一种新型检测铜离子和磷酸氢根阴离子的荧光逻辑门探针
周晓彦 吕树升 刘 泉 沈 珍
(南京大学化学化工学院,配位化学国家重点实验室,南京 210093)
一种新型检测铜离子和磷酸氢根阴离子的荧光逻辑门探针
周晓彦 吕树升 刘 泉 沈 珍*
(南京大学化学化工学院,配位化学国家重点实验室,南京 210093)
报道了一种中位-联吡啶取代的4,4-二氟-4-硼-3a,4a-二氮-s-因达省(简称BODIPY)1的合成与离子识别性能。化合物1在加入Cu2+后,荧光被猝灭,加入HPO42-能使这种荧光恢复,而且对Cu2+和HPO42-有着很高的专一性识别。表明化合物1是一种新型的荧光逻辑门探针。
硼二吡咯亚甲基;荧光逻辑门;离子识别;铜离子;磷酸氢根离子
Boradiazaindacenes (BODIPYs)are well-known fluorescent dyes that possess highly desirable characteristics such as high fluorescence quantum yield and extinction coefficient,high stability against light and heat and easy modificative structure.These properties facilitate their extensive application as chemosensors,fluorescent switches,labeling reagents,OLEDs,and laser dyes[1].
Copper is one of the essential trace elements of transition metals and responsible for the function of manycellularenzymesand protein.However,it becomes toxic when excessive amounts of copper ion are accumulated.It is believed that the disruption of copper homeostasis is associated with neurodegenerative diseases like Parkinsons disease (PD),Alzheimers disease (AD),and Amyotrophic lateral sclerosis(ALS)[2].
Monohydrogenphosphate(MHP)ion exists as one form of phosphates,which is an essential nutrient and energy carrier for both human and plants[3].The products containing phosphate are wildly used in many areas.
Because of the important role of copper ion and monohydrogenphosphate anion in biological,industrialand environmental systems,there are many literatures reported about the fluorescent probe for Cu2+ion[4]and the electrochemical recognition of HPO42-anion[3,5].
Most fluorescent sensors reported have one input and one output,however in fluorescence logic gates there are two inputs and one output as shown in Fig.1 and Table 1.At any given moment,every terminal is in one of the two binary conditions,low(0)or high(1),represented by different emission state.In most logic gates,the low state is approximately low emission,while the high state is high emission.
Herein,we report a meso-bipyridine substituted BODIPY 1 that shows Cu2+-induced fluorescence“turn-off” response (0)and fluorescence “turn-on”property (1)after addition of HPO42-,which is a new type of logic gate from the seven basic logic gates[6].Such type of fluorescent probe may have potential applications in clinical chemistry, biomedical research, pharmacology, industrial process and environmental monitoring,etc[7].
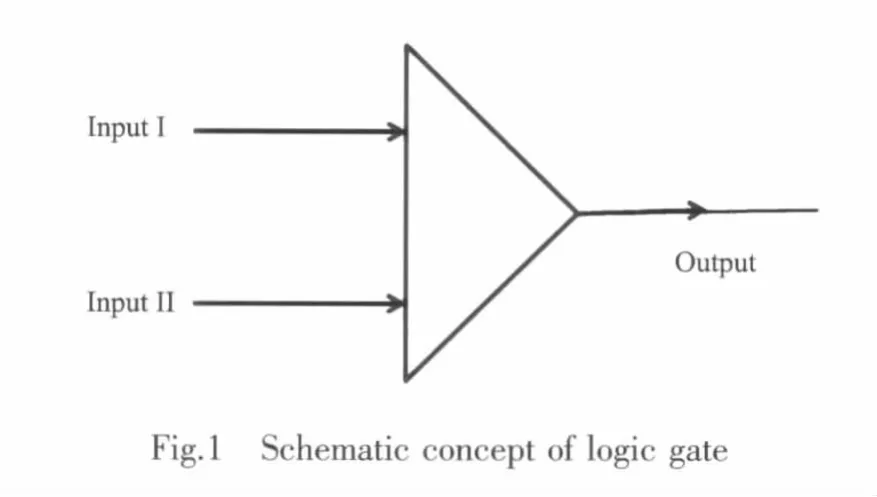
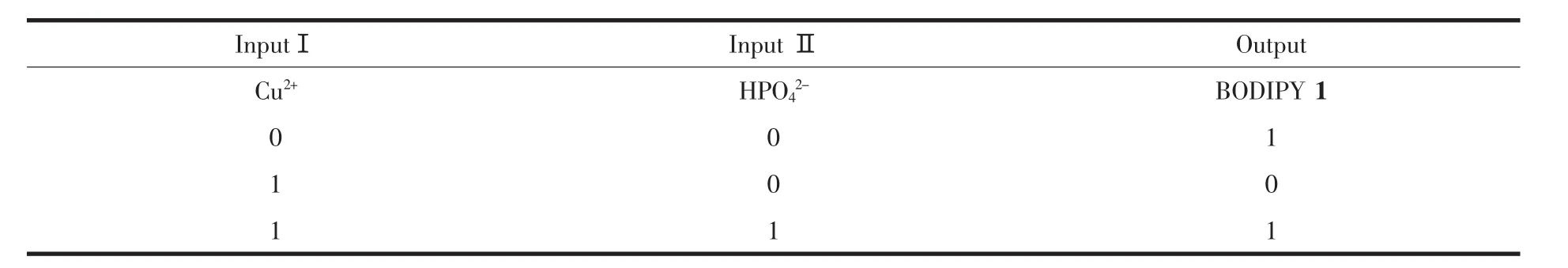
Table 1 Operation of meso-bipyridine substituted 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene(BODIPY)1 as a logic gate
1 Experimental
1.1 Instruments
1H NMR spectra were recorded on a Bruker DRX500 spectrometer at ambient temperature.NMR chemical shifts are expressed in ppm relative to TMS as the internal standard.Mass spectra were measured on a Bruker Daltonics AutoflexⅡTMMALDI-TOF spectrometer.UV-Visible spectra were recorded on a UV-2550 spectrophotometer.Fluorescence spectra were measured on an F-4600 spectrophotometer with a xenon arc lamp as the light source.
1.2 Reagents and materials
All syntheses were carried out under an atmosphere of nitrogen.Unless otherwise noted,all reagents or solvents were of analytical grade and were used as received.Dry CH2Cl2was obtained by refluxing and distilling over CaH2under an inert atmosphere.Dioxane was obtained by simple reflux and distillation process.The solvents used for photophysical measurements were of spectroscopic grade and used without further purification.SeO2,which is a colorless crystal,was obtained by sublimation purification.
1.3 Procedures for metal ion sensing
Stock solutions of the metal ions (10-2mol·L-1)and anions(10-2mol·L-1)were prepared in anhydrous ethanol.A stock solution of 1 (8.867×10-4mol·L-1)was prepared in anhydrous ethanol.In titration experiments,each time a 2 mL solution of 1(1 μmol·L-1)was filled in a quartz optical cell of 1 cm optical path length,and the Cu2+stock solution was added into the quartz optical cell gradually by using a micropippet.In selectivity experiments,the test samples were prepared by placing appropriate amounts of metal ion stock solution into 2 mL solution of 1(1 μmol·L-1).During fluorescence measurements,the excitation wavelength was 470 nm and the emission spectra were collected between 480~900 nm.
1.4 DensityFunctional Theory(DFT)calculation
The G03W software package[8]was used to carryout a DFT geometry optimisation for 1 using the B3LYP functionalwith 6-31G*basissets.While B3LYP functional with LanL2DZ basis set was used for Cu atom and 6-31G*basis sets for the other atoms.
1.5 Synthesis of 1
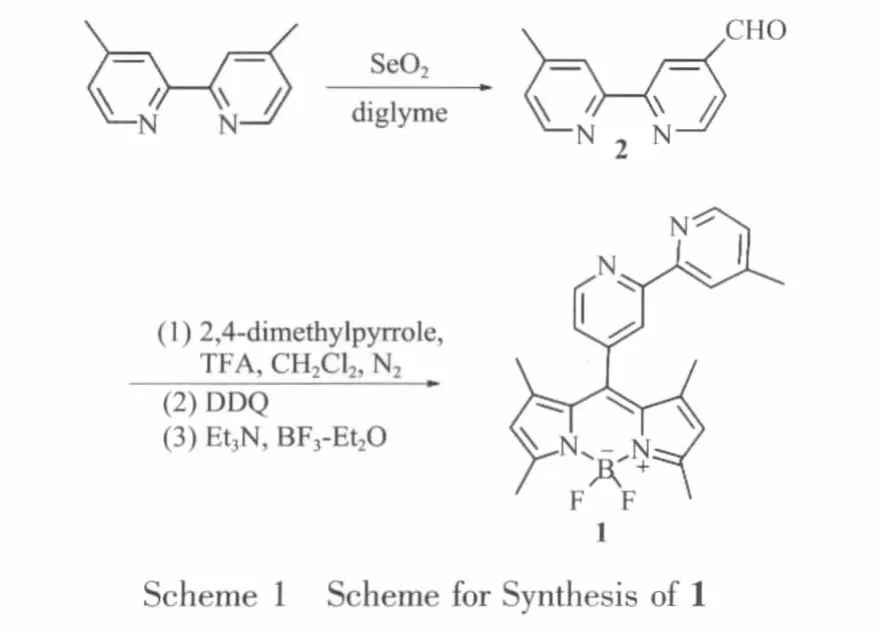
2 and two folds of 2,4-dimethylpyrrole were dissolved in dry CH2Cl2(150 mL)under nitrogen in the presence of a catalytic amount of triuoroacetic acid (TFA)and the solution was stirred for 24 h at ambienttemperaturein the dark untilcomplete consumption of the aldehyde as indicated by TLC.2,3-Dichloro-5,6-dicyanoquinone (DDQ)was added,and the mixture was stirred for an additional 2.5 h.The reaction mixture was then treated with triethylamine and boron triuoride etherate.After stirring for another 3 h,an obvious intense green fluorescence can be seen.The dark brown solution was washed with waterand brine,dried over anhydrous magnesium sulfate,and concentrated at reduced pressure.After purification on silica gel column and recrystallization, compound 1 was obtained as a salmon pink solid[10].1H NMR(500 MHz,CDCl3) δ 8.83(d,J=5,2H),8.53(d,J=5,2H),8.50(s,1H),8.32(s,1H),7.33(d,J=5,1H),7.19(d,J=5,1H),6.00(s,2H),2.57(s,6H),2.48(s,3H),1.47(s,6H).MS(MALDI TOF):m/z 418.43[M]+,Calcd.:418.29.
2 Results and discussion
2.1 Spectroscopic properties of 1
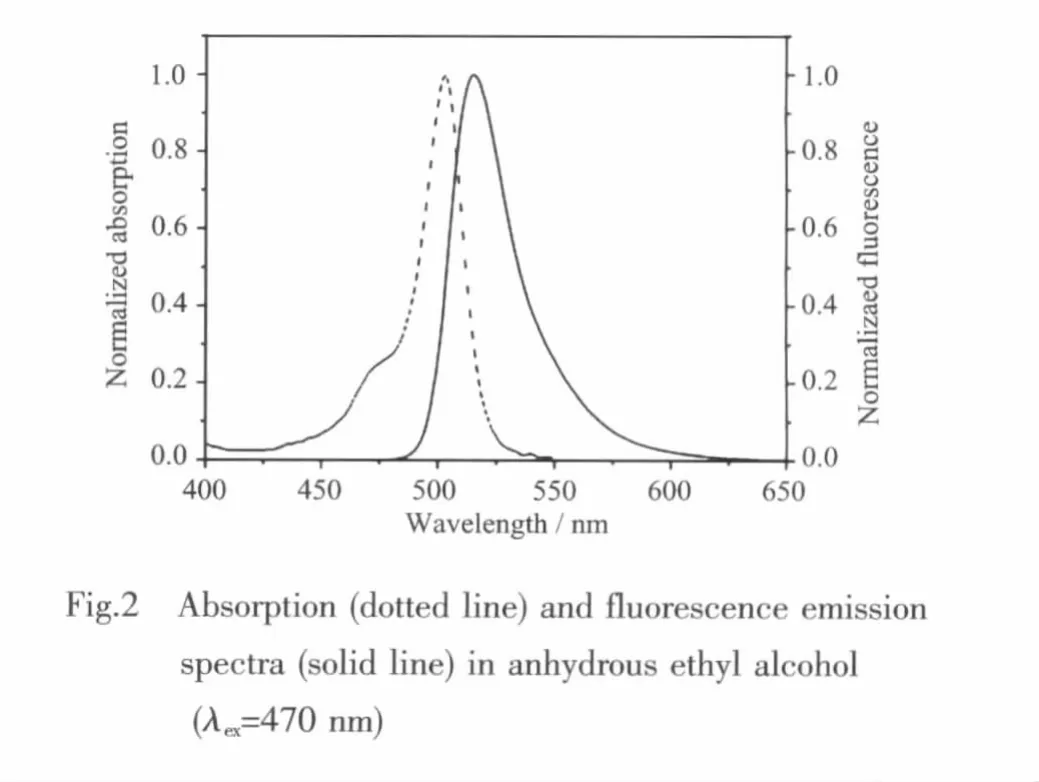
The absorption and steady-state fluorescence emission spectra of 1 are given in Fig.2,which show the characteristic spectroscopic propertiesofthe BODIPY chromophore with slight Stokes shift[11].In C2H5OH,a strong transition with a maximum at 502 nm (ε=11 765 L·mol-1·cm-1)is observed,which can be attributed to the 0-0 vibrational band of a strong S0-S1transition and a shoulder (more or less pronounced)at a shorter wavelength is ascribed to the 0-1 vibrational band of the same transition.The absorption spectrum of 1 is similar to that of classical BODIPY dyesindicating the absence ofdonoracceptor interaction in the ground state,which is due to the decoupled structure between the meso-subunit and indacene plane[12].Excitation of 1 yields the typical BDP fluorescence spectrum with mirror image of the absorption band,the maximum appears at 515 nm with slight Stokes shift in C2H5OH.
The synthetic procedure for the preparation of 1 was outlined in Scheme 1.4-methyl-2,2′-bipyridine-4-carbaldehyde 2 was prepared by oxidation reaction of 4,4′-dimethyl-2,2′-bipyridine with SeO2in dioxane.The reaction mixture was heated at reflux for 24 h and then cooled to room temperature.The solvent was removed by rotate evaporation.The resulting mixture was dissolved in ethyl acetate and filtrated to remove SeO2.Na2S2O5was added to form aldehyde bisulfate.The system was adjusted to the pH value of 9~10 with an aqueous NaOH solution (10%)and extracted with CH2Cl2three times,after column chromatography on silica gel 2 was obtained with a yield of 25%.[9]1H NMR(500 MHz,CDCl3) δ 10.19(s,1H),8.91(s,1H),8.85(s,1H),8.59(d,J=5,1H),8.29(s,1H),7.73(d,J=5,2H),7.20(d,J=5,2H),2.48(s,3H).MS(MALDI TOF):m/z 199.25[M+H]+,calcd.:198.08
2.2 Fluorometric titration experiments of 1
1 shows bright green fluorescence in C2H5OH.The uorescent intensity at 515 nm is quenched upon the addition of Cu2+ion.However,the fluorescence intensity of 1 is almost not influenced by the addition of 10-fold of other metal ions such as Mg2+,Ca2+,Cr3+,Cd2+,Al3+,Fe2+,Mn2+,Hg2+,K+,Pb2+,Zn2+,Fe3+,Na+,Ni2+,respectively.To evaluate the utility of 1 as a Cu2+-selective fluorescent sensor, ion interference experiments were carried out.As shown in Fig.3,its Cu2+response is not interfered in the background containing appropriate metal ions.

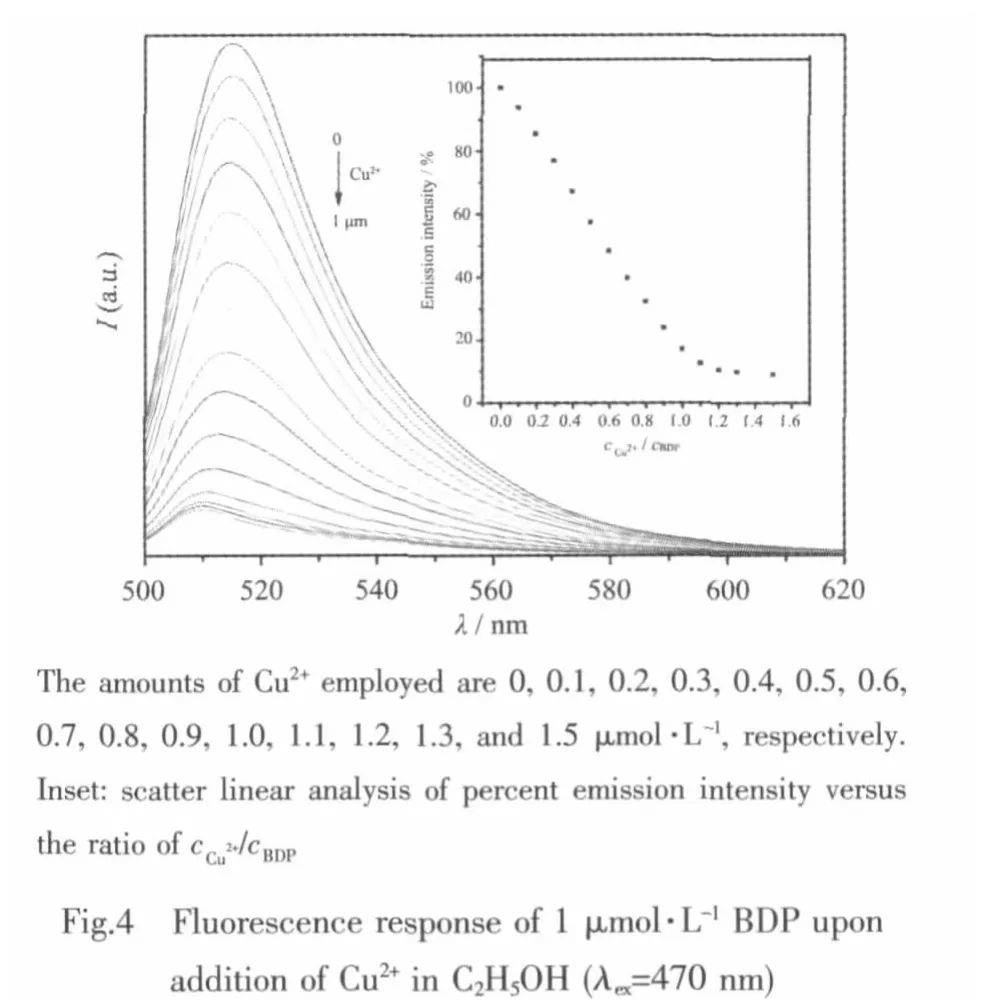
The sensitivity of 1 toward Cu2+was further investigated by fluorescence titration experiment.As shown in Fig.4,thefluorescenceintensity of1 decreases gradually upon the addition of Cu2+.According to the DFT calculation,both HOMO and LUMO energy levels of the copper complex become lower than that of the free ligand (Fig.5),therefore an oxidative electron transfer from the excited BDP fluorophore to the LUMO of the bipyridine metal complex occurs and causes the fluorescent quenching.Moreover,before reaching the stoichiometric point,the observed fluorescent intensity is proportional to the concentration of copper ion;subsequent addition of Cu2+(>1 μmol·L-1)causes an unchanged behaviour in the fluorescence intensity,indicating that the Cu2+sensor has a 1∶1 stoichiometry[13].
To explore the anion response of the metal complex,we have prepared a solution of the complex in anhydrous ethanol.To a 1 μmol·L-1solution of BODIPY 1,1 μmol·L-1Cu2+was added in the form of acetate salt,according to the stoichiometry of Cu2+and BODIPY.The metal complex was then titrated with F-,Cl-,Br-,SO42-,HPO42-and N3-.Among these anions,only phosphate can enhance the fluorescence intensity.Also,phosphate has a lower detection limit.When 25-fold phosphate was added,the fluorescence intensity of the complex was nearly restored.The changes in the fluorescence intensity of metal complex upon successive addition of HPO42-anion are shown in Fig.6.The added anion is not a strong enough chelator to remove the Cu(Ⅱ)from the complex,butthro ugh simple electrostatic interactions it can,at least partially,neutralizes the charge on the metal center[14].

In summary,a highly selective and sensitive fluorescent logic gate for Cu2+and HPO42-based on meso-bipyridine substituted BODIPY has been synthesized. DFT calculation indicates that coordination of Cu2+influences the frontier molecular orbital of the ligand,thus causes an oxidative PET quenching ofthe fluorescence.Furtheraddition of HPO42-anion restores the fluorescence of BDP chromophore.
[1](a)Loudet A,Burgess K.Chem.Rev.,2007,107:4891-4932
(b)Ulrich G,Ziessel R,Harriman A.Angew.Chem.,Int.Ed.,2008,47:1184-1201
(c)Shen Z,Roehr H,Rurack K,et al.Chem.Eur.J.,2004,10:4853-4871
(d)Yu Y H,Descalzo A B,Shen Z.et al.Chem.Asian J.,2006,1:176-187
(e)Yu Y H,Shen Z,Xu H Y,et al.J.Mol.Struc.,2007,827:130-136
(f)WANG Yan-Wei(王艳玮),LI Min(李敏),SHEN Zhen(沈珍).Chin.J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(8):1247-1252
(g)XU Hai-Yun(徐海云),SHEN Zhen(沈珍),OKUJIMA Tetsuo(奥岛铁雄),et al.Chinese.J.Inorg.Chem.(Wuji Huaxue Xuebao),2006,22(5):801-807
(h)Wang Y W,Descalzo A B,Shen Z,et al.Chem.Eur.J.,2010,9:2887-2903
(i)JIANG Jia-Li(姜佳丽),LU Hua(卢华),SHEN Zhen(沈珍).Chinese.J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(6):1105-1108
[2](a)Mertz W.Science,1981,213:1332-1338
(b)Frausto da Silva J,Williams R J P.The Biological Chemistry of the Elements:The Inorganic Chemistry of Life;Clarendon:Oxford,1993.
(c)Lonnerdal B.Am.J.Clin.Nutr.,1996,63:821S-829S
(d)Linder M C,Hazegh-Azam M.Am.J.Clin.Nutr.,1996,63:797S-811S
(e)Uauy R,Olivares M,Gonzalez M.Am.J.Clin.Nutr.,1998,67:952S-959S
(f)US EPA,Lead and Copper Rule:EPA 816-F-04-009
(g)Georgopoulos P G,Roy A,Yonone-Lioy M J,et al.P.J.J.Toxicol.Environ.Health B,2001,4:341-394
(h)Pang Y,MacIntosh D L,Ryan P B.J.Nutr.,2001,131:2171-2176
(i)Wu J,Ricker M,Muench J.J.Am.Board Fam.Med.,2006,19:191-194
(j)Gaggelli E,Kozlowski H,Valensin D,et al.Chem.Rev.,2006,106:1995-2044
[3]Kivlehan F,Wade J M,Moynihan H A,et al.Anal.Chim.Acta,2007,585:154-160
[4](a)Wang H H,Xue L,Fang Z J,et al.New J.Chem.,2010,34:1239-1242
(b)Jung H S,Kwon P S,Lee J W,et al.J.Am.Chem.Soc.,2009,131:2008-2012
(c)Kim S H,Kim J S,Park S M,et al.Org.Lett.,2006,8:371-374
(d)Kim H J,Park S Y,Yoon S,et al.Tetrahedron,2008,64:1294-1300
(e)Xiang Y,Tong A J,Jin P Y,et al.Org.Lett.,2006,8:2863-2866
(f)Mei L,Xiang Y,Li N,et al.Talanta,2007,72:1717-1722
(g)Zhao Y,Zhang X B,Han Z X,et al.Anal.Chem,.2009,81:7022-7030
(h)Zhou Y,Wang F,Kim Y,et al.Org.Lett.2009,11,4442-4445
(i)Goswami S,Sen D,Das N K,et al.Tetrahedron Lett.,2010,51:5563-5566
[5](a)Paul D B,Zheng C,Michael G B Drew,et al.Inorg.Chim.Acta,1996,246:143-150
[6]Glazier S A,Arnold M A.Anal.Chem.,1998,60:2540-2542
[7](a)de Silva A P,Gunaratne H Q N,McCoy C P.Nature,1993,364:42-44
(b)Iwata S,Tanaka K J.Chem.Soc.,Chem.Commun.,1995,15:1491-1492
(c)de Silva A P,Gunaratne H Q N,McCoy C P.J.Am.Chem.Soc.,1997,119:7891-7892
(d)Baytekin H T,Akkaya E U.Org.Lett.,2000,2:1725-1727
(e)Ghosh P,Bharadwaj P K,Mandal S,et al.J.Am.Chem.Soc.,1996,118:1553-1554
(f)Gunnlaugsson T,MacDonail D A,Parker D.J.Am.Chem.Soc.,2001,123:12866-12876
(g)Credi A,Balzani V,Langford S J,et al.J.Am.Chem.Soc.,1997,119:2679-2681
(h)de Silva A P,Rupasinghe R A D.J.Chem.Soc.,Chem.Commun.,1985,16:1669-1670
(i)de Silva A P,Gunnlaugsson T,McCoy C P.J.Chem.Educ.,1997,74:53-58
[8]Frisch M J,Trucks G W,Schlegel H B,et al.Gaussian 03,Gaussian,Inc.,Wallingford CT,2004.
[9](a)Polin J,Schmohel E,Balzani V.Synthesis.1998:321-324
(b)Dupau P,Renouaed T,Le Bozec H.Tetrahedron Lett.,1996,37:7503-7506
[10]Turfan B,Akkaya E U.Org.Lett.,2002,4:2857-2859
[11](a)Qin W,Baruah M,Van der Auweraer M,et al.J.Phys.Chem.A,2005,109:7371-7384;
(b)Descalzo A B,Xu H J,Xue Z L,et al.Org.Lett.,2008,10:1581-1584
[12]Kollmannsberger M,Rurack K,Resch Genger U,et al.J.Phys.Chem.A,1998,102:10211-10220
[13]Yang Y K,Yook K J,Tae J.J.Am.Chem.Soc.,2005,127:16760-16761
[14]Coskun A,Baytekin B T,Akkaya E U.Tetrahedron Lett.,2003,44:5649-5651
A Novel Fluorescence Logic Gate for Cu2+and HPO42-Recognition
ZHOU Xiao-Yan LU Shu-Sheng LIU Quan SHEN Zhen*
(School of Chemistry and Chemical Engineering,State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210093,China)
A meso-bipyridine substituted 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY)1 has been synthesized and its response for metal cations and anions has been investigated.1 shows fluorescence “turn-off”response toward Cu2+.Further addition of HPO42-regenerates the fluorescence intensity,indicating that 1 can be used as a logic gate with high selectivity for Cu2+and HPO42-ions.
boron-dipyrromethene;fluorescent logic gate;ion recognition;Cu2+;HPO42-
O621.2
A
1001-4861(2012)07-1483-06
2012-01-14。收修改稿日期:2012-04-08。
国家自然科学基金面上基金(No.20971066)资助项目。
*通讯联系人。E-mail:zshen@nju.edu.cn;Tel:025-83686679

