Event-related potentials during mental rotation tasks in patients with first-episode depression
Jiu CHEN, Laiqi YANG*, Guangxiong LIU, Yan ZHANG, Xinqu WU, Wentao MA, Zihe DENG
· Original article ·
Event-related potentials during mental rotation tasks in patients with first-episode depression
Jiu CHEN, Laiqi YANG*, Guangxiong LIU, Yan ZHANG, Xinqu WU, Wentao MA, Zihe DENG
Background:The ability to mentally rotate images is impaired in depressed patients but the electrophysiological abnormalities in the brain related to this impairment remain unclear.
Aim:To compare the event-related potentials (ERPs) of depressed patients and control subjects during the completion of a mental rotation (MR) task.
Methods: Thirty-two inpatients and outpatients with first-episode depression and twenty-nine control subjects were administered an MR task that presented test stimuli at different angles of orientation. During the test ERPs were measured in four regions of the brain (PZ, CZ, P3 and P4). Outcome variables included the error rate, reaction time, P500 latency and maximum P500 amplitude.
Results:Compared to control subjects, patients with depression completing the MR test had a significantly longer mean (sd) reaction time (689 [98] ms vs. 569 [55] ms; t=4.36, p<0.001) and a significantly higher mean percent error rate (30.2% [11.4%] vs. 20.3% [7.2%]; t=3.61, p=0.015); these differences were also significant at each of the four orientation angles assessed. The ERP assessment during the MR test found that patients had a non-significant increase in the overall P500 latency and a significant reduction in the mean maximum P500 amplitude at each of the four brain regions assessed. For both patients and controls the error rate, reaction time, P500 latency and P500 amplitude increased significantly in a stepwise fashion as the angle of orientation of the presented stimulus increased from 0° to 180°. In the control group there was a positive peak in the averaged ERP waveforms at about 700 ms that was not present in the patient group.
Conclusion:Our study confirms previous work on the usefulness of MR tests to assess the cognitive deficits in depression. We find that the electrophysiological measures provided by ERP assessments during MR tasks, particularly maximum P500 amplitude and maximum P700 amplitude, are potential biological markers for depression. Prospective studies that assess changes in these measures over the course of a depressive illness will be needed to confirm their usefulness.
1. Introduction
First introduced by Shepard and colleagues in the 1970s,[1]mental rotation (MR) tasks involve mentally rotating a two-dimensional or three dimensional object (e.g. a letter, number, or cube) or, alternatively, mentally rotating oneself around a mental image of the object. Individuals with depression have prolonged reaction times and higher error rates when performing MR tasks than normal controls.[2]Previous studies have used event-related potential (ERP) topographic mapping techniques to assess depressed patients in China[3]and studies from other countries have used ERP techniques to assess cognitive functioning during MR tasks.[4]The present study compares the ERP characteristics of depressed patients and control subjects while performing MR tasks.
2. Subjects and Methods
2.1 Subjects
The enrollment of patients and controls in the study is shown in Figure 1.
Depressed subjects were identified from inpatients and outpatients treated at the Department of Psychiatry of the Third Hospital of the People’s Liberation Army in Baoji, Shaanxi from January 2011 to September 2011 who had a diagnosis of depression based on the criteria in the third edition of the Chinese Classification of Mental Disorders.[5]Eligible patients met the following criteria: a) currently in the first episode of depression; b) duration of illness less than one year; c) had never taken anti-depressant medication; d) right-handed; e) normalvisual acuity; f) no co-morbid mental or physical illness; g) no history of substance abuse or severe suicidal tendency; and h) subject and guardian signed informed consent to participate in the study. A total of 15 eligible inpatients and 33 eligible outpatients were identified. Two-thirds of the eligible patients were randomly selected to participate, that is, 10 inpatients and 22 outpatients. These 32 patients included 16 males and 16 females from 18 to 30 years of age with a mean (sd) age of 27.0 (6.0) years, a mean (sd) duration of education of 12.6 (3.4) years, a mean duration of depression of 13 (5) weeks, and a mean score on the Hamilton Depression Rating Scale[6](HAMD) of 29 (5) at the time of admission (inpatients) or enrollment (outpatients).
A convenience control group of 29 medical students, trainees and interns at the Baoji Hospital who responded to a recruitment notice and who had no personal or family history of mental illness was identified. They included 15 men and 14 women 18 to 37 years of age; their mean (sd) age was 24.2 (5.2) years and their mean years of schooling were 14.3 (2.1) years. There were no significant differences between cases and controls by gender (χ2=0.08, p=0.121), age (t=0.26, p=0.157), or mean years of education (t=0.38, p=0.118).
This study was approved by the Medical Ethics Commission of the Baoji Third Hospital of the People’s Liberation Army.
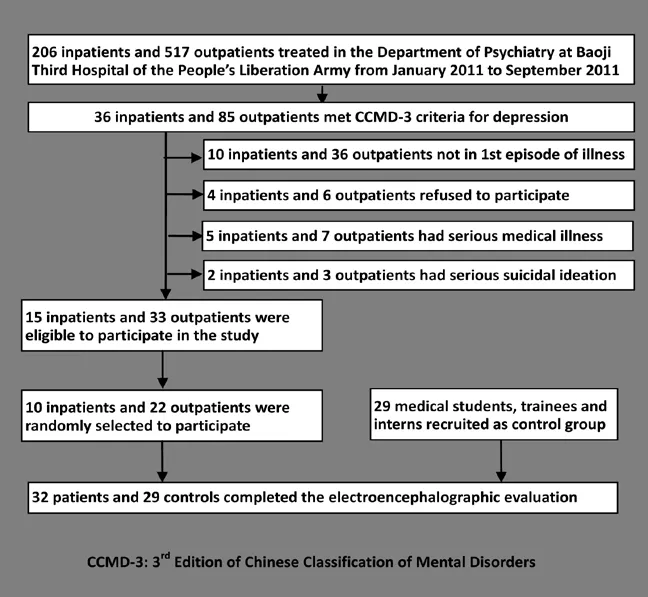
Figure 1. Flowchart of subject enrollment
2.2 Research Methods
2.2.1 Electrophysiological measurement
The ERPs were recorded in the morning using a BrainAMP MR32 32-channel amplifier (BrainProducts, Munich, Germany). Participants were asked to wear a BrainCAP-MR 32-electrode cap to record EEG signals. Recording electrodes were placed at P3, P4, CZ, PZ, O1, OZ, and O2 according to the ten-twenty electrode system of the International Federation of Clinical Neurophysiology placement system;[7]two ear electrodes served as reference electrodes, and the AFz electrode was used for grounding. The scalp was cleaned to maintain an impedance of <5 kΩ for each electrode. The stimuli were presented for 1200 ms and the resulting ERP signals were sampled (frequency: 500 Hz, sensitivity: 5 μV) and bandpass filtered (low cut-off frequency: 0.1 Hz; high cut-off frequency: 30 Hz). Signals >200 ms before the stimulus presentation were regarded as the baseline, and signals between 200 ms before stimulus presentation and 1000 ms after the presentation were recorded as responses. Artifacts (e.g. blinks, signals with an absolute peak amplitude greater than 70 μV) were identified off-line and cleared. The P500 component (400–600 ms) was monitored and analyzed for latency and amplitude. Reaction time and the number of incorrect responses were automatically recorded and the ERP signals fromtrials that had a correct response were averaged. All measurement and signal analysis were performed by two clinical psychiatrists who were blind to the status of the subject (patient or control); they had received training using the apparatus and had good inter-rater reliability when independently assessing the wave amplitudes of ten patients (r=0 .85-0.90).
2.2.2 Test procedures
MR tests were performed in a quiet, dark room (soundproofed and maintained at 24 °C) using E-Prime 2.0 (Psychology Software Tools Inc., Pittsburgh, USA). The subject was seated in a soft chair 60 cm away from a 17-inch color display monitor (75 Hz refresh rate) and instructed to look at the ‘+’ symbol displayed in the center of the screen. In each trial, after 500 ms the‘+’ symbol was replaced by the letter ‘R’ or the letter‘F’ (size: ≤1.58 cm2; with a vertical visual angle of 0°, height of 1.1° and width of 1.0° ). The two letters were presented as normal images or as mirror images at six different degrees of rotation (0°, 60°, 120°, 180°, 240°, 300°); the 24 different test samples were randomly presented. The subject indicated if the letter shown was normally oriented or a mirror-image by clicking the left or right button of a mouse.
Subjects were trained in the test procedure until they reached 60% accuracy and then the formal part of the MR test started. In the first part of the formal test, each letter was displayed at random rotation angles 360 times, and the subject gave 720 reponses (each of the 24 test samples was presented 30 times). After a rest period (the duration was determined by the subject) the same procedure was repeated. The whole experiment lasted about 20 minutes. The ERPs were recorded during the MR test.
2.3 Statistical analysis
Data were analyzed by analysis of variance (ANOVA), using SPSS 17.0 software (SPSS, Chicago, IL, USA). Twenty seven data on reaction times (<5% of total data) were considered outliers — deviating from the mean value by more than three standard deviations— and discarded. The ANOVAs indicated that the main effects of the graphic stimuli (R or F) were not statistically significant (F=0.76, p=0.428 for reaction time; F=0.42, p=0.723 for error rate; F=1.24, p=0.156 for P500 amplitude; and F=0.58, p=0.306 for P500 latency), so results from these two stimuli were pooled for analysis. Additionally, the rotation angles 60° and 300°, and the rotation angles 120° and 240° are equal distances from 0° so they are considered equivalent.[4]The ANOVA results indicated that the results for rotation angles 60° and 300°, and those for angles 120° and 240° were not significantly different (t=1.01, 1.32, p=0.082, 0.075, respectively, for reaction time; t=0.62, 1.15, p=0.102, 0.079 for error rate; t=1.47, 1.68, p=0.076, 0.070 for P500 amplitude; t=0.51, 0.37, p=0.126, 0.162 for P500 latency), so the results for 60° and 300° were pooled and the results for 120° and 240° were pooled. Thus results are presented for four different angles: 0°, 60°(300°), 120°(240°), and 180°. For all four measurements (error rate, reaction time, P500 latency, P500 amplitude) an ‘overall’ value was computed combining the results for all angles.
We used two-way ANOVA to assess reaction time and error rate with one between-subjects factor (group membership: patients vs. controls) and one withinsubjects factor (rotation angle: 0°, 60°, 120°, and 180°). We used three-way ANOVA to assess P500 latency and mean peak P500 amplitude with one betweensubjects factor (group membership) and two withinsubjects factors (rotation and electrode placement) and using the Greenhouse–Geisser method for correction of significance levels.[8]The three-way analysis found that the interaction between electrode placement and group membership was not significant for P500 latency (F=1.53, p=0.058) or for peak P500 amplitude (F=1.26, p=0.063) so results for the four electrode placements were pooled and the results for the two groups at the four angles were assessed using two-way ANOVA. The significance levels of post-hoc comparisons of patientcontrol differences at different angles were adjusted using the Bonferroni correction.
Two-sample t-tests were used to compare group differences at each angle. One-way ANOVA was used to compare results at different angles in each group (i.e., in patients and separately in controls). Paired t-tests were used to compare the patient-control difference in P500 amplitude to the patient-control difference in P700 amplitude at each of the four electrode placements. Between-group differences in age and mean years of education were analyzed using two-tailed t-tests. Sex ratios were checked using a χ2test, and data normality was checked using a probability-probability plot test. A two-tailed α value of less than 0.05 was considered statistically significant.
3. Results
3.1 MR test performance
Table 1 summarizes the MR test performance of subjects in the depressed group and the control group. At all angles of presenting the stimuli the error rate in the depressed group is significantly higher than in the control group and the reaction time in the depressed group is significantly longer. In both the patient group and the control group as the rotation angle increased from 0° to 180° there was a significant stepwise increase in the error rate (F=12.16, p<0.001 and F=10.21, p<0.001, respectively) and a significant stepwise lengthening of the reaction time (F=26.33, p<0.001, and F=18.05, p<0.001, respectively).
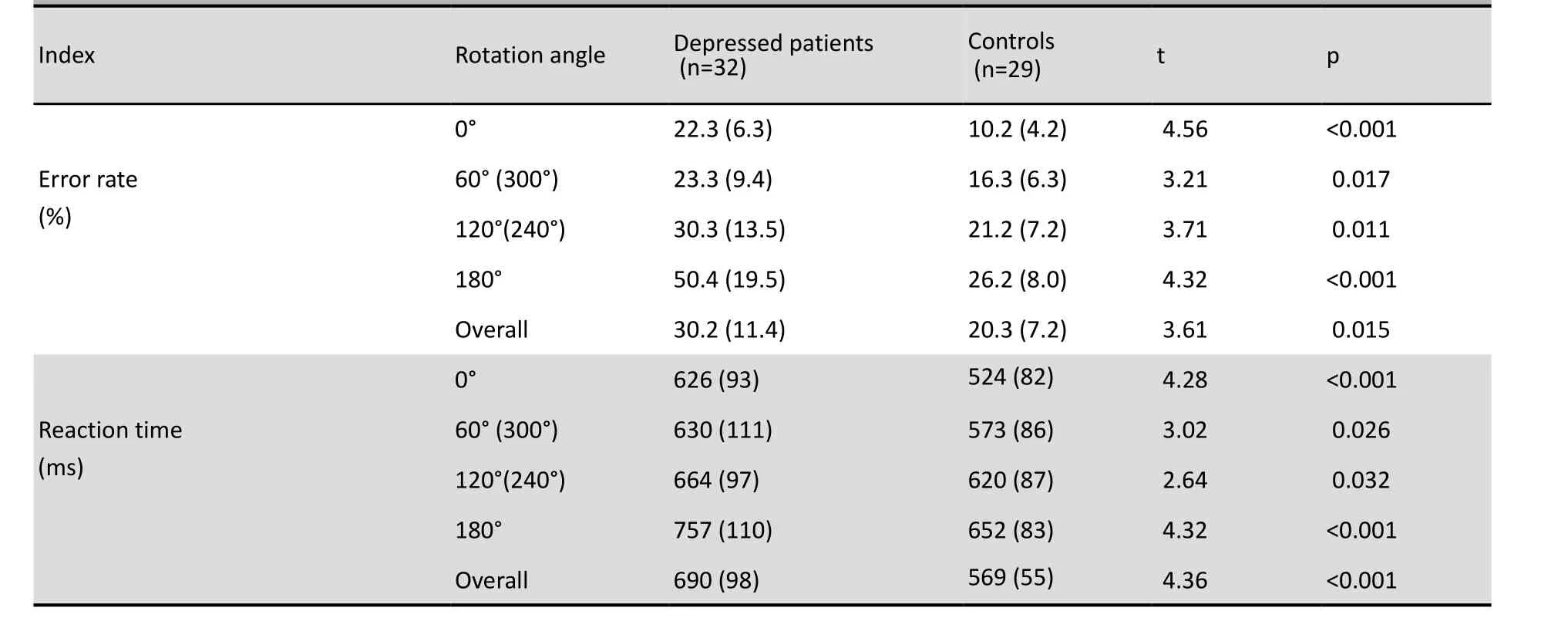
Table 1. Mean (sd) error rates and reaction times for depressed patients and controls
The two-way ANOVA of the MR test results found that the main effects for group membership (F=12.76, p=0.003) and rotation angle (F=21.38, p<0.001) on the mean error rate were both significant. Similarly, the main effects for group membership (F=6.26, p=0.013) and rotation angle (F=18.46, p<0.001) on the mean reaction time were both significant. Moreover, there is a significant interaction between group membership and rotation angle both for the error rate (F=21.19, p<0.001) and for the reaction time (F=24.51, p<0.001). The largest between group (patient vs. control) difference in the error rates was at 180°, followed by the differences at 0°, 120° and 60°; the difference at 180° was significantly greater than those at the other three rotation angles but none of the other pairwise comparisons were significant. The largest between group difference in the reaction time was at 180°, followed by the differences at 0°, 60° and 120°. The differences at 180° and 0° were both significantly greater than the differences at 60° and 120°, but the difference between 180° and 0° and the difference between 60° and 120° were not statistically significant. (Detailed statistical results provided on request.)
3.2 Event-related potentials during the MR test
Figure 2 shows the grand average (by group) ERP waveforms recorded at PZ, CZ, P3, and P4. For both depressed patients and normal controls at all four electrode placements there were positive peaks between 200 and 400 ms and between 400 and 600 ms. In the patient group the ERPs drifted negatively after 500 ms but in the control group there was a third positive peak at about 700 ms.
Table 2 shows the P500 latency and amplitude results for patients and controls at the different rotation angles for each of the four electrode placements. At all four electrode placements for all four rotation angles the P500 latency is longer in the patient group than in the control group and peak P500 amplitude is lower in the patient group than in the control group. These differences are all significant for the peak P500 amplitude but for P500 latency they are only statistically significant for rotation angles 0° and 180°. For all four electrode placements the average P500 latency is longer in the patient group than in the control group and the average P500 amplitude is lower in the patient group than in the control group. These differences were only statistically significant for P500 amplitude, not for P500 latency. Despite the relatively small sample size the power for these comparisons were all >79%.
For both patients and controls at all four electrode placements the P500 latency and the P500 amplitude increased significantly in a stepwise manner as the rotation angle increased from 0° to 180°. In the patient group the main effect of rotation angle for P500 latency at the different electrode placements were as follows: PZ, F=15.21; CZ, F=14.75; P3, F=15.61; P4, F=15.31; and the main effect of rotation angle for P500 amplitude were as follows: PZ, F=8.41; CZ, F=8.22; P3, F=8.62; P4, F=8.29 (all p-values<0.001). In the control group the main effect of rotation angle for P500 latency at the different electrode placements were as follows: PZ, F=8.46; CZ, F=9.31; P3, F=8.20; P4, F=8.96; and the main effect of rotation angle for P500 amplitude were as follows: PZ, F=10.82; CZ, F=10.46; P3, F=10.63; P4, F=11.03 (all p-values<0.001).
Three-way ANOVA revealed that the main effect of rotation angle on P500 latency was statistically significant (F=2.68, p=0.023) and the main effect ofelectrode placement was statistically significant (F=2.52, p=0.033), but the main effect of group membership was not statistically significant (F=1.24, p=0.078). For P500 amplitude the main effect of rotation angle (F=20.16, p<0.001), electrode placement (F=6.12, p=0.028), and group membership (F=10.26, p=0.010) were all statistically significant. The interaction between group membership and electrode placement for peak P500 amplitude was not statistically significant (F=1.26, p=0.063), but the interaction between group membership and rotation angle was statistically significant (F=23.79, p<0.001). Pooling results for the four electrode placements the two-way ANOVA of group by rotation angle found that the largest between group difference in the P500 amplitude was at 180°, followed by the differences at 0°, 60° and 120°. The differences at 180° and 0° were both significantly greater than the differences at 60° and 120°, but the difference between 180° and 0°, and the difference between 60° and 120° were not statistically significant. (Detailed statistical results provided on request.)
Based on the results for recorded waveforms (Figure 2) we also compared peak P700 amplitudes between patients and controls. The mean peak amplitude of P700 was significantly smaller in the patient group than in the control group at all four electrode placements (Pz: 3.27 [1.32] μV vs. 7.89 [2.11] μV, respectively, t=3.19, p=0.016; Cz: 1.32 [0.53] μV vs. 4.12 [1.35] μV, t=2.56, p=0.025; P3: 3.68 [1.20] μV vs. 8.25 [2.27] μV, t=3.20, p=0.015; P4: 4.38 [1.64] μV vs. 7.02 [2.36] μV, t=2.45, p=0.027). Moreover, the difference between patients and controls in peak P700 amplitude was greater than the patient-control difference in peak P500 amplitude at all four electrode placements (Pz: 4.55 [1.68] μV vs. 1.86 [1.01] μV, respectively, paired-t=2.36, p=0.035; Cz: 2.79 [1.13] μV vs. 2.40 [1.10] μV, paired-t=1.28, p=0.069; P3: 4.46 [1.13] μV vs. 1.65 [0.84] μV, paired-t=2.41, p=0.033; P4: 2.76 [1.53] μV vs. 2.68 [1.36] μV, paired-t=0.86, p=0.088).

Figure 2. Grand average ERP waveforms in normal and depressed subjects

Table 2. Mean (sd) P500 latency and peak amplitude for depressed patients and controls
4. Discussion
4.1 Main findings
Consistent with the findings of Rogers and colleagues[2]the current study found increased errors and delayed reaction time in depressed patients administered the MR test compared to normal control subjects, suggesting that they have deficiencies in cognitive processing time and in spatial orientation skills.
Many studies have demonstrated that the P500 component is related to MR tasks.[4,9-11]In our study the overall latency (i.e., combining results for all angles of rotation) of P500 at the four electrode placements assessed was longer in the depressed group than in the control group, but this difference was not statistically significant. Consistent with previous studies[12-14]the latency was significantly longer among depressed patients at rotations of 0° and 180° (i.e., no rotation and maximum rotation), but not significantly longer at rotations of 60° and 120°. Thus the behavioral measure for assessing mental processing time (i.e., the reaction time) was significantly impaired but the electrophysiological measure of mental processing time (i.e., P500 overall latency) was not significantly impaired. Despite the relatively small sample size, the power for these comparisons was satisfactory so it is unlikely that the difference between the behavioral and electrophysiological results is due to Type II errors. This result suggests that the processing time impairment may be state-dependent (possibly associated with the severity of depression), not a fundamental underlying trait.
For both of the behavior measures (error rate and reaction time) and for the P500 amplitude the difference between patients and controls was greater at 180° and 0°, and smaller at 60° and 120°. The consistency of this pattern across different measures suggests some underlying mechanism; further research would be needed to clarify why different rotation angles for presenting the stimuli would have such an effect.
The decrease in the P500 amplitude for depressed patients was statistically significant for all rotation angles at all four electrode placements, suggesting that this is a potential biomarker for the cognitive dysfunction associated with depression. But it is also possible, as suggested by Liu and colleagues,[15]that the slower reaction time in patients with depression increases the spread (standard deviation) of the P500 wave and thus results in a decrease in the maximum amplitude of the P500 wave. Combination of the MR test behavior measures (i.e., error rates and reaction time) and electrophysiological measures (i.e., P500 latency and amplitude) may help to distinguish the trait-dependent and state-dependent components of attention deficits in patients with depression.
For both depressed subjects and control subjects, the error rate, reaction time, P500 latency and peak P500 amplitude increased as the rotation angle increased, suggesting that the difficulty of the discrimination tasks increases as the angle of rotation increases. Previous studies have also found this relationship for the behavior measures[16,17](i.e., error rate and reaction time) and for the electrophysiological measures[10,12,13,17](i.e., P500 latency and amplitude).
The average ERPs measured from the PZ, CZ, P3, and P4 electrodes of both depressed subjects and control subjects showed two positive peaks, one between 200 and 400 ms and another between 400 and 600 ms. These features have consistently been reported in other studies[4,9-12,17-19]and taken together provide strong evidence for a distinct electrophysiological marker for the MR task: a significant positive peak in the parietal region with a latency of 500 ms and a peak amplitude of 8 to 15 μV.
We also identified a positive ERP peak between 600 and 800 ms in the control group that was not found in the patient group, a finding that was also reported by Harris and colleagues.[20]The difference in the peak P700 amplitude between patients and controls was significantly greater than the difference in peak P500 amplitude between patients and controls at the PZ and P3 electrode placements. This is an interesting finding that suggests that the decrease in the peak P700 amplitude may be a more robust marker for cognitive impairment in depression than the decrease in peak P500 amplitude.
4.2 Limitations
This study used first-episode, drug-naïve patients with depression so we are certain that the observed results are not confounded by medication effects. But we did not correlate the magnitude of the decrement in the MR ability with the magnitude of the ERP changes or with the severity of the depressive symptoms, so the correlation between these three phenomena remains uncertain. Identifying the causal relationships between these variables will require prospective follow-up studies that assess changes in MR test results, ERP parameters and depressive symptoms over time. Our results are only relevant for those who receive psychiatric treatment for first-episode depression. These relationships may be different in persons with chronic depression or in those who have relatively mild depression that does not require mental health care.
We did not assess potential differences in results for normally oriented versus mirror-image stimuli and we limited our assessment of ERP parameters to two P500 measures, though we also conducted a post-hoc analysis of P700 amplitude. A wider selection of outcome measures may have generated different results. The relatively small sample made it impossible to adjust theresults for potential confounders such as gender, age, duration of illness and severity of depressive symptoms. MR tests only assess one component of cognition, so results for different measures of cognition may be different.
4.3 Significance
Our study confirms previous work on cognitive deficits in depression and provides some new insights that may aid in the understanding of the electrophysiological mechanisms underlying impaired brain function in depressed patients. The finding of a greater decrement in the peak P700 amplitude than in the peak P500 amplitude during MR tests in patients with depression has not been highlighted in previous reports so it certainly deserves further study. Prospective studies that assess changes in these complex relationships over time will help determine whether or not the behavioral and ERP measures during MR tests can be used as biological markers for depression that could clarify the diagnosis, predict outcome, or be used to assess the effectiveness of treatments.
Conflict of interest
The authors report no conflict of interest.
Funding
This study was supported by the Traditional Chinese Medicine Research Program of the People’s Liberation Army (Grant No: 10ZYX108).
1. Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science 1971; 171(3972): 701-703.
2. Rogers MA, Bradshaw JL, Phillips JG, Chiu E, Mileshkin C, Vaddadi K. Mental rotation in unipolar major depression. J Clin Exp Neuropsychol 2002; 24(1): 101-106.
3. Yao SQ, Wu DX, Liang BY, Guo WB. Relationship between automatic thoughts, depression and event-related potentials of Chinese emotional words in major depression. Chinese Journal of Clinical Psychology 2003; 11(3): 192-194. (in Chinese)
4. Wijers A, Otten L, Feenstra S, Mulder G, Mulder L. Brain potentials during selective attention, memory search, and mental rotation. Psychophysiology 1989; 26(4): 452-467.
5. Psychiatric Branch of Chinese Medical Association. Chinese Classification of Mental Disorder. 3rd ed. Jinan: Shandong Science and Technology Press, 2004. (in Chinese)
6. Zheng YP, Zhao JP, Phillips M, Liu JB, Cai MF, Sun SQ, et al. Validity and reliability of the Chinese Hamilton Depression Rating Scale. Br J Psychiatry 1988; 152(5): 660-664.
7. Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol 1958; 10(2): 371-375.
8. Bathke AC, Schabenberger O, Tobias RD, Madden LV. Greenhouse–Geisser Adjustment and the ANOVA-Type Statistic: Cousins or Twins? Am Stat 2009; 63(3): 239-246.
9. Milivojevic B, Johnson BW, Hamm JP, Corballis MC. Non-identical neural mechanisms for two types of mental transformation: Event-related potentials during mental rotation and mental paper folding. Neuropsychologia 2003; 41(10): 1345-1356.
10. Núñez-Peña ML, Aznar J, Linares D, Corral MJ, Escera C. Effects of dynamic rotation on event-related brain potentials. Cogn Brain Res 2005; 24(2): 307-316.
11. Chen J, Yang LQ, Zhao J, Li LL, Liu GX, Ma WT, et al. Hemispheric dominance in the mental rotation task in schizophrenia. Shanghai Arch Psychiatry 2012; 24(2): 76-82. (in Chinese)
12. Milivojevic B, Hamm JP, Corballis MC. Hemispheric dominance for mental rotation: it is a matter of time. Neuroreport 2009; 20(17): 1507-1512.
13. Núñez-Peña M, Aznar-Casanova J. Mental rotation of mirrored letters: evidence from event-related brain potentials. Brain Cogn 2009; 69(1): 180-187.
14. Kawamichi H, Kikuchi Y, Ueno S. Spatio-temporal brain activity related to rotation method during a mental rotation task of threedimensional objects: an MEG study. NeuroImage 2007; 37(3): 956-965.
15. Liu LK, Huang-Fu E, Miao DM, Liu XF. The progress of studies on event-related potentials P300 and mental rotation. Chinese Journal Aerospace Medicine 2004; 15(4): 248-250. (in Chinese)
16. Cooper LA, Shepard RN. Turning something over in the mind. Sci Am 1984; 251(6): 106-107, 110-114.
17. Hamm JP, Johnson BW, Corballis MC. One good turn deserves another: an event-related brain potential study of rotated mirrornormal letter discriminations. Neuropsychologia 2004; 42(6): 810-820.
18. Overney LS, Blanke O. Impaired imagery for upper limbs. Brain Topogr 2009; 22(1): 27-43.
19. Logie R, Pernet C, Buonocore A, Della Sala S. Low and high imagers activate networks differentially in mental rotation. Neuropsychologia 2011; 49(11): 3071-3077.
20. Harris IM, Miniussi C. Parietal lobe contribution to mental rotation demonstrated with rTMS. J Cogn Neurosci 2003; 15(3): 315-323.
· 论著 ·
背景抑郁症患者的心理旋转意象能力受损,但与此相关联的异常脑电生理机制仍不清楚。
目的比较抑郁症患者与健康对照完成心理旋转任务时的事件相关电位(event-related potentials,ERPs)的差异。
方法对32例住院或门诊首发抑郁症患者和29名对照进行心理旋转任务的ERP测定,给予不同旋转角度的测试刺激。测定4个脑区的ERPs(PZ、CZ、P3和P4)。测量指标包括错误数、反应时以及P500潜伏期和波峰值。
结果与对照组相比,患者组完成心理旋转任务的反应时显著延长[689(98)ms 比 569 (55)ms; t=4.36, p<0.001],错误率显著升高[30.2%(11.4%)比20.3%(7.2%); t=3.61, p=0.015],且在所有4个旋转角度均有差异。完成心理旋转任务时的事件相关电位测定发现,抑郁症患者在4个脑区的总P500潜伏期有所增加,但无统计学意义,而P500波峰值显著降低。患者组与对照组错误率、反应时、P500潜伏期及波峰值均随着旋转角度(0°~180°)的递增而呈逐渐增加。对照组平均ERP波形在700ms处出现一个正波峰,而在患者组未见。
结论本研究证实了既往研究利用心理旋转任务评定抑郁症患者认知功能缺陷的有效性。本研究发现心理旋转任务诱发的事件相关电位的电生理指标,特别是P500及P700波峰值,可能是抑郁症的潜在生物学标记。需要前瞻性研究测定上述指标在抑郁症病程中的变化,以证实其有效性。
首发抑郁症患者心理旋转任务的事件相关电位研究
陈玖 杨来启* 刘光雄 张彦 吴兴曲 马文涛 邓自和
中国人民解放军第三医院全军精神疾病防治中心,陕西宝鸡
*通信作者:ericcst@yahoo.cn
2011-12-06; accepted: 2012-03-28)
10.3969/j.issn.1002-0829.2012.04.005
Center for Mental Disease Control and Prevention, Third Hospital of the PLA, Baoji, Shaanxi, China
*Correspondence: ericcst@yahoo.cn
- 上海精神医学的其它文章
- Prevention and management of missing data during conduct of a clinical study
- Cross-sectional assessment of the factors associated with occupational functioning in patients with schizophrenia
- Cross-sectional study of executive functioning in children with developmental coordination disorders
- Meta-analysis of studies in China about changes in P300 latency and amplitude that occur in patients with schizophrenia during treatment with antipsychotic medication
- Research in China on the molecular genetics of schizophrenia
- Psychiatric symptoms in an individual with tuberous sclerosis

