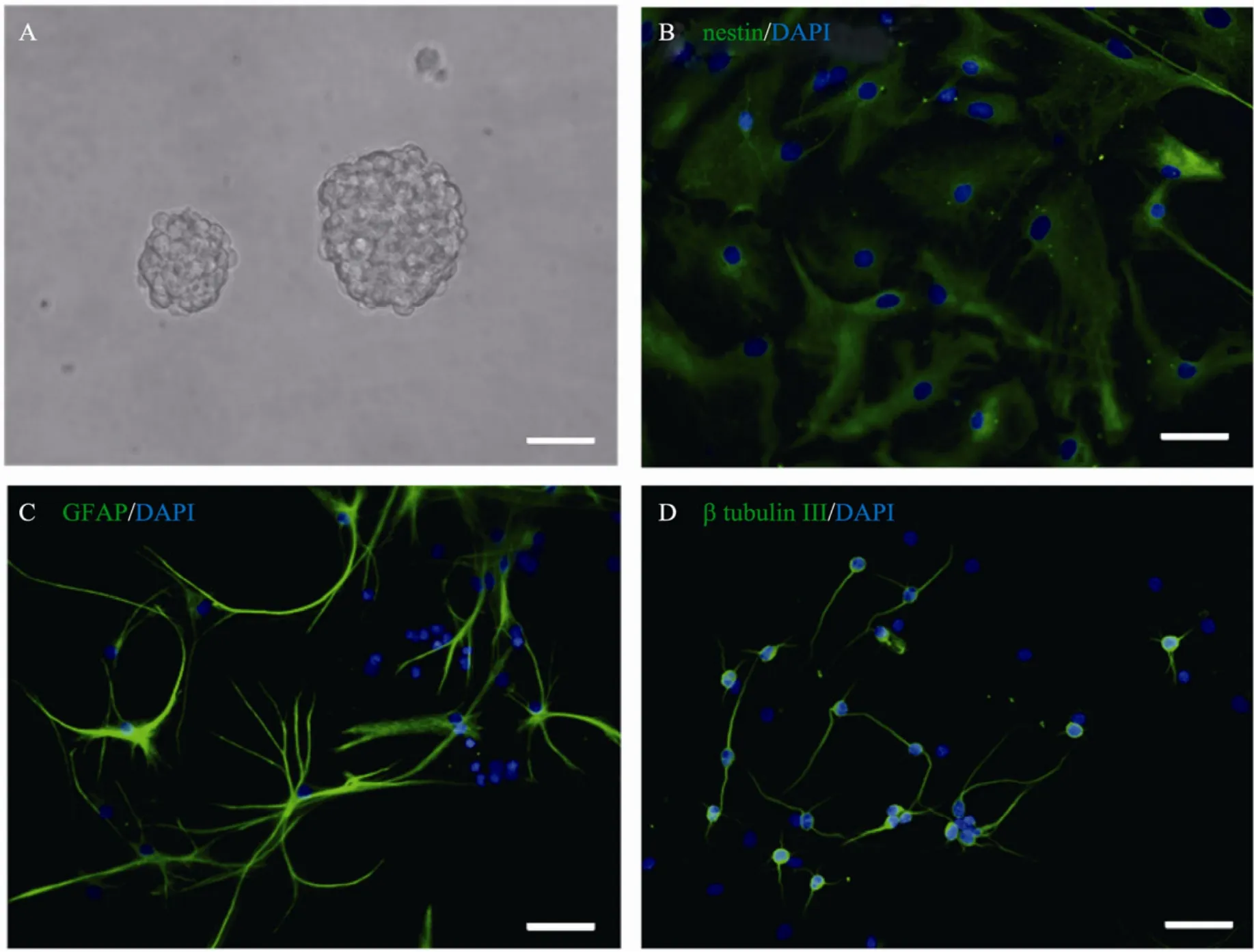
图2 NSCs体外培养及鉴定Fig.2 Cultivation and identification of NSCsin vitro. (A) Neurosphers. (B) Nestin positive NSCs. (C) GFAP positive astrocytes. (D) β-tubulin Ⅲ positive neurons. Cell nucli stained with DAPI (blue). Nestin, GFAP and β-tubulin Ⅲwere in green. Scale bar=50 μm.
2.4 NSCs在改性 PHBHHx膜表面的增殖及分化情况
将单个NSCs接种于PHBHHx膜,并以经多聚赖氨酸包被的盖玻片为对照,分化培养基常规培养3 d后,MTT检测PHBHHx膜表面贴附生长的 NSCs活力,结果提示:在改性PHBHHx膜上生长的 NSCs细胞活力明显增强(图 4)。
分化培养基中培养7 d,以免疫细胞化学染色法观察 NSCs分化情况,结果显示:细胞在PHBHHx膜表面发生多向分化 (图5),分化为星形胶质细胞 (GFAP阳性,A、B、C) 和神经元 (btubulin Ⅲ阳性细胞,D、E、F)。随机选择的视野中,显微镜下计数全部细胞、GFAP阳性细胞、b tubulin Ⅲ阳性和nestin阳性细胞,计算分化细胞比例,结果显示在改性的 PHBHHx膜表面生长细胞约有30%发生分化,其中多数为b tubulinⅢ阳性神经元,而其余的 70%仍维持在未分化状态;而在盖玻片上培养的细胞则有约90%的细胞发生不同方向的分化 (图6),相对来讲,分化为b tubulin Ⅲ阳性神经元和GFAP阳性细胞胶质细胞的比例 (1.37) 明显低于其在PHBHHx膜上的分化 (2.14)。

图3 NSCs在改性处理后的PHBHHx膜表面的贴附生长情况Fig.3 Attachment and survival of NSCs on alkali-treated PHBHHx film. Scale bar in A and B =100 μm.

图4 在改性的PHBHHx膜表面贴附生长的NSCs活力Fig.4 NSCs viability on the surface of modified PHBHHx films.

图5 NSCs在改性PHBHHx膜表面的分化情况Fig.5 Differentiation of NSCs on the surface of PHBHHx films. DAPI was in blue, nestin was in green, GFAP and β-tubulin Ⅲ were in red. Scale bar=50 μm.
3 讨论
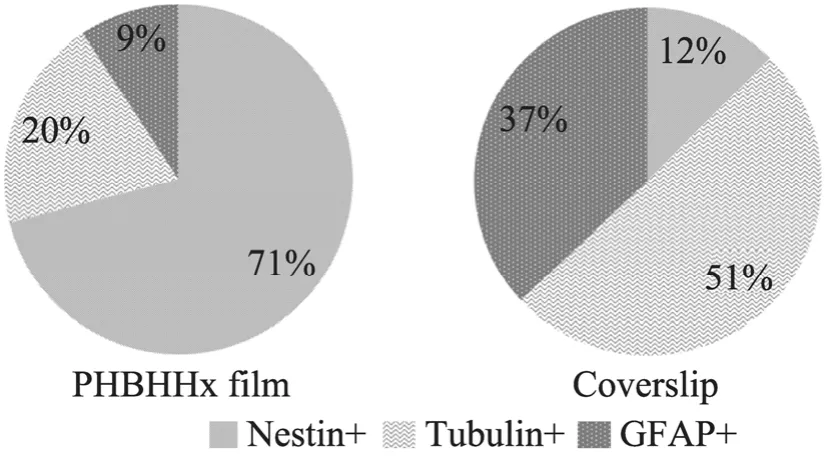
图6 NSCs在改性后PHBHHx表面分化比例Fig.6 Differentiation of NSCs on PHBHHx films.
生物可降解聚合物材料是组织工程学材料研究中最活跃的领域[37]。PHA家族是近年来在组织工程中被广泛关注的新型生物材料[19,26],由于各种 PHA材料单体组成的不同,其机械性能和生物相容性也有很大差异。PHBHHx是 PHA家族中具有很好生物相容性的一员,由于其主链含有短链的 3-羟基丁酸单体和中长链的 3-羟基己酸单体,其机械性能和可加工性能均优于只含有短链单体的 PHA材料,如聚羟基丁酸酯(Polyhydroxybutyrate,PHB) 和羟基丁酸-羟基戊酸共聚酯 (poly(3-hydroxybutyrate-co-3-hydroxvalerate),PHBV)。但由于PHBHHx的聚酯性质,其表面疏水性仍比较强,不利于细胞的贴附[29]。Zhao等采用NaOH和脂肪酶分别处理PHB膜,结果显示脂肪酶处理能够显著改善 PHB膜表面与成纤维细胞的生物相容性,NaOH处理对PHB生物相容性的改善不明显[38-39]。本实验中以碱(NaOH) 和脂肪酶对PHBHHx膜表面进行处理,结果显示两种处理方式都可以通过打断 PHA材料主链中的酯键,增加被处理表面的羟基和羧基含量,从而提高 PHBHHx膜表面的亲水性。扫描电镜的结果提示NaOH处理后PHBHHx膜表面出现很多细小的微孔,膜表面粗糙程度增加,最终促进了细胞在膜表面的贴附和生长。其中单纯的NaOH处理效果更为显著,而且处理方法简单,成本低廉,耗时较短,因此不失为一种简便而有效的PHBHHx膜表面改性手段。
NSCs联合生物材料移植治疗神经损伤已成为神经损伤修复以及功能重建领域新的热点。作为可用于促进神经损伤修复的生物材料,必须与其负载细胞NSCs有良好的相容性,既不产生细胞毒性,也不影响细胞功能。PHA家族中多个成员,包括PHB,PHBHHx和PHBVHHx均被尝试用于中枢及外周神经的支架材料[27-28,40-42],其中 PHBHHx材料显示出与神经干细胞、神经胶质细胞的良好生物相容性[27]。最近,Xu等[27-28]的研究表明PHBHHx作为NSCs体外培养支架材料具有低细胞毒性、与NSCs有很好的相容性,并能够诱导NSCs向神经元方向分化。本研究结果提示,对 PHBHHx膜表面的改性处理可以增加NSCs在其表面的贴附生长,而且NSCs活力明显高于玻璃片表面,细胞保持其原有的多向分化能力;在发生分化的细胞中以b tubulin Ⅲ阳性的神经元为主,这与Xu等报道的研究结果一致。除此之外,本研究还通过降低培养体系中的血清浓度,(由Xu等采用的 10%FBS降至1%),能够更好地保留 NSCs原有的增殖能力,使大约70%的细胞仍处于nestin阳性的未分化状态,这部分细胞能够在体内外进一步增殖,一方面为获得足量的移植细胞提供了保证,同时也为其在体内分化成“神经功能重建所需的特定细胞类型”奠定了基础。
本研究通过采用NaOH对PHBHHx膜进行表面处理,增加了 PHBHHx膜表面亲水性和与神经干细胞的生物相容性,促进NSCs的贴附生长;在合适的培养条件下,NSCs在PHBHHx表面不仅可以很好的存活,而且能够保持持久的增殖能力和多向分化能力。以上结果进一步提示PHBHHx是一种非常有潜力的中枢神经系统组织工程支架材料和神经干细胞移植的细胞载体材料,在神经损伤修复和功能重建方面有非常广泛的应用前景。
REFERENCES
[1] Tsuji O, Miura K, Fujiyoshi K, et al. Cell therapy for spinal cord injury by neural stem/progenitor cells derived from iPS/ES cells. Neurotherapeutics,2011, 8(4): 668−676.
[2] Mochizuki N, Moriyama Y, Takagi N, et al.Intravenous injection of neural progenitor cells improves cerebral ischemia-induced learning dysfunction. Biol Pharm Bull, 2011, 34(2):260−265.
[3] Thonhoff JR, Lou DI, Jordan PM, et al.Compatibility of human fetal neural stem cells with hydrogel biomaterialsin vitro. Brain Res, 2008,1187: 42−51.
[4] Arias-Carrion O, Yuan TF. Autologous neural stem cell transplantation: a new treatment option for Parkinson's disease? Medical Hypoth, 2009, 73(5):757−759.
[5] Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exper Neurol, 2005, 194(1): 230−242.
[6] Lepore AC, Neuhuber B, Connors TM, et al.Long-term fate of neural precursor cells following transplantation into developing and adult CNS.Neuroscience, 2006, 142(1): 287−304.
[7] Lepore AC, Han SSW, Tyler-Polsz CJ, et al.Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol, 2004, 1(2): 113−126.
[8] Wang D, Zhang JJ, Ma JJ, et al. Neural stem cell transplantation with Nogo-66 receptor gene silencing to treat severe traumatic brain injury.Neural Regeneration Res, 2011, 6(10): 725−731.
[9] Shi Y, Zhou L, Tian J, et al. Transplantation of neural stem cells overexpressing glia-derived neurotrophic factor promotes facial nerve regeneration. Acta Oto-Laryngol, 2009, 129(8):906−914.
[10] Lee SI, Kim BG, Hwang DH, et al. Overexpression of bcl-X(L) in Human neural stem cells promotes graft survival and functional recovery following transplantation in spinal cord injury. J Neurosci Res, 2009, 87(14): 3186−3197.
[11] Zhang Y, Zhu C, Tao Y, et al. Differentiation of fetal rat neural stem cells into neurons induced by brain-derived neurotrophic factor. J Clinical Rehab Tissue Eng Res, 2008, 12(51): 10073−10076.
[12] Yu HW, Cao B, Feng MY, et al. Combinated transplantation of neural stem cells and collagen type I promote functional recovery after cerebral ischemia in rats. Anat Rec, 2010, 293(5): 911−917.
[13] Cholas RH, Hsu HP, Spector M. The reparative response to cross-linked collagen-based scaffolds in a rat spinal cord gap model. Biomaterials, 2012,33(7): 2050−2059.
[14] Hansen K, Mueller FJ, Messing M, et al. A 3-dimensional extracellular matrix as a delivery system for the transplantation of glioma-targeting neural stem/progenitor cells. Neuro Oncol, 2010,12(7): 645−654.
[15] Wang YS, Zhou CW, Yao M, et al. Biodegradable parallel and porous HSPG/collagen scaffolds for the in vitro culture of NSCs for the spinal cord tissue engineering. J Porous Materials, 2012, 19(2):173−180.
[16] Shen CC, Yang YC, Liu BS. Peripheral nerve repair of transplanted undifferentiated adipose tissue-derived stem cells in a biodegradable reinforced nerve conduit. J Biom Materials Res Part A, 2012, 100A(1): 48−63.
[17] Wang W, Lin JH, Tsai CC, et al. Biodegradable glutaraldehyde-crosslinked casein conduit promotes regeneration after peripheral nerve injury in adult rats. Macromol Biosci, 2011, 11(7): 914−926.
[18] Wu Q, Wang Y, Chen GQ. Medical application of microbial biopolyesters polyhydroxyalkanoates.Artif Cells Blood Substit Biotechnol, 2009, 37(1):1−12.
[19] Chen GQ, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials, 2005, 26(33): 6565−6578.
[20] Zhao K, Deng Y, Chen JC, et al.Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility.Biomaterials, 2003, 24(6): 1041−1045.
[21] Wang YW, Wu Q, Chen GQ. Attachment,proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrateco-3-hydroxyhexanoate) scaffolds. Biomaterials,2004, 25(4): 669−675.
[22] Wang Y, Bian YZ, Wu Q, et al. Evaluation of three-dimensional scaffolds prepared from poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials, 2008, 29(19):2858−2868.
[23] Wu S, Liu Y, Tang Y, et al. Intravascular biocompatibility of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). J Clin Rehab Tissue Eng Res,2011, 15(38): 7066−7070.
[24] Zhou J, Peng SW, Wang YY, et al. The use of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)scaffolds for tarsal repair in eyelid reconstruction in the rat. Biomaterials, 2010, 31(29): 7512−7518.
[25] Qu XH, Wu Q, Chen GQ.In vitrostudy on hemocompatibility and cytocompatibility of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). J Biomater Sci, 2006, 17(10): 1107−1121.
[26] Chen GQ, Wu Q, Wang YW, et al. Application of microbial polyesters-polyhydroxyalkanoates as tissue engineering materials. Key Eng Mater, 2005,288/289: 437−440.
[27] Bian YZ, Wang Y, Aibaidoula G, et al. Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)conduits for peripheral nerve regeneration.Biomaterials, 2009, 30(2): 217−225.
[28] Xu XY, Li XT, Peng SW, et al. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials, 2010, 31(14): 3967−3975.
[29] Shen F, Zhang E, Wei ZJ.In vitroblood compatibility of poly (hydroxybutyrate-cohydroxyhexanoate) and the influence of surface modification by alkali treatment. Materials Sci Engineering C, 2010, 30(3): 369−375.
[30] Shangguan YY, Wang YW, Wu Q, et al. The mechanical properties andin vitrobiodegradation and biocompatibility of UV-treated poly(3-hydroxybutyrate-co-3-hydroxyhexanoate).Biomaterials, 2006, 27(11): 2349−2357.
[31] Sun M, Zhou P, Pan LF, et al. Enhanced cell affinity of the silk fibroin-modified PHBHHx material. J Mater Sci Mater in Med, 2009, 20(8):1743−1751.
[32] Li XT, Sun J, Chen S, et al.In vitroinvestigation of maleated poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) for its biocompatibility to mouse fibroblast L929 and human microvascular endothelial cells. J Biomed Mater Res A, 2008,87A(3): 832−842.
[33] Zheng Z, Bei FF, Tian HL, et al. Effects of crystallization of polyhydroxyalkanoate blend on surface physicochemical properties and interactions with rabbit articular cartilage chondrocytes.Biomaterials, 2005, 26(17): 3537−3548.
[34] Xuerong FAN, Liuliu SU, Qiang W, et al.Modification of polylactic acid fibers with lipases.J Textile Res, 2009, 30(3): 58.
[35] Lu H, Liu Y, Li M, et al. Isolation, culture and identification of neural stem cells in rats. J Xi'an Jiaotong Univ, 2002, 23(5): 428.
[36] Zhang P, Li W, Gao M, et al. Culture and identification of neural stem cells from mouse embryos. Chin J Contemp Pediatrics, 2011, 13(3):244−247.
[37] Wu S, Liu Y, Cui B, et al. Intravascularbiocompatibility of decellularized xenogenic vascular scaffolds/PHBHHx hybrid material for cardiovascular tissue engineering. Chin J Biotech,2008, 24(4): 610−616.
吴松, 刘迎龙, 崔彬, 等, 3-羟基丁酸与3-羟基已酸共聚酯在心血管组织工程中的应用. 生物工程学报, 2008, 24(4): 610−616.
[38] Zhao K, Yang X, Chen GQ, et al. Effect of lipase treatment on the biocompatibility of microbial polyhydroxyalkanoates. J Materials Sci Mater Med,2002, 13(9): 849−854.
[39] Yang XS, Zhao K, Chen GQ. Effect of surface treatment on the biocompatibility of microbial polyhydroxyalkanoates. Biomaterials, 2002, 23(5):1391−1397.
[40] Wang L, Wang ZH, Shen CY, et al. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials, 2010,31(7): 1691−1698.
[41] Chen WH, Tong YW. PHBV microspheres as neural tissue engineering scaffold support neuronal cell growth and axon-dendrite polarization. Acta Biomaterialia, 2012, 8(2): 540−548.
[42] Novikova LN, Pettersson J, Brohlin M, et al.Biodegradable poly-beta-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials, 2008, 29(9): 1198−1206.
Biocompatibility of surface modified PHBHHx with rat embryonic neural stem cells
Haixia Lü1, Zhiqian Yang2, Xiaoyun Lu2, Mingchuan Li2, Qian Jiao1, Xinlin Chen1,Yuanyuan Wang1, and Yali Zhang2
1Institute of Neurobiology,Xi’an Jiaotong University College of Medicine,Xi’an710061,Shaanxi,China
2Department of Biological Science and Bioengineering,Key Laboratory of Biomedical Information Engineering of Ministry of Education,School of Life Science and Technology,Xi’an Jiaotong University,Xi’an710049,Shaanxi,China
吕海侠, 杨志倩, 卢晓云, 等. PHBHHx膜表面亲水改性及其与神经干细胞生物相容性. 生物工程学报, 2012, 28(10):1216−1226.
Lü HX, Yang ZQ, Lu XY, et al. Biocompatibility of surface modified PHBHHx with rat embryonic neural stem cells. Chin J Biotech, 2012, 28(10): 1216−1226.
Received:April 17, 2012;Accepted:June 6, 2012
Supported by:National Natural Science Foundation of China (Nos. 81070998, 31070943, 31101266), Fundamental Research Funds for the Central Universities.
Corresponding author:Xiaoyun Lu. Tel/Fax: +86-29-82668463-423; E-mail: luxy05@mail.xjtu.edu.cn
国家自然科学基金 (Nos. 81070998,31070943,31101266),西安交通大学中央高校基本科研业务费专项资金资助。