Research in China on the molecular genetics of schizophrenia
Donghong CUI, Kaida JIANG*
· Review ·
Research in China on the molecular genetics of schizophrenia
Donghong CUI, Kaida JIANG*
Summary:Schizophrenia is a complex disease caused by genetic and environmental factors with a global heritability of more than 80%. By the end of the 1970s, Chinese scientists reported a heritability of schizophrenia of 82.9% in the Chinese Han population. Continuous improvements in research techniques and the recruitment of larger samples have made it possible for Chinese scientists to identify a number of candidate susceptibility genes for schizophrenia. This article reviews the results in genetic research of schizophrenia by Chinese scientists over the last five decades.
1. Introduction
Schizophrenia (SZ) is a severe mental disorder characterized by decrements in cognitive functioning, emotional responsiveness, and behavior. The global lifetime prevalence of this disorder is about 1%. The first onset of SZ is usually during adolescence or adulthood, typically between 15 and 35 years of age. About 50% of SZ patients develop this disorder between 20 and 30 years of age, and smaller fractions of patients develop it before 10 years of age (childhood-onset SZ) or between 40 and 50 years of age (late-onset SZ). In most patients the progression is continuous, with episodes of recurrent symptoms interspersed with periods of partial remission. Consequently, the illness is usually chronic and the prognosis is poor in terms of social development and cognitive functioning. Although the exact pathogenesis of SZ remains unclear there is general consensus that the onset and course of schizophrenia is controlled by both genetic and environmental factors. The genetic basis of SZ has been a topic of intense research worldwide; family, twin, and adoption studies have demonstrated that the heritability of SZ is greater than 80%.
In China SZ has been recognized as a major public health issue. A substantial body of research has developed that aims to clarify the molecular genetic mechanisms underlying the disorder. Improved research techniques and large samples of patients have made it possible to identify a number candidate susceptibility genes for SZ in China. The current article reviews the progress in this field made by Chinese scientists.
2. Classical genetics
In the 1970s, the Shanghai Polygenic Inheritance Collaborative Research Group investigated the heritability of SZ in Chinese individuals using classical genetic methods (i.e., family surveys, twin and adoption studies) and estimated a heritability of 82.9% in the Chinese population. Subsequent studies reported heritability for schizophrenia of 63 to 78% in Chinese individuals[1]and heritability of 70% in Chinese children.[2]Similar to results from international studies, these heritability estimates for SZ became widely accepted in China. But the genetic complexity of schizophrenia and the limitations of research techniques and equipment in China in the 1970s and 1980s made it impossible to conduct more detailed studies of potential susceptibility genes for SZ. By the early 1990s molecular biological techniques had advanced in China, so Chinese neuroscientists started to explore the genes associated with SZ.
3. Family linkage analysis
In the late 1980s, Jiang and colleagues[3]con-ducted a pioneer linkage study in China to locate SZ susceptibility genes in 11 families using HLA antibodies and blood groups (i.e., ABO, P, MN, and Rh) as genetic markers, but they found no association between SZ and these markers, with logarithms of odds (LOD) scores between 0.083 and -0.296. In a subsequent study[4]they analyzed the restriction-fragment length polymorphisms (RFLPs) of the Ha-ras-1 genes in 14 SZ patients and 12 controls by enzymatic restriction and Southern blotting and found a significant association between SZ susceptibility and a 1.7-kb restriction fragment, with a relative risk of 14.47. In 2001, Yu et al.[5]conducted a linkage disequilibrium analysis using microsatellite markers and PCR techniques among male SZ patients in 70 families and found a significant association between SZ and HSU93305 (a locus on the short arm of the X chromosome). One year later, in 2002, Deng et al.[6]performed gene scans for 137 sib-pair families with SZ based on 28 microsatellitemarkers on chromosome 6 and found a relationship between D6S1960 and the positive symptoms of SZ (LOD, 1.39), and a relationship between D6S291 and both the general pathology symptoms of SZ (LOD, 2.5) and the negative symptoms of SZ (LOD, 1.56). Their findings suggested the existence of SZ susceptibility genes on the short arm of chromosome 6. Chen et al.[7]investigated 60 sibling pairs with chronic SZ and 120 patients with sporadic SZ using 60 unaffected sibling pairs and 120 controls as the respective controls; they found significantly higher frequencies of 264 bp and 278 bp alleles at D6S296 in the sibling pairs with chronic SZ than in the control pairs (264 bp, χ2=18.84, p<0.01; 278 bp, χ2=4.59, p<0.05, respectively). Cai et al.[8]performed a linkage analysis and quantitative trait locus study of SZ susceptibility genes for 32 families with familial SZ by analyzing 29 microsatellite markers distributed on chromosome 1. They detected an association between SZ and D1S206 (LOD, 1.71, p=0.046) and D1S425 (LOD, 1.37, p=0.086), suggesting the presence of SZ susceptibility genes on the long arm of chromosome 1. Their results also suggested that the negative symptoms of SZ may be related to independent quantitative trait loci in the chromosomal region 1q21-23. Tang et al.[9]studied a number of candidate chromosomal regions (e.g., 1q21-22, 1q32-44, 5q21-33, 6p24-22, 8p22-21, 10p15-11, 11q23-24, 11p15, 12q23-24, 13q32-34, 22q11-12, 9q34, 16p13, 12q13, 17q25 and 19q13) for a SZ family with 26 members by capillary electrophoresis and only detected an association between SZ and one chromosomal region (i.e, 11q22.1-24.2). In contrast, Zhu et al.[10]and Chen et al.[11]performed microsatellite scans for 119 SZ patients and 119 controls and found positive associations between SZ and several chromosomal regions (i.e., D13S265, D21S1256, D1S2878, D8S264, and D9S273; see Table 1).
4. Genetic association analysis
The progress of molecular biological techniques and, particularly, the use of single nucleotide polymorphisms (SNPs) as genetic markers has greatly improved the efficiency of genotyping and expedited the application of genetic association analysis. In the late 1990s, based on the mechanisms of action of antipsychotic medications, studies on candidate genes for SZ were focused on 5-hydroxytryptamine (5-HT) and dopamine (DA) receptors. The most commonly used designs for association studies were case-control analyses and family-based transmission disequilibrium tests.Subsequent work investigated candidate genes related to various theories about the causes of SZ (e.g., the glutamate hypothesis, neurodevelopmental hypothesis, neurodegenerative hypothesis, immune abnormality hypothesis). Within a relatively short period of time several groups produced results about the association of various genes with SZ. With the exception of chromosomes 16 and Y, all other chromosomes have been examined for linkage with SZ. Table 2 summarizes the genes that have been extensively studied in China.
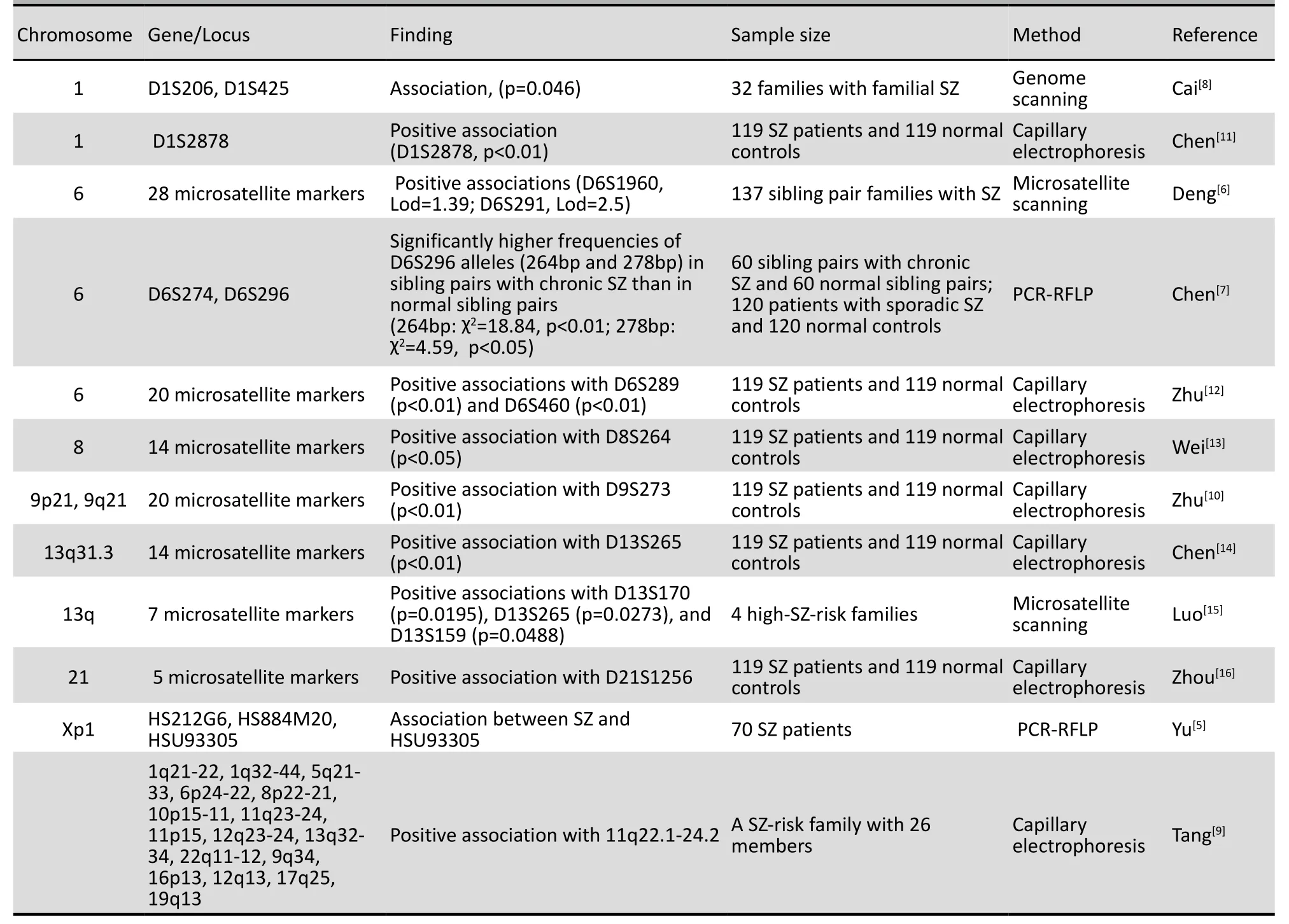
Table 1. Family linkage analysis
4.1 Neurotransmitter-related genes
4.1.1 Dopamine receptor D (DRD) genes
The DRD system consists of two families: the D1-likefamily receptors and the D2-like family receptors. The D1-like family receptors include the DRD1 and DRD5 genes. The D2-like family receptors include the DRD2, DRD3, and DRD4 genes. Between 2000 and 2005 research on the DRD systems in Chinese SZ patients focused on the DRD2, DRD3, and DRD4 genes. Studies first examined DRD2 because of the well-established linear relationship between the effects of antipsychotic medications and their binding affinities to DRD2. These studies investigated the A-241G polymorphism, TaqIA RFLP, C ins/del mutation in the promoter region (position -141), and the (CA)n dinucleotide-repeat polymorphism in intron 1. However, these studies did not find a significant relationship between SZ and DRD2, and only one study (with 101 families) found an association between the DRD2-A241G genotype and the clinical characteristics of SZ (e.g., delusion score, hallucination score, emotional abnormalities, thought form abnormalities, aphasia and the duration of symptoms).[17]
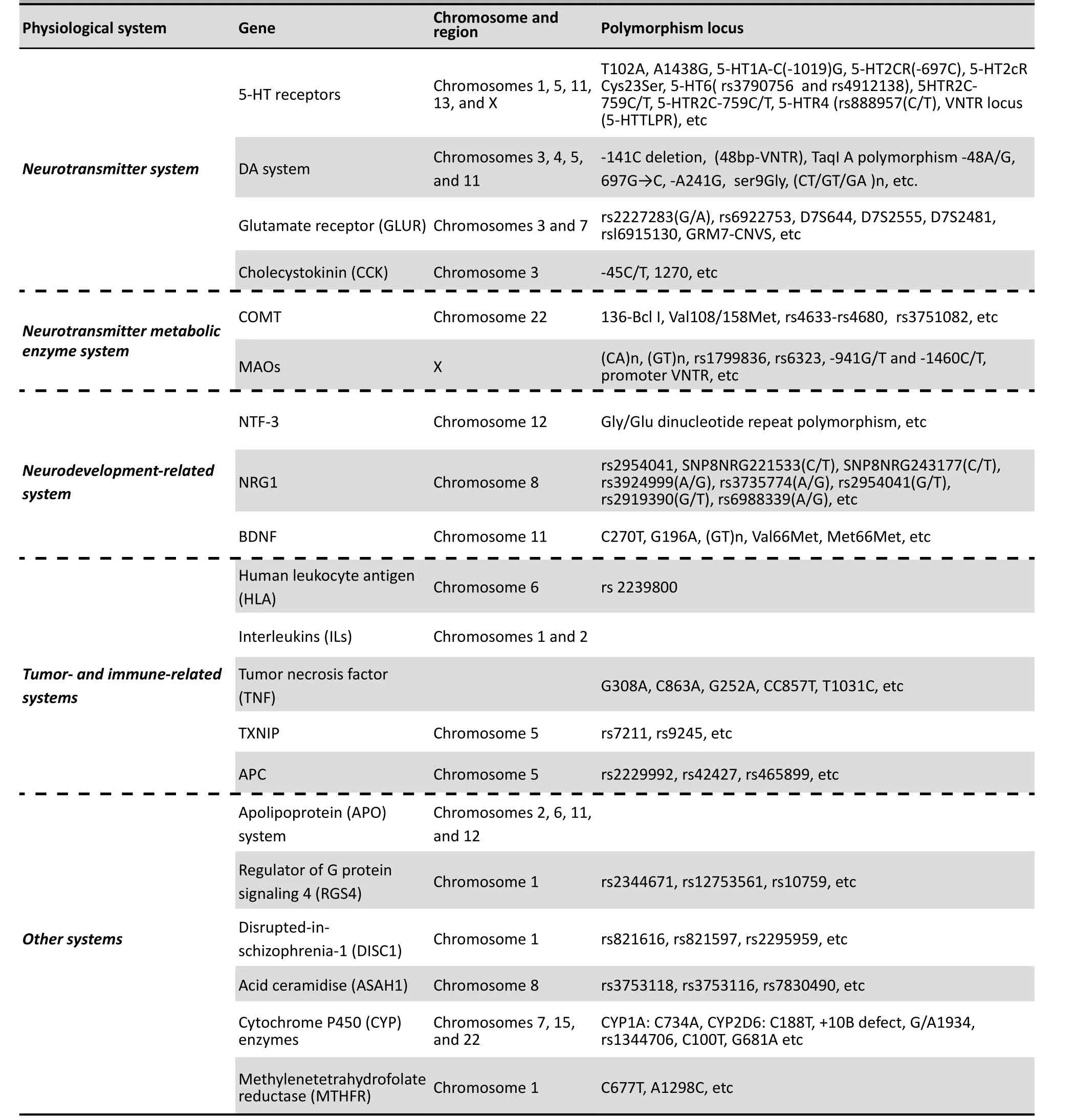
Table 2. Extensively analyzed genes for association with schizophrenia
DRD3 is mainly expressed in the ventral parts of the basal ganglia (e.g., nucleus accumbens). Most studies in China have focused on the Ser9Gly polymorphism of the DRD3 gene. Three out of eight studies[18-20]found associations between the Ser9Gly polymorphism and SZ, while the other five failed to detect an association.
The DRD4 gene is also a major candidate susceptibility gene for SZ because of its predominant expression in the frontal cortex and the high affinity of DRD4 receptors to clozapine, a potent atypical antipsychotic medication. Studies in China have focused on a 48-bp repeating sequence in the third exon of the DRF4 gene, known as 48-bp variable number tandem repeat (VNTR). In 2000, Luo et al.[21]discovered an association between 48-bp VNTR and refractory SZ in a study of 104 SZ patients and 76 controls. In another study of 510 patients and 171 controls in 2001, Tang et al.[22]observed SZ to be related to four repeats of 48-bp VNTR (χ2=13.00, df=1, p<0.001). However, this association was not confirmed in smaller subsequent studies of 38 patients and 76 controls[23]and 162 patients and 162 controls.[24]
After 2005 studies in China increasingly focused on the genes encoding DRD1, DRD5, and DA transporter 1 (DAT1). In 2005, Hu et al.[25]reported a higher frequency of the DRD1 AA genotype in SZ patients than in controls (χ2=6.621, p<0.01), suggesting that the allele A was a susceptibility gene for SZ. In 2011, Zhu et al.[26]studied 385 SZ patients and 350 controls and found an association between SZ and two SNP of the DRD1 gene (i.e., rs686 and rs10063995). However, in another study of 373 patients and 379 controls,[27]Zhang et al. failed to detect an association between SZ and DRD1 SNPs (i.e., rs4532, rs5326, rs2168631, rs6882300 and rs267418). Compared with the extensive research on DRD1, few studies have investigated the DRD5 gene in Chinese individuals, and the studies that do exist have small sample sizes; no significant associations have been found between DRD5 and SZ in Chinese patients.[28,29]
In 2010, Huang et al.[30]performed genotyping for 352 Chinese SZ patients and 311 controls to identify six promoter polymorphisms of the DAT1 gene (i.e., rs6413429, rs2652511, rs2975226, rs6347 and rs27072) and a VNTR of this gene. They found a positive association between SZ and two polymorphisms (rs2652511 and rs2975226) and an association between SZ and a promoter region haplotype (rs2652511-rs2975226-rs6413429).
4.1.2 5-HTR receptors
Human 5-HTR receptors are divided into seven subtypes. One of these subtypes, the 5-HT2A receptor (5-HTR2A), is the target of many antipsychotic medications. Therefore, the 5-HTR2A gene has been the target of extensive research, much of which has focused on the T102C polymorphism. In a 1997 study of 223 patients and 162 controls, Luo et al.[31]first reported that the A1/A1 genotype at the T102C locus was a risk factor for SZ. Some subsequent studies supported this finding,[32,33]but most did not. Zhang et al.[34]conducted a small-scale study and found an association (p<0.05) between 5-HTR2A-A1438G and tardive dyskinesia in chronic SZ, but subsequent larger studies failed to confirm this association. Yi et al.[35]reported an association between SZ and the 5-HTR6 gene (χ2=5.16, p<0.05). A research group at Shanghai Jiao Tong University performed several studies with large samples and found that a repeating sequence (STin2) in the 5-HT transporter (5-HTT) gene was associated with SZ in Chinese patients,[36]but they found no significant associations between SZ and other loci of this gene.[37,38]Studies in China have also investigated the relationship between SZ and 5-HT polymorphisms (e.g., 5-HT1A-C-1019G, 5-HTR1B, 5-HTR2C, 5-HTR4), but the results are inconclusive. A few studies suggested that the polymorphisms of the genes encoding 5-HTT[39]and 5-HTR3A[40]may be useful for predicting the therapeutic response of Chinese patients with SZ to treatment with risperidone.
4.1.3 Neurotransmitter metabolic enzymes
After release into the extracellular space, neurotransmitters are degraded by their metabolic enzymes. The genes encoding these enzymes have also been considered candidate susceptibility genes for SZ. Existing studies have focused on the genes encoding catechol-O-methyltransferase (COMT, an enzyme catalyzing dopamine) and monoamine oxidases (MAOs, key enzymes in the catabolism of monoamine neurotransmitters).
Many studies have focused on the Val158Met polymorphisms of the COMT gene. In 2002, Tang et al.[41]investigated 476 SZ patients and 207 controls, and identified associations between early-onset SZ and the Val158Met polymorphism. Shen Yan’s research group revealed that COMT may play important rolesin the development of paranoid SZ[42,43]and that the COMT gene may be associated with the negative symptoms of chronic SZ.[44]Kang et al.[45]examined the relationship between the P300 event-related potential (ERP) component and the Val158Met polymorphism in Chinese SZ patients and found significantly shorter P300 latencies (at Cz and Pz) in Met homozygotes than those in Val/Met carriers and Val/Val carriers, and significantly shorter P300 latencies (Cz and Pz) in Val/Met carriers than in Val/Val carriers; taken together these results suggest a relationship between the Val158Met polymorphism and P300 abnormalities in patients with SZ. Other studies suggested that this polymorphism may be implicated in cognitive functions,[46]propensity for violence,[47]and tardive dyskinesia[48]in Chinese SZ patients, although a few studies failed to detect such relations.[49,50]Additionally, Liu et al.[51]and Lü et al.[52]reported that SZ is associated with the 900 ins/del and the 287A/G polymorphisms of the COMT gene. In a relatively small-scale study (82 patients and 88 controls) in 2003 Jiang et al.[53]observed an association between SZ and the gene encoding monoamine oxidase B (MAOB), but there was no association between SZ and the gene encoding monoamine oxidase A (MAOA). Subsequently, Wei et al.[54]reported an association between SZ and the rs1799836 polymorphism of the MAOB gene (p=0.00001). Shi et al.[55]performed genetic association analysis for 212 SZ patients and 168 controls, but they detected no association between SZ and the MAOA gene polymorphism. However, a study of Chinese SZ patients in Taiwan[56]reported that SZ was associated with the 941T/G polymorphism of the MAOA gene in male patients, but not in female patients.
4.2 Neuroimmune- and endocrine-related genes
In the mid-1990s the emergence of the immune hypothesis of SZ lead to the realization that genes related to the immunoendocrine system may be SZ susceptibility genes. Human leukocyte antigens (HLAs) are an important component of the human immune system. The genes encoding the HLAs are located on the short arm of chromosome 6. Some large-sample nuclear-family studies and case-control association analyses in China have suggested a relation between SZ and the HLA class-II genes in immune cells.[57,58]Yu et al.[59]analyzed 190 nuclear families with SZ and suggested an association between the rs2239800 SNP of the HLA-DQA1 gene and positive symptoms in SZ. Yu et al.[60]subsequently confirmed this association in 195 nuclear families.
The results from other studies on the association between SZ and HLA class-II genes are inconclusive.[58-61]Yang et al.[61]analyzed 277 SZ patients and 355 controls and observed significant associations between SZ and HLA class-I genes, but a subsequent study[62]of 355 patients and 321 controls failed to confirm these associations. The potential relationship between SZ and genes encoding other immune factors (e.g., IL-2R, L-1β, IL-10, and TNF-α) has also been examined, but the results are inconsistent.
Cholecystokinin (CCK) is a peptide hormone responsible for stimulating the digestion of fat and proteins. The promoter region polymorphisms (i.e., -333G/T and -286A/C) of the CCK receptor gene have also been suggested as susceptibility genes for SZ.[63]Song et al.[64]studied 94 nuclear families with SZ and identified the CCK gene as an important factor affecting the severity of negative symptoms in SZ. Lü et al.[65]analyzed 77 nuclear families and another 32 SZ patients and found an association between the CCK gene polymorphism and the positive symptoms of SZ; this association was confirmed in a subsequent study[66]of 207 female SZ patients and 202 female controls. In contrast, several other studies[67,68]failed to confirm such an association. In addition, Itokawa[69]suggested that the polymorphism of the neuropeptide Y (NPY) gene is associated with SZ, but a subsequent study of 583 Chinese SZ patients failed to find this association.[70]The inconsistency between these studies may be partially attributed to their relatively small sample sizes.
4.3 Cancer-related genes
International epidemiological surveys have revealed that SZ patients have lower incidences of cancers than the general population, suggesting a potential antagonism between SZ and cancers. Using DNA microarray analysis, Cui et al.[71]discovered significantly different expression of the adenomatous polyposis coli (APC) and thioredoxin interacting protein (TXNIP) genes in the peripheral blood leukocytes of Chinese SZ patients compared to controls. The APC gene is a key factor in the Wnt signaling pathway and is located in 5q21-22, a chromosomal region reported to host many SZ susceptibility genes. The TXNIP gene is located in 1q21.1 (another region containing SZ susceptibility genes) and participates in cell proliferation and differentiation. In particular, this gene also participates in suppressing tumor metastasis and is involved in neuron damage. In another study,[72]the same research group analyzed 169 nuclear families and found significant associations between SZ and three SNPs of the APC gene (rs2229992, χ2=4.23, p<0.05; rs42427, χ2=4.15, p<0.05; rs465899 χ2=8.49, p<0.01), and a significant association between SZ and the APC haplotypes from rs2229992-rs42427-rs465899 (χ2=44.376, p<0.05). Subsequently, this group investigated[73]182 nuclear families and identified associations between SZ and the APC rs7211 SNP (χ2=6.32, p=0.012), as well as between SZ and the haplotypes from rs7211-rs9245 (χ2=5.01, df=1, p=0.024).
4.4 Neurodevelopment-related genes and others
After 2000, with the development of the neurodevelopment hypothesis for SZ, a series ofneurodevelopment-related genes were investigated as possible SZ susceptibility genes. Brain-derived neurotrophic factor (BDNF) is a protein with important functions in the growth, survival, and differentiation of neurons and has vital roles in the hippocampus and the dopaminergic system. Studies have focused on the C270T, Val66Met, and (GT)n dinucleotiderepeat polymorphisms of the BDNF gene. In 2005, He et al.[74,75]reported an association between SZ and the C270T polymorphism. The association was confirmed by subsequent studies.[76-78]Xiu et al.[79]identified the G196A and C270T polymorphisms of this gene as risk factors in SZ patients who were smokers. Two studies[80,81]found no direct association between SZ susceptibility and the Val66Met polymorphism in Chinese patients, but both studies found that the polymorphism was related to the age of onset of SZ. Xu et al.[82]performed case-control association analysis and meta-analysis for Chinese patients but detected no association between SZ susceptibility and the C270T or Val66Met polymorphism. In another study, these authors[83]identified an association between BDNF (GT)n dinucleotide-repeat polymorphism and SZ, and associations between the polymorphism and chlorpromazine-induced extrapyramidal adverse effects and therapeutic response to treatment with risperidone. Collectively, the available results on Chinese patient groups support the roles of the BDNF gene in the pathogenesis of SZ.
Neuregulin 1 (NRG1) is another protein essential for the development and differentiation of neurons. Only a few studies have investigated the relation between NRG1 and SZ in Chinese individuals. A study of 258 nuclear families with SZ[84]and another study examining 315 patients and 347 controls[85]found an association between NRG1 and SZ in Chinese patients.
Methylenetetrahydrofolate reductase (MTHFR) is a key methyl transferase affecting the development of the nervous system and also the principal methyl donor in the human body. The C/T polymorphism at the 677 bp (T677C) of the MTHFR gene has been found to affect the enzymatic activity of MTHFR. Several case-control studies have supported the association between SZ susceptibility and the T677C polymorphism in Chinese individuals.[86,87]The study by Yang et al.[86]with 100 firstepisode SZ patients and 100 controls suggested that T677C polymorphism is associated with SZ, a finding that was confirmed by the study of Feng et al.[88]among 123 patients and 123 controls. But Shi et al.[89]did not detect this association in a study of 106 nuclear families with SZ.
Regulator of G protein signaling 4 (RGS4) is a protein that participates in neuron differentiation and modulates serotonergic (5-HTergic) receptors and metabolic glutamate receptors. The RGS4 gene is located in a chromosomal region containing candidate SZ genes, and its expression is regulated by dopaminergic neurotransmitters. In two studies (one including 386 SZ patients and 390 controls[90]; the other 315 patients and 347 controls[84]) Yue et al. found associations between SZ and two polymorphisms of the RGS4 gene (rs12753561: χ2=8.97, p=0.002; rs10759: χ2=13.77, F=0.002, p=0.002). In a study of 504 SZ patients and 531 controls, So et al.[91]analyzed the relation between SZ and four rGS4 SNPs (rs10917670(G/A), rs951436(G/T), rs951439(A/G), rs2661319(A/G). Their results suggested that the GGGG haplotype may be a risk factor for the development of SZ, but studies by Zhang et al.[92]and Guo et al.[93]did not confirm these results.
Neurotrophin-3 (NTF3) is a protein closely related to the survival, proliferation, and migration of neurons. International studies have revealed the association between SZ susceptibility and the G304A polymorphism of the NTF3 gene. Few studies, however, have examined the role of NFT3 in Chinese SZ patients. Wu et al.[94]compared 80 patients with 81 controls and found an association between SZ and the Gly-63/ Glu-63 polymorphism of the NTF3 gene. Du et al.[95]confirmed this association (χ2=6.86, p<0.05) in a largerscale study in children with SZ. Studies on other NTF-3 gene polymorphisms, such as the dinucleotide-repeat polymorphism examined by Deng et al.,[96]failed to detect an association with SZ in Chinese patients.
Disrupted-in-schizophrenia-1 (DISC1) is a multifunctional regulator of cell activities, such as neuronal axis and dendrite outgrowth. The DISC1 gene, first discovered in a family with SZ in Scotland, is characterized by a balanced (t[1;11] q[42.1;14.3]) chromosomal translation. In 2007, Qu et al.[97]compared 313 SZ patients and 317 controls, and identified associations between SZ susceptibility and two DISC1 gene SNPs (rs821616: χ2=7.8006, p= 0.0052; rs821597: χ2=9.5404, p=0.0020). These findings were confirmed in a subsequent study[98]of 466 patients and 551 controls (rs821616: χ2=7.063, p=0.008; rs821597: χ2=6.009, p=0.014); this second study also reported that the GA haplotype was a risk factor for SZ (χ2=6.01, p=0.014). Several small-scale case-control studies[99,100]also found an association between the rs821616 polymorphism and SZ. But another study[101]of 560 patients and 576 controls failed to detect significant associations between SZ and the two loci; this study found a weak association between the rs2295959 polymorphism and SZ in the female patients (χ2=6.188, p=0.0135, OR=0.728, 95% confidence level: 0.567–0.935).
N-acylsphingosine amidohydrolase 1 (ASAH1, also known as acid ceramidase) is a critical enzyme that catalyzes sphingomyelin synthesis and participates in brain neuronal development and in the metabolism of sphingomyelin. The ASAH1 gene is located in 8p22-21.3, a ‘hotspot’ region of SZ susceptibility genes. Zhang et al.[102]investigated ASAH1 SNPs in 254 SZ families and identified two haplotypes (rs3753118T-rs7830490A and rs3753118T-rs3753116G-rs7830490A) that were riskfactors for SZ. In another study, they also identified[103]linkage between the SNPs and the quantitative traits loci (QTL) in SZ (rs3753118: p=0.030; rs3753116: p=0.030; rs7830490: p=0.036).
The roles of other genes in SZ have also been examined in Chinese patients, such as the genes encoding proline dehydrogenase (PRODH), tryptophan hydrogenase (TPH), apolipoprotein D(APOD), apolipoprotein E(APOE), neuronal acetylcholine receptor subunit α-7(CHRNA7), and dopamine β–hydroxylase(DBH). For example, Yue et al.[104]detected an association between SZ susceptibility and the PRDH gene in a study of 330 patients and 334 controls. This association was confirmed by another study of paranoid SZ patients.[105]Three separate studies[106-108]suggested that TPH gene polymorphisms may be associated with SZ susceptibility in female patients, male patients, and minority ethnic groups of Chinese citizens.
Despite a large number of studies, the available studies on candidate SZ susceptibility genes for Chinese individuals have a common limitation of relatively small sample sizes. Consequently, results from these studies need to be verified by larger scale in-depth research.
5. Expression of schizophrenia-related genes
In the 2000s, the development of DNA microarrays provided an effective tool for clarifying the genetic mechanisms of SZ. Cui et al.[71]first analyzed the peripheral blood leukocyte gene expression patterns of six Chinese patients with SZ and six controls using cDNA microarrays with 8,464 genes. Compared with the patterns of the controls, the SZ patients showed 31 differentially expressed genes (DEGs, 29 downregulated; the other 2 up-regulated). These DEGs (Table 3) included cancer suppressor genes, cancerrelated genes, and neurodevelopment-related genes. Subsequently, this research group compared the gene expression patterns of 30 drug-naïve, first-episode SZ patients, 30 medicated patients with recurrent SZ, and 30 controls. They confirmed changes in the expression levels of APC, TXN1, and ASH1 genes by quantitative realtime PCR and were the first to report the relationship of the three genes to SZ.[72,102,94]
The emergence of quantitative real-time PCR around 2005 offered more opportunities for genetic research. Correspondingly, many studies in China have applied quantitative (or semi-quantitative) real-time PCR to investigate the expression of candidate SZ susceptibility genes in peripheral blood cells, such as genes related to the dopamine system and immune factors. Liu et al.[109]analyzed the expression of the genes encoding interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and tyrosine hydrogenase (TH) in 30 SZ patients, 25 control siblings, and 30 controls. The expression levels of L-1β, TNF-α, and TH were similar in the SZ patients and their siblings, but substantially lower in the controls, suggesting the presence of dopaminergic hyperfunction and inflammatory cytokine overexpression in SZ patients. In another study, Liu et al.[110]compared the expression levels of the nitric oxide synthase (NOS) gene in 63 SZ patients, 54 control siblings, and 51 controls. Their results indicated a NOS overexpression in the SZ patients that was positively correlated with the severity of positive symptoms.
Despite these positive results, the exact relations between SZ susceptibility gene expression in the peripheral blood and the expression in the central nervous system (e.g., to what extent the former represents the latter) remains unclear. So after 2007 the number of studies solely focused on the expression of SZ susceptibility genes rapidly decreased. One exception is the 2010 study by Zhang et al.[111]which found increased DRD1 gene expression in patients with first-episode SZ compared with controls, a positive correlation between the level of DRD1 expression and the severity of negative symptoms, and an inverse relationship between the level of DRD1 expression level and the level of aggressiveness of the patient. Another exception is the study by Zhang et al.[112]that found no significant association between SZ and the expression of the G72 gene.
6. Genome-wide association studies (GWASs)
With rapid development of DNA microarray techniques, particularly since 2007, several GWASs have been conducted to investigate the genetic mechanisms of SZ. In 2011 two research groups in China independently conducted large-sample GWASs in Chinese SZ patients. As shown in Table 4, Shi et al.[113]analyzed 546,561 SNPs in 3,570 individuals with schizophrenia and 6,468 controls using Affymetrix 6.0 microarrays and discovered associations between SZ and five SNPs on chromosomes 1 and 8. Yue et al.[114]analyzed 620,901 SNPs and copy number variations in 746 individuals with schizophrenia and 1,599 controls using Illumina 610 microarrays and independently validated the findings in 4,027 patients and 5,603 controls, identifying significant associations between SZ and 6 SNPs on chromosomes 6 and 11. However, there was no overlap in the identified SNPs between these two large-sample studies.
7. Challenges and prospects in molecular genetics of schizophrenia
7.1 Challenges
Chinese scientists have conducted a large number of studies on the molecular genetics of SZ and have identified or confirmed a large number of susceptibility genes. However, many of these studies are repetitive or superficial and most of them have relatively small sample sizes so the results all need to be verified by multiple, large-scale studies. The two available GWASs studies used large samples but they still had inconsistent
results; this may have been due to different recruitment procedures or heterogeneity within the samples.
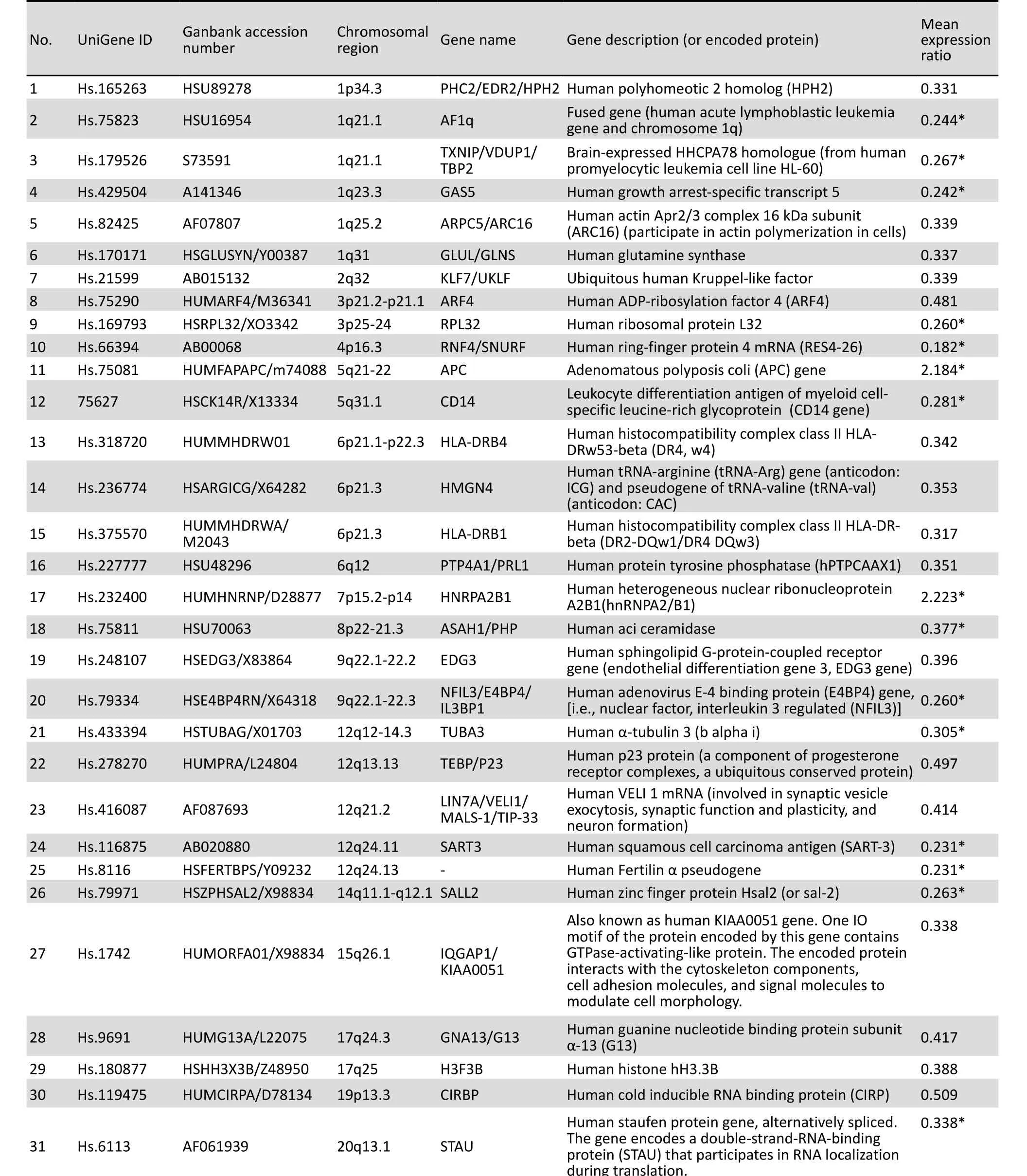
Table 3. Summary of 31 differentially expressed genes screened by DNA microarray assays
Furthermore, the GWAS methodology has several weaknesses: a) Simultaneously analyzing tens of thousands of loci inevitably results in severe error accumulation that may not be fully resolved by using statistical corrections for multiple testing. There is no standard method available to differentiate true positives from false positives; b) As a multifactorial disorder, SZ is only weakly associated with individual genes so these weak associations may be missed; c) The GWAS may not be appropriate for complex diseases like SZ that probably involve the interaction of different genes; d) Microarrays for GWAS may have flaws that adversely affect the accuracy of genotyping analysis; e) Effective research strategies and statistical methods for GWAS are, as yet, imperfect.
7.2 Prospects: exome sequencing, whole-genome sequencing, and applications
Since 2000, the introduction of the Roche 454, Illumina Solexa, and ABI SOLiD platforms has marked the appearance of second-generation sequencing technologies. The rapid advance of high-throughput sequencing platforms multiplies sequencing speed and reduces cost. This technical progress has made exome sequencing and whole-genome sequencing possible, thereby allowing accurate localization of pathogenic genes of SZ. Relevant studies are underway worldwide, including in China, but no results have been reported. We believe that in the next several years these secondgeneration sequencing technologies will lead to breakthrough discoveries in the molecular genetics of SZ in China and elsewhere.

Table 4. Two available genome-wide association studies on Chinese SZ patients
1. Lou HM, Wu YB, Zhao YZ. An investigation of the heritability of schizophrenia in northeast China.Chinese Journal of neurology and psychiatry1983;16(1):49-50. (in Chinese)
2. Xia ZY, Jiang SD, Bei GP, Lin ZG, Feng GY, Li CF. Genetic factors and hereditary patterns of pediatric schizophrenia.Chin J Nerv Ment Dis1982;8(1): 18-21. (in Chinese)
3. Jiang SD, Feng GQ, Bei GP, Lin ZG. Genetic marker studies in the families with schizophrenia.Hereditas1991;13(3): 31-32. (in Chinese)
4. Jiang SD, An X, Ren DM. RFLP of a rare Ha-ras-1 gene in schizophrenic patients.Nature Magazine1990;13(2): 126-127. (in Chinese)
5. Yu YQ, Li B, Shi JP, Zhang M, Wei J, Quo YJ. Susceptibility locus for schizophrenia possibly located within chromosome Xp11.China Public Health2001;17(10): 879-880. (in Chinese)
6. Deng H, Liu XH, Cai GQ, Terwedow H, Wang CY, Xu X. Susceptibility of schizophrenia on chromosome 6 in affected-sib-pair families.Chin J Psychiatry2002;35(2): 69-72. (in Chinese)
7. Chen JF, Wang WY, Wang SH, Han G, Wang GQ, Jiao GK, et al. Association between schizophrenia sibling and the chromosome D6S274 and D6S296 markers.Chin J Psychiatry2005;38(2):73-75. (in Chinese)
8. Cai GQ, Wu XR, Li T, Collier DA, Liu XH, Feng BJ, et al. Linkage analysis of susceptibility genes for familial schizophrenia on chromosome 1 in Chinese population.Chin J Med Gener2002;19(6):491-494. (in Chinese)
9. Tang JS, Chen XG, Xu XJ, Xu L, Xia K. A linkage study of schizophrenia candidate regions in a Chinese multiplex pedigree.Chin J Psychiatry2006;39(3): 145-148. (in Chinese)
10. Zhu HN, Chen G, Wen XY, Lin RX, Zhu JP, Wei R, et al. A screen study for genetic loci associated with schizophrenia on chromosome 9. J Clin Psychol Med 2007; 17(3): 151-153. (in Chinese)
11. Chen G, Wen XY, Zhu HN, Wei R, Luan M, et al. Association mapping of schizophrenia loci on chromosome 1 by use of pooled DNA genomic screening in eastern Shandong peninsula. Chin J Med Gent 2007; 24(3): 288-292. (in Chinese)
12. Zhu HN, Chen G, Wen XY, Lin RX, Zhu JP, Wei R et al. A genomic screen for genetic loci associated with schizophrenia on chromosome 6 in certain region of Shandong Province. Chin J Psychiatry 2007; 40(2): 82-85. (in Chinese)
13. Wei R, Zhou P, Wen XY, Lin RX, Luan M, Zhu HN et al. the study on chromosome 8 of schizophrenia in eastern Shandong peninsula. Chin J Nerv Ment Dis 2007; 33(2): 77-81. (in Chinese)
14. Chen G, Wen XY, Lin RX, Luan M, Zhou P, Yu X et al. The screen for genetic loci associated with schizophrenia on chromosome 13 in eastern Shandong peninsular. J Psychiatry 2007;20(2): 65-68.
15. Luo J, Cai Z, Dong F, Hu Z, Jiang T, Nie C, et al. Linkage analyses of 13q in schizophrenia complex pedigrees. J Capital Med Uni 2008; 29(4): 412-415.
16. Zhou P, Wei R, Wen XY, Lin RX, Zhu JP, Weng Z, et al. Association mapping of schizophrenia on chromosome 21, by use of pooled DNA genomic screen in eastern Shandong peninsular. Shandong Arch Psychiatry 2006; 19(4): 241-244.
17. Hu XZ, Zhou RL, Zhou CF, Chen CH, Wang YF, Han YH, et al. Association between A-241G polymorphism of dopamine D2 receptor promoter gene and schizophrenia in Chinese. Journal of Beijing Medical University 2000; 32(6): 551-554. (in Chinese)
18. Tang YL, Wang YF, Cai ZJ, Zhou RL, Zhou CF. Association study of dopamine D3 receptor gene with schizophrenia subtypes. Chin J Psychiatry 2002; 35(1): 11-14. (in Chinese)
19. Yao ZJ, Zhang ZJ, Sun J, Chen JF, Zhu RX, Yao H, et al. Association of dopamine D3 receptor gene functional polymorphism with response to antipsychotics in first-episode schizophrenic patients. Chin J Psychiatry 2003; 36(4): 200-203. (in Chinese)
20. Wang YH, Shi YZ, Lü LX, Zhao GQ, Li WQ, Guo SQ, et al. Association between dopamine D3 receptor Ser9Gly polymorphisms and schizophrenia. J Clin Psychol Med 2006; 16(1): 38-39. (in Chinese)
21. Luo XG, Jiang KD, Jiang SD, Gu NF, Yang XM, Zhu SY. Factors for the D4 receptor gene in refractory schizophrenic patients. Modern Rehabilitation 2000; 4(10): 1522-1523. (in Chinese)
22. Tang YL, Wang YF, Cai ZJ, Zhou RL, Zhou CF. Schizophrenia and dopamine D4 gene polymorphism in Chinese population: association analysis. Nati Med J China 2001; 81(16): 995-998. (in Chinese)
23. Jiang KD, Luo XG, Jiang SD, Yang XM, Zhang YH, Qian YP, et al. A study on the association between D4 receptor gene and schizophrenia. Shanghai Arch Psychiatry 2001; 13(2): 63-66. (in Chinese)
24. Zhao AL, Zhao JP, Xue ZM, Chen JD, Chen XG, Liu ZN, et al. Association between polymorphism in the dopamine D4 receptor gene and qualitative and quantitative characters of schizophrenia in Chinese. Chin J Psychiatry 2005; 38(1): 3-6. (in Chinese)
25. Hu YW, Wang GH, Wang HL, Liu ZC, Tang JH. Association between dopamine D1 receptor -48A/G gene polymorphism and schizophrenia in healthy Chinese Han population. Chinese Journal of Clinical Rehabilitation 2005; 9(24): 118-119. (in Chinese)
26. Zhu F, Yan CX, Wang Q, Zhu YS, Zhao Y, Huang J, et al. An association study between dopamine D1 receptor gene polymorphisms and the risk of schizophrenia. Brain Res 2011; 1420: 106-113.
27. Zhang C, Fang Y, Xie B, Cheng W, Du Y, Wang D, et al. No genetic association between dopamine D1 receptor gene and [early onset] schizophrenia. Psychiatry Res 2010; 177(3): 350-353.
28. Chen YQ, Xu XF, Zhao XD, Wang YM, Duan Y, Liu H, et al. Dopamine D5 receptor gene polymorphism and schizophrenia in a Chinese population. Chin J Psychiatry 2003; 36(4): 204-206. (in Chinese)
29. Wang YM, Du Y, Xu XF, Liu H, Chen YQ. Dinucleotide repeat polymorphism in dopamine D5 receptor gene and patients of Han nationality with schizophrenia in Kunming. Chinese Journal of Medical Laboratory Technology 2004; 5(3): 172-174. (in Chinese)
30. Huang SY, Chen HK, Ma KH, Shy MJ, Chen JH, Lin WC, et al. Association of promoter variants of human dopamine transporter gene with schizophrenia in Han Chinese. Schizophr Res 2010; 116(1): 68-74.
31. Luo XG, Jiang SD, Jiang KD, Gu NF, Lin SC, Qian YP, et al. Association between schizophrenia and T102C polymorphisms of the 5HT1a receptor gene. Shanghai Arch Psychiatry 1997; 9(4): 266-268. (in Chinese)
32. Wang YH, Li WQ, Huang Z, Shi YZ, Wang XY, Huang JS, et al. 5-HT2A receptor gene polymorphism and negative symptoms in first episode (drug-naïve) Chinese HAN nationality individuals with schizophrenia. J Cent South Univ (Med Sci) 2008; 33(4): 293-298. (in Chinese)
33. Yang XH, Luo XG, Fang YR, Yang XM, Jiang KD. 5-HT 2A receptor gene polymorphisms and schizophrenia. J Clin Psychol Med 2001; 11(5): 288-289. (in Chinese)
34. Zhang XB, Sha WW, Hou G. Association between the serotonin 2A receptor gene and tardive dyskinesia in chronic schizophrenia. Shanghai Arch Psychiatry 2004; 16(3): 145-148. (in Chinese)
35. Yi ZH, Wang ZW, Wang DY, Yu SY, Jiang SD, Fang YR. Association study between 5-HTR6 gene polymorphisms with schizophrenia and mood disorders. Shanghai Arch Psychiatry 2005; 17(6): 346-348. (in Chinese)
36. Lin C, Tang W, Hu J, Gao L, Huang K, Xu Y, et al. Haplotype analysis confirms association of the serotonin transporter (5-HTT) gene with schizophrenia in the Han Chines population. Neurosci Lett 2009; 453(3): 210-213.
37. Duan S, Yin H, Chen W, Xing Q, Chen Q, Guo T, et al. No association between the serotonin 1B receptor gene and schizophrenia in a case-control and family-based association study. Neurosci Lett 2005; 376(2): 93-97.
38. Sun WW, Fan JB, Qian XQ, Tang JX, Xing YL, Shi JG, et al. Transmission disequilibrium test of polymorphisms of serotonin transporter gene and schizophrenia based on family trios. Chin J Med Genet 2003; 20(4): 342-344. (in Chinese)
39. Li D, Duan Y, He L. Association study of serotonin 2A receptor (5-HT2A) gene with schizophrenia and suicidal behavior using systematic mtea-analysis. Biochem Biophys Res Commun 2006; 340(3): 1006-1015.
40. Gu B, Wang L, Zhang AP, Ma G, Zhao XZ, Li HF, et al. Association between a polymorphism of the HTR3A gene and therapeutic response to risperidone treatment in drug-naïve Chinese schizophrenia patients. Pharmacogenet Genomics 2008; 18(8): 721-727.
41. Tang YL, Wang YF, Cai ZJ, Zhou RL, Wang B, Zhou CF. Association analysis of schizophrenia with catechol-O-methyltransferase gene polymorphism. Journal of Beijing Medical University 2003; 34(4):342-344. (in Chinese)
42. Li JN, Xu Q, Shen Y, Ji L. An association study between paranoid schizophrenia and four genes involved in dopamine metabolism. Hereditas 2006; 28(4): 403-406. (in Chinese)
43. Wang Y, Hu Y, Fang Y, Zhang K, Yang H, Ma J, et al. Evidence of epistasis between the catechol-O-methyltransferase and aldehyde dehydrogenase 3B1 gene in paranoid schizophrenia. Biol Psychiatry 2009; 65(12): 1048-1054.
44. Wang Y, Fang Y, Shen Y, Xu Q. Analysis of association between the catechol-O-ethyltransferase (COMT) gene and negative symptoms in chronic schizophrenia. Psychiatry Res 2010; 179(2): 147-150.
45. Kang C, Xu X, Liu H, Yang J. Association study of catechol-O-methyltransferase (COMT) gene Val158Met polymorphism with auditory P300 in Chinese Han patients with schizophrenia. Psychiatry Res 2010; 180(2-3): 153-155.
46. Ma XH, Wang YC, Wang Q, Sun XL, Li T, Deng W, et al. Asssociation study of clinical presentation in first-episode schizophrenia and possible candidate genes in chromosome 22. Chin J Psychiatry 2004; 37(3): 145-148. (in Chinese)
47. Jiang HY, Xu XF, Cheng YQ, Liu H, Yang JZ. Association study between cognitive function in schizophrenics and catechol-O-methyltransferase gene polymorphism. Chin J of Behavioral Med Sci 2006; 15(12): 1090-1092. (in Chinese)
48. Hu WH, Jiang KD, Wang DY, Qian YP, Chen HF, Ding HR. The association study between catechol-O-methyltransferase gene polymorphism and cognitive function in schizophrenia patients with tardive dyskinesia. Shanghai Arch Psychiatry 2007; 19(4): 193-196. (in Chinese)
49. Jia MZ, Lü LX, Li WQ, Guo SQ, Zhong ZX, Wang CH. Association study of Val/Met polymorphism of catechol-O-methyltransferase gene and schizophrenia. Chin J of Behavioral Med Sci 2007; 16(11): 972-974. (in Chinese)
50. Tang YL, Wang YF, Cai ZJ, Zhou RL, Zhou CF. Association between schizophrenia and catachol-O-methyltransferase gene polymorphism. Chin J Psychiaty 2002; 35(4): 195-197. (in Chinese)
51. Liu S, Liu Y, Wang H, Zhou R, Zong J, Li C, Zhang X, Ma X.Association of catechol-O-methyl transferase (COMT) gene -287A/G polymorphism with susceptibility to obsessivecompulsive disorder in Chinese Han population. Am J Med Genet B Neuropsychiatr Genet 2011,156B(4):393-400.
52. Lü L, Zhang Y, Jia M, Yu J, Zhang H, Zhong Z. Association study of polymorphism of catechol-O-methyltransferase gene and schizophrenia. China Journal of Modern Medicine 2006;16(16):2415-2418,2422.
53. Jiang S, Tang G, Qian Y, Zhang Y, Wang D, Li F, Lin S, Wu X. Linkage disequilibrium between polymorphic loci for monoamine oxidases A and B and schizophrenia. Chin J Psychiatry 2003;36(1):11-13. (in Chinese)
54. Wei YL, Li CX, Li SB, Liu Y, Hu L. Association study of monoamine oxidase A/B genes and schizophrenia in Han Chinese. Behav Brain Funct 2011; 7: 42.
55. Shi YZ, Wang CH, Lü LX, Wang YH, Hang HX, Lou BY. Association study of the polymorphisms of monoamine oxidase A with schizophrenia. Chin J Med Genet 2007; 24(4): 457-459. (in Chinese)
56. Qiu HT, Meng HQ, Song C, Xiu MH, Chen DC, Zhu FY, et al. Association between monoamine oxidase (MAO)-A gene variants and schizophrenia in a Chinese population. Brain Res 2009; 1287: 67-73.
57. Li SJ, Liu H, Yuan H, Cao HJ. Relationship between HLA-DRB1 and schizophrenia. Chinese Journal of Medical Laboratory Technology 2002; 3(6): 376-378. (in Chinese)
58. Huang WJ, Liu ZC, Wang GH, Wang HL, Wu JG, Hou XH, Zhu F. Detection of HERV pol gene in blood samples from first-episode schizophrenic patients. Chinese J Exp Clin Virol 2007; 21(2): 199. (in Chinese)
59. Yu YQ, Shi JP, Yu Q, Tao R, Li J. Genetic association between DQA2 locus in human MHC region and psychotic symptoms of schizophrenia. Journal of Jilin University (Medicine Edition) 2004; 30(4): 495-498. (in Chinese)
60. Yu Q, Chen Y, Kou CG, Yu YQ. Relationship between HLA class II gene polymorphism and schizophrenia. Journal of Jilin University (Medicine Edition) 2007; 33(2):306-309. (in Chinese)
61. Yang RY, Du GY, Zhang BW. HLA gene frequency in Han Chinese individuals with schizophrenia from Henan Province, China. Journal of Medical Forum 2008; 29(17): 29;31.
62. Chao YL, Shen YC, Liao DL, Chen JY, Wang YC, Lai IC, et al. Association study of HLA-A gene and schizophrenia in Han Chinese from Taiwan. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32(8):1834-1837.
63. Ventriglia M, Bocchio Chiavetto L, Bonvicini C, Tura GB, Bignotti S, Racagni G, et al. Allelic variation in the human prodynorphin gene promoter and schizophrenia. Neuropsychobiology 2002; 46(1): 17-21.
64. Song YQ, Zhou DF, Wei J, Zhang XY, Guan ZQ. Cholecystokinin-A receptor gene polymorphism and schizophrenia: a transmission disequilibrium test. Chin J Psychiatry 2002; 35(2):73-76. (in Chinese)
65. Lü WT, Liu LL, Zhang M, Gong SL, Wei J. Association of cholecystokinin A receptor gene with schizophrenia. Journal of Jilin University (Medicine Edition) 2003; 29(4):398-401. (in Chinese)
66. Yang J, Ding M, Sun Y, Pang H, Xing JX, Xuan JF, et al. Relationship between cholecystokinin gene-45C/T polymorphism and schizophrenia and its application in forensic medicine. Journal of Forensic Medicine 2011; 27(1): 22-24. (in Chinese)
67. Luo XG, Jiang KD, Gu NF. A study on the relationship between the responses to clozapine and the 5-hydroxytryptamine type 2A receptor gene in chronic refractory schizophrenia. Chin J Psychiatry 2000; 33(3): 141-144. (in Chinese)
68. Zhang XY, Zhou DF, Zhang PY, Wei J. Association of cholecystokinin A receptor gene polymorphism with the symptoms of schizophrenia. Chin J Psychiatry 2000; 33(3):138-140. (in Chinese)
69. Itokawa M, Arai M, Kato S, Ogata Y, Furukawa A, Haga S, Ujike H, Sora I, Ikeda K, Yoshikawa T.Association between a novel polymorphism in the promoter region of the neuropeptide Y gene and schizophrenia in humans. Neurosci Lett 2003; 347(3): 202-204.
70. Wang HS, Duan SW, Xing QH, Du J, Li XW, Xu YF, et al. Association study between NPY and YWHAH gene polymorphism and schizophrenia. Acta Genetrica Sinica 2005; 32(12): 1235-1240. (in Chinese)
71. Cui DH, Yao H, Wang XY, Zhu B, Jiang KD. Microarray analysis of altered gene expression in schizophrenia. Chin J Psychiatry 2004; 37(1): 4-8. (in Chinese)
72. Cui DH, Jiang KD, Jiang SD, Xu YF, Yao H. The tumor suppressor adenomatous polyposis coli gene is associated with susceptibility to schizophrenia. Mol Psychiatry 2005; 10(7): 669-677.
73. Su YS, Qiu JY, Zhang HH, Wang ZC, Xie B, Cui DH. Analysis of association between thioredoxin interacting protein gene polymorphism and schizophrenia. Shanghai Arch Psychiatry 2008; 20(3): 146-148. (in Chinese)
74. He XL, Zhao JP, Liu TQ, Liu T, Zhang XH. Association of schizophrenia and the C270T polymorphism of brain-derived neurotrophic factor gene. Chinese Journal of Clinical Rehabilitation 2005; 9(28): 245-247.
75. He XL, Zhao JP, Liiu TQ, Liu T, Zhang XH. An association study of schizophrenia and the C270T polymorphism of brain-derived neurotrophic factor gene. Chin J of Behavioral Med Sci 2005; 14(7): 623-624. (in Chinese)
76. Ding Q, Jiang KD, Tang GM, Qian YP, Wang DY, Lin SC, et al. An association between the 5-HT2A receptor gene T102C polymorphism and schizophrenia. Shanghai Arch Psychiatry 1999; 11(3): 140-142. (in Chinese)
77. Wang WY, Chen JF, Wang GQ, Wang SH, Jiao GK, Han G. Association between schizophrenia and the serotonin 2A receptor gene in co-morbid siblings. Chin J Psychiatry 2004; 37(4): 224-227. (in Chinese)
78. Wang YH, Li WQ, Huang Z, Shi YZ, Wang XZ, Huang JS, et al. 5-HT2A receptor gene polymorphism and negative symptoms in first episode (drug-naïve) Chinese Han nationality individuals with schizophrenia. J Cent South Univ (Med Sci) 2008; 33(4): 293-298. (in Chinese)
79. Xiu MH, Chen DC, Li YL, Wang N, Hui L, Liu HB, et al. Association between smoking and brain-derived neurotrophic factor gene in male patients with schizophrenia. Chin J Nerv Ment Dis 2010; 36(12): 760-761. (in Chinese)
80. Yi Z, Zhang C, Wu Z, Hong W, Li Z, Fang Y, Yu S. Lack of effect of brain derived neurotrophic factor (BDNF) Val66Met polymorphism on early onset schizophrenia in Chinese Han population. Brain Res 2011; 1417: 146-150.
81. Zhou DH, Yan QZ, Yan XM, Li CB, Fang H, Zheng YL, et al. The study of BDNF Val66Met polymorphism in Chinese schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34(6): 930-933.
82. Xu MQ, St Clair D, Feng GY, Lin ZG, He G, Li X, et al. BDNF gene is a genetic risk factor for schizophrenia and is related to the chlorpromazine-induced extrapyramidal syndrome in the Chinese population. Pharmacogenet Genomics 2008; 18(6): 449-457.
83. Xu M, Li S, Xing Q, Gao R, Feng G, Lin Z, et al. Genetic variants in the BDNF gene and therapeutic response to risperidone in schizophrenia patients: a pharmacogenetic study. Eur J Hum Genet 2010; 18(6): 707-712.
84. Yue WH, Kang GL, Zhang HD, Tang FL, Qu M, Han YH, et al. Association study between schizophrenia and NRG1, G72, RGS4 polymorphisms. Chin J of Behavioral Med Sci 2007; 16(5): 418-420. (in Chinese)
85. Zhang HX, Zhao JP. Neuregulin-1 gene and schizophrenia. Foreign Medical Science: Section of Psychiatry 2005; 32(4): 203-206. (in Chinese)
86. Yang DY, Lu XB, Li YH, Tong ZS, Fu Y, Yang MX, et al. The association of methylenetetrahydrofolate reductase gene polymorphism, plasma homocysteine level and first-episode schizophrenia. Chin J of Behavioral Med Sci 2007; 16(10):901-902. (in Chinese)
87. Ye XM, Zhang X, Wang HL. Study association of MTHFR C677T polymorphism and schizophrenia. Heilongjiang Medical Journal 2012; 34(9): 641-645. (in Chinese)
88. Feng LG, Song ZW, Xin F, Hu J. Association of plasma homocysteine and methylenetetrahydrofolate reductase C677T gene variant with schizophrenia: A Chinese Han population-based case–control study. Psychiatry Research 2009; 168(3): 205-208.
89. Shi N, Kang WH, Lü SM, Li Q, Gao CG, Ma XC, et al. Study between methylenetetrahydrofolate reductase (MTHFR) gene and schizophrenia core families. Chin J Nerv Ment Dis 2007; 33(10): 597-600. (in Chinese)
90. Yue WH, Zhang HD, Tang FL, Qu M, Han YH, Zhang DR, et al. Association study of the regulator of G-protein signaling 4 (RGS4) polymorphisms with schizophrenia. Chinese Mental Health Journal 2007; 21(3): 181-185. (in Chinese)
91. So HC, Chen RY, Chen EY, Cheung EF, Li T, Sham PC. An association study of RGS4 polymorphisms with clinical phenotypes of schizophrenia in a Chinese population. Am J Med Genet B Neuropsychiatr Genet 2008; 147B(1): 77-85.
92. Zhang F, St Clair D, Liu X, Sun X, Sham PC, Crombie C, et al. Association analysis of the RGS4 gene in Han Chinese and Scottish populations with schizophrenia. Genes Brain Behav 2005; 4(7): 444-448.
93. Guo S, Tang W, Shi Y, Huang K, Xi Z, Xu Y, et al. RGS4 polymorphisms and risk of schizophrenia: an association study in Han Chinese plus meta-analysis. Neurosci Lett 2006; 406(1-2): 122-127.
94. Wu CY, Wang ZC, Jiang SD. The relationship between the Gly/ Glu polymorphism of the neurotrophin-3 and schizophrenia in Chinese. Chin J Psychiatry 2000; 33(4): 207-209.
95. Du HX, Guo SQ, Su LY, Pan WM, Guo JH, Guo F, et al. The Gly/ Glu polymorphism of the neurotrophim 3 gene and its effect on ventricular enlargement in childhood schizophrenia. Chin J Nerv Ment Dis 2007; 33(11): 648-651. (in Chinese)
96. Deng H, Terwe H, Cai GQ, Xu X, Wang CX, Liu XH, et al. A linkage disequilibrium study of the dinucleotide repeat polymorphism in the promoter region of neurotrophin-3 gene and schizophrenia. Chin J Psychiatry 2000; 33(4): 203-206. (in Chinese)
97. Qu M, Tang F, Yue W, Ruan Y, Lu T, Liu Z, et al. Positive association of the Disrupted-in-Schizophrenia-1 gene (DISC1) with schizophrenia in the Chinese Han population. Am J Med Genet B Neuropsychiatr Genet 2007; 144B(3): 266-270.
98. Sun W, Yan J, Wang LF, Qu M, Ruan YY, Lu TL, et al. Association study of the disrupted in schizophrenia 1 (DISC 1) gene polymorphisms with schizophrenia. Chinese Mental Health Journal 2009; 23(8): 590-594. (in Chinese)
99. Yin Y, Huang LQ, Huang SQ, Shao FY, Zhang P, Tan LW. Association analysis of cytosolic phospholipase A2 and disrupted-inschizophrenia-1 gene polymorphism in schizophrenia and type 2 diabetes. Chin J Nerv Ment Dis 2008; 34(4): 221-224. (in Chinese)
100. Zhai JG, Chen M, Su ZH, Li W, Yu Q, Li Q, et al. Association study between disrupted in schizophrenia 1 (DISC 1) gene polymorphism and schizophrenic and different subtype depressive patients. Chin J Behav Med & Brain Sci 2011; 20(7):605-607. (in Chinese)
101. Chen QY, Chen Q, Feng GY, Lindpaintner K, Wang LJ, Chen ZX, et al. Case-control association study of Disrupted-in-Schizophrenia-1 (DISC1) gene and schizophrenia in the Chinese population. J Psychiatr Res 2007; 41(5): 428-434.
102. Zhang H, Li D, Su Y, Jiang S, Xu Y, Jiang K, Cui D. Identification of the N-acylsphingosine amidohydrolase 1 gene (ASAH1) for susceptibility to schizophrenia in a Han Chinese population. World J Biol Psychiatry 2012; 13(2): 106-113.
103. Zhang HH, Cui DH, Zhu ZQ, Jiang KD, Jiang SD. Association analysis between N-acylsphingosine amidohydrolase gene and symptom quantitative trait of schizophrenia in Chinese Han population. Chin J Psychiatry 2008; 41(1): 5-9. (in Chinese)
104. Yue WH, Tang FL, Qu M, Zhang HD, Yan J, Liu C, et al. Association study of RPODH 1945(T/C) polymorphism with schizophrenia. Progress in Morden Biomedicine 2007; 7(3): 332-334. (in Chinese)
105. Kong FZ, Hong XH, Wang CQ, Peng ZZ, Cheng L, Sun BL. Association study of proline dehydrogenase gene polymorphism and paranoid schizophrenia. Journal of Shantou University Medical College 2009; 22(1): 18-20;28. (in Chinese)
106. Wang CH, Shi YZ, Lü LX, Zuo JH, Chen HH. Association between tryptophan hydroxylase gene polymorphism and schizophrenia. Chin J Nerv Ment Dis 2007; 33(8): 478-479. (in Chinese)
107. Guo JX, Liu EY, Lin ZQ, Xiao AX, Jiang ZY, Lu XB, et al. A study on association between the polymorphism of the tryptophan hydroxylase gene and the monoamine oxidase A gene and aggression in schizophrenia. Chin J Nerv Ment Dis 2009; 35(2): 84-87. (in Chinese)
108. Chen ZF, Zeng Y, Xu XF, Guo WJ, Yang JZ, Zhang XJ, et al. Association study between schizophrenia and tryptophan hydroxylase 2 gene (rs1386494, G1463A) polymorphism in Yunnan Han and Jinuo population. Chin J Clinicians (Electronic Edition) 2009; 3(1): 42-47. (in Chinese)
109. Liu L, Jia FJ, Li HF. Gene expression level of inflammation cytokine and tyrosine hydroxylase in peripheral blood in schizophrenia. J Clin Psychol Med 2007; 17(4): 223-225. (in Chinese)
110. Liu XX, Li HF, Zhang ZJ, Guo XS, Jia FJ. The relationship of gene expression levels of nitric oxide synthase of schizophrenic and PANSS score. Chin J Behavioral Med Sci 2008; 17(12): 1083-1085. (in Chinese)
111. Zhang HX, Li WQ, Zhang HS, Lü LX, Yang G. The expression of DRD1 gene mRNA in peripheral blood lymphocytes of schizophrenia. J Clin Psychosom Dis 2010; 16(5): 385-387;390. (in Chinese)
112. Zhang Y, Qian YP, Hong W, Yu SY, Cui DH, Wang DY, et al. Study of the expression levels of G72 gene in the patients with schizophrenia. Int J Genet 2007; 30(2): 85-87; 84. (in Chinese)
113. Shi Y, Li Z, Xu Q, Wang T, Li T, Shen J, et al. Common variants on 8p12 and 1q24.2 confer risk of schizophrenia. Nat Genet 2011; 43(12): 1224-1227.
114. Yue WH, Wang HF, Sun LD, Tang FL, Liu ZH, Zhang HX, et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet 2011; 43(12): 1228-1231.
10.3969/j.issn.1002-0829.2012.04.001
Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
*Correspondence: jiangkaida@yahoo.com.cn
- 上海精神医学的其它文章
- Prevention and management of missing data during conduct of a clinical study
- Cross-sectional assessment of the factors associated with occupational functioning in patients with schizophrenia
- Cross-sectional study of executive functioning in children with developmental coordination disorders
- Event-related potentials during mental rotation tasks in patients with first-episode depression
- Meta-analysis of studies in China about changes in P300 latency and amplitude that occur in patients with schizophrenia during treatment with antipsychotic medication
- Psychiatric symptoms in an individual with tuberous sclerosis

