凝胶-溶胶法制备单分散菱柱形草酸镍的研究
徐 毅,刘志宏
1.深圳市中金岭南有色金属股份公司丹霞冶炼厂,广东仁化512300;2.中南大学冶金科学与工程学院,湖南长沙410083
凝胶-溶胶法制备单分散菱柱形草酸镍的研究
徐 毅1,刘志宏2
1.深圳市中金岭南有色金属股份公司丹霞冶炼厂,广东仁化512300;2.中南大学冶金科学与工程学院,湖南长沙410083
以Ni(OH)2凝胶为前驱体,采用凝胶-溶胶法制备了单分散菱柱形二水草酸镍.采用SEM,XRD等方法及Imagetool图像分析软件,对所制备的NiC2O4·2H2O粉末形貌及形成机理等进行了研究,结果表明,控制适当条件,可使NiC2O4·2H2O颗粒在低过饱和度下均匀生长,其形成机理为溶解-再结晶;随温度升高,粉末粒度增大,单分散性降低;温度较低时,粉末颗粒接近球形;在313K制备的粉末为α型NiC2O4·2H2O,粉末的粒度分布范围为0.45~0.90μm.
制备;二水草酸镍;凝胶-溶胶法;菱柱状形貌
微米或纳米尺度的镍及其化合物粉末,在电子、磁性材料、催化剂等领域应用广泛,其制备技术一直是研究的热点[1-4].为满足不同应用的要求,制备中必须对粉末形貌、粒度及晶体结构等进行控制,单分散粉末的制备是研究者们长期追求的目标之一[5].LaMer[6]提出了湿化学法制备单分散粉末的模型,其要点为:通过对体系过饱和度的精确控制,将形核与晶核长大过程分开,粉末颗粒在一次形核的基础上,按生长而非团聚方式长大.LaM er模型一般只适用于极稀溶液体系,难以应用于工业生产.在浓度较高的体系中,颗粒间静电斥力较弱,其相互间团聚难以避免.为了解决这一问题,Tadao Sagamoto[7]提出了制备单分散粉末的凝胶-溶胶法(Gel-Sol),其思路为:首先通过水解或其他沉淀方法,将高浓度金属盐溶液制备成凝胶,然后再将凝胶进一步转化为溶解度更低的溶胶,实现粉末颗粒形核与生长在低的过饱和度下进行,满足LaM er模型制备单分散粉末条件的要求.此外,在溶胶颗粒形成中,由于凝胶的阻隔作用,也可避免其颗粒间的团聚发生.截至目前,已采用凝胶-溶胶法制备出单分散
Al3(SO4)2(OH)5·2H2O[8], CdS[9-11], Fe2O3[7,12-17],TiO2[18-22]等粉末.
金属镍及其氧化物粉末可通过前驱体草酸镍热分解制备,其形貌与粒度对前驱体具有“继承性”.因此,单分散草酸镍粉末的制备,是制备高质量金属镍及其氧化物粉末的关键步骤.本文研究了一种以Ni(OH)2凝胶为前驱体,加入 H2C2O4使其转化为NiC2O4·2H2O溶胶,即凝胶-溶胶法制备单分散二水草酸镍粉末的方法.同时,研究了粉末颗粒的形成机理,以及温度对粉末形貌与粒度的影响.
1 实验部分
1.1 原料
NiCl2·6H2O,NaOH,Na2C2O4·2H2O,H2C2O4·2H2O均为试剂级(由日本 Nacalai Tesque公司生产,未进一步提纯);去离子水.
1.2 粉末制备
粉末样品制备分为3步,各步骤及其固定条件为:
第1步:将100m L 0.2mol/L NiCl2溶液加入反应器中加热至313K,溶液p H313K值为5.75.然后,在搅拌条件下,快速加入30 m L 1 mol/L NaOH溶液,按反应(1)生成浅绿色Ni(OH)2凝胶,其溶度积KSP,298K,Ni(OH)2=5.48 ×10-16,平衡后体系 p H313K值增至6.64.
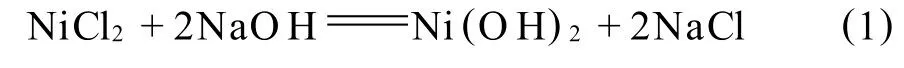
第2步:由于NaOH的加入量不足以使全部Ni2+水解沉淀,根据加入的 NaOH量,可计算出Ni2+剩余浓度为0.0385 mol/L;以1 m L/min的速度滴加20 mL 0.25 mol/L Na2C2O4溶液,剩余的游离Ni2+依反应(2)生成 NiC2O4·2H2O晶核,其溶度积 KSP,298K,NiC2O4=4×10-10,至此,体系 p H313K增加至7.36.

第3步:在控制体系p H313K值为6.0的条件下,将0.2mol/L H2C2O4溶液通过带p H控制器的计量泵(EH/W,日本 Iwaki公司),滴入反应器中,使Ni(OH)2凝胶通过下列反应(3)~(6)逐步转化为NiC2O4·2H2O溶胶.
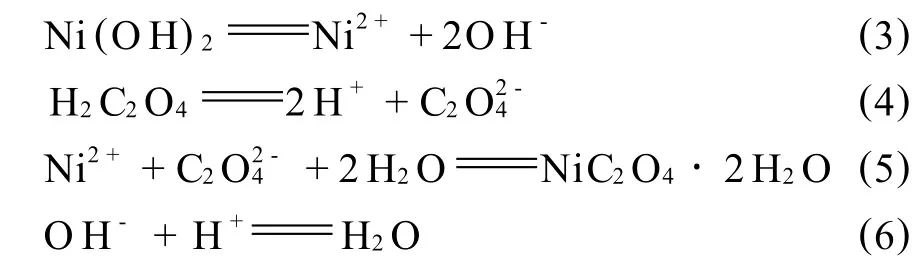
H2C2O4溶液的滴加速度,可根据p H值偏离设定值的大小,通过反应(3)~(6)的耦合自动调节.当H2C2O4溶液的滴加速度快,使体系的p H值达到或低于设定值时,自动停止滴加,此时,反应(3)会加速正向进行,体系p H值增高,又恢复滴加,滴加速度与p H值正向偏离设定值的大小成正比.因此,反应可在基本恒定的p H值下进行,保证了NiC2O4·2H2O颗粒在稳定的过饱和度下生长.
1.3 样品表征
采用SEM(Hitachi S-800)观察样品形貌;采用Imagetool图像分析软件,通过测量 SEM照片中100个粉末颗粒来确定其长度、宽度及轴向比(长度/宽度);采用 XRD(Shimadzu XRD-600,铜靶Kα)对样品进行物相鉴定.
2 实验结果及分析
2.1 粉末颗粒的形成机理
图1(a)为第1步制得的Ni(OH)2凝胶的形貌图.图1(a)与图3 XRD分析表明,凝胶的物相为β型Ni(OH)2(JCPDS 14-117),其形貌为直径30~100 nm的球形颗粒,颗粒间呈网状结构.图1(b),(c)分别为第3步加入 H2C2O4溶液20,40 min后,制得的NiC2O4·2H2O颗粒的SEM照片.图1(b),(c)及图 3 XRD分析表明,随 H2C2O4加入,Ni(OH)2凝胶逐渐消失,至40 min后,已完全转化为α型NiC2O4·2H2O(JCPDS25-0281).图1(b)还表明,二水草酸镍颗粒在生长过程中被Ni(OH)2凝胶所包裹,避免了颗粒团聚.对图1(c)所示样品陈化2 h后,其SEM和XRD分析表明,粉末颗粒的形貌与晶体结构没有变化.以上研究结果表明,NiC2O4·2H2O粉末形成的机理为溶解-再结晶(Dissolution-Recrystallization),如式 (3)~ (6)所示.

图1 固定条件下样品的SEM照片(a)加入NaOH溶液后;(b)加入 H2 C2 O4溶液20min后;(c)加入 H2 C2O4溶液40min后Fig.1 SEM photographs of p recipitates under control conditions(a)after adding NaOH solution;(b),(c)after adding H2 C2 O4 solution fo r 20 and 40 min,respectively
2.2 粉末的表征
所制备的粉末形貌如图2所示.图2(b)为采用图像处理软件 Imagetool,对图2(a)中的颗粒进行测量,以此为依据绘制的粉末颗粒形貌示意图.图2表明,颗粒形貌为菱柱形,菱形底面的两对夹角分别为106°和74°,已知菱边长b和柱高l,可推导出颗粒体积V的计算公式,如式(7)所示.

对Imagetool测定结果进行统计处理得到,固定条件下制备的粉末颗粒底面菱边长平均值为0.32μm,柱高平均值为0.65μm,菱边长与柱高的比值(b/l)为0.49,考虑测量与统计误差,取值0.5.
图3为所制备的NiC2O4·2H2O颗粒的XRD图.图3表明,制备的样品为α型 NiC2O4·2H2O(JCPDS 25-281),属单斜(Monoclinic)晶系,a=1.1775×10-9m,b=5.333×10-10m,c=9.833×10-10m,β=127.2°.
测量粉末SEM照片中100个颗粒的粒径,所得结果绘制成粉末粒度频率分布图,如图4所示.由图4可知,粉末的平均粒度为0.65μm,粒度分布范围为0.45~0.90μm.根据统计结果计算,其单分散性指标σ(σ=D84.13/D50)值为 1.22,这表明按1.2节所述条件制备的粉末的单分散性较好.

图2 粉末形貌(a)SEM照片;(b)颗粒形貌示意图Fig.2 Morphology of pow ders(a)SEM photographs;(b)Schematic diagram of typical particle morphology
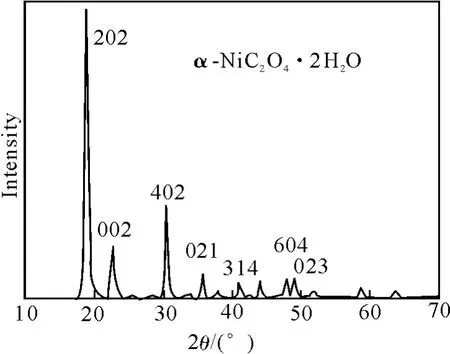
图3 粉末的XRD图谱Fig.3 XRD pattern of pow der p repared under control conditions

图4 粉末的粒度分布Fig.4 Size distribution of pow ders
2.3 温度的影响
在1.2节粉末的制备中,将反应温度分别改为293K和333K进行试验,所制得的粉末形貌如图5所示.粉末粒度与形貌参数的统计结果列于表1.
图5与表1表明,温度对颗粒的形貌与粒度均有较大影响.随温度升高,粉末粒度增大、单分散性降低.图5显示,不同温度下,颗粒形貌均为菱柱形,但在293K低温下,菱边长与柱高的比值(b/l)较大,形貌接近类球形,而在313K和333K下,其值均为0.5左右.
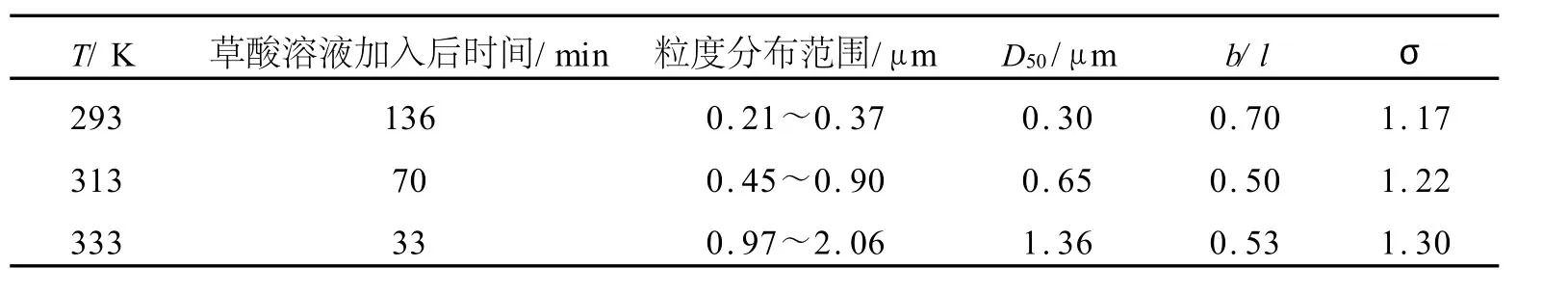
表1 温度对粉末形貌及粒度的影响Table 1 Effect of temperatures onmorphology and size of pow ders p repared
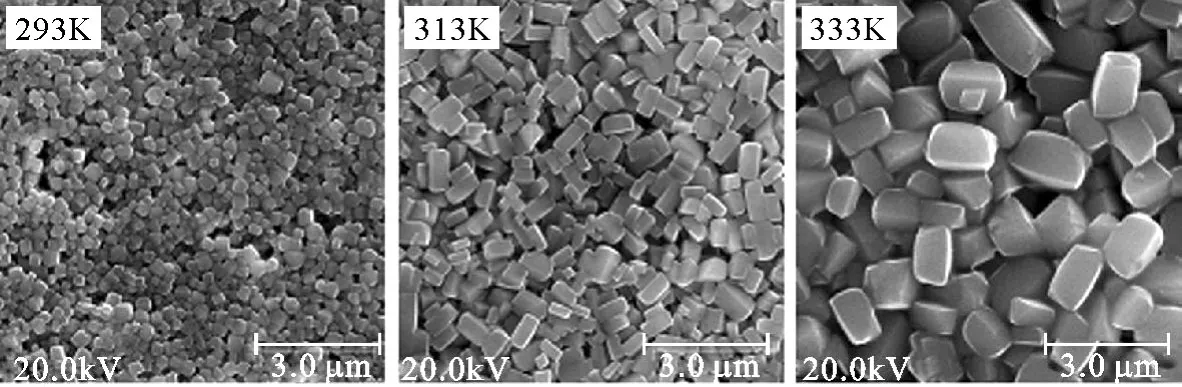
图5 不同温度下样品的SEM照片Fig.5 SEM photographs of pow ders p repared at different temperatures
3 结 论
(1)二水草酸镍粉末颗粒的形成机理为溶解-再结晶.控制适当条件,可使颗粒在低过饱和度下均匀生长.颗粒生长过程中,Ni(OH)2凝胶包覆在NiC2O4·2H2O颗粒表面,一方面作为 Ni2+的“缓释源”,另一方面,起到了阻隔颗粒碰撞团聚的作用.
(2)在 313K制备的粉末为α型 NiC2O4·2H2O,呈菱柱形,菱形底面的两对夹角分别为74°和106°.粉末的粒度分布范围为 0.45~0.90μm,D50=0.65μm,单分散性指标为1.22.
(3)温度对颗粒的形貌与粒度均有较大影响.随温度升高,粉末粒度增大,单分散性降低;温度较低时,粉末颗粒接近球形.
[1]WANGWeining,ITOH Yoshifumi,WULED ILengoro,et al.Nickel and nickeloxide nanoparticles p repared from nickel nitrate hexahydrate by a low p ressure sp ray pyrolysis[J].M aterials Science and Engineering B,2004(111):69-76.
[2]CHOU Kansen,HUANG Kuocheng.Studies on the chemical synthesis of nanosized nickel pow der and its stability[J].Journal of Nanoparticle Research,2001(3):127-132.
[3]L I Y D,L IC W,WANG H R,et al.Preparation of nickel ultrafine pow der and crystalline film by chemical control reduction[J].Materials Science and Physics,1999(59):88-90.
[4]GAO Jinzhang,GUAN Fei,ZHAO Yanchun,et al.Preparation of ultrafine nickel pow der and its catalytic dehydro-genation activity[J].Materials Chemistry and Physics,2001(71):215-219.
[5]GO IA V Dan,MA TIJEV IC Egon.Preparation of monodispersed metal particles[J].New J Chem,1998:1203-1215.
[6]PRIVMAN V ladimir,GO IA V Dan,PARK Jongsoon,et al.Mechanism of formation of monodispersed colloids by aggregation of nanosize p recursors[J].Journal of Colloid and Interface Science,1999(213):36-45.
[7]SUGIMOTO Tadao,WANG Yingsheng,ITOH Hiroyuki,et al.Systematic control of size,shape and internal structure of mono-disperseα-Fe2O3particles[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,1998(134):265-279.
[8]SUGIMOTO Tadao,ITOH Hiroyuki,M IYA KIHideaki.Formation of monodisperse microcrystals of basic aluminum sulfate by the gel-solmethod[J].Journal of Colloid and Interface Science,1997(188):101-114.
[9]SUGIMOTO Tadao,D IRIGE E Grace,MURAMU TSU A tsushi.Synthesis of uniform CdS particles from condensed Cd(OH)2suspension[J].Journal of Colloid and Interface Science,1995(173):257-259.
[10]SUGIUMOTO Tadao,D IRIGE E Grace,MURAMU TSU A tsushi.Formation mechanism of uniform CdSparticles from condensed Cd(OH)2suspension[J].Journal of Colloid and Interface Science,1995(176):442-453.
[11]YANGJunmo,SH INDO Daisuke,D IRIGE E Grace,et al.High-resolution electron microscopy on thin sections of monodisperse CdS particles[J].Journal of Colliod and Interface Science,1996(183):295-298.
[12]SUGIMOTO Tadao,MURAMA TSU A tsushi.Fo rmation mechanism of monodispersedα-Fe2O3particles in dilute FeCl3solutions[J].Journal of Colloid and Interface Science,1996(184):626-638.
[13]SUGIMOTO Tadao,WAL I Shuzo,ITOH Hiriyuki,et al.Preparation of monodisperse p latelet-type hematite particles f rom a highly condensedβ-FeOOH sus-pension[J].Colloids and Surfaces A:Physicochenical and Engineering Aspects,1996(109):155-165.
[14]SUGIMOTO Tadao,WANG Yinsheng.Mechanism of the shape and structure control of monodispersedα-Fe2O3particles by sulfate ions[J].Journal of Colloid and Interface Science,1998(207):137-149.
[15]SUGIMOTO Tadao,ITOH Hiroyuki,MOCH IDA Takeaki.Shape control of monodisperse hematite particles by organic additives in the gel-sol system[J].Journal of Colloid and Interface Science,1998(205):42-52.
[16]KUROKAWA Haruki,SENNA Mamoru.Self-stabilization of green rust(II)as a p recursor of acicular goethite particles w ith highest possible aspect ratio[J].Pow der Technology,1999(103):71-79.
[17]L IU Qingyuan,OSSEO-ASARE K.Synthesis of monodisperse al-substituted hematite particles from highly condensed metal hydroxide gels[J].Journal of Colloid and Interface Science,2000(231),401-403.
[18]SUGIMOTO Tadao,OKADA Kazumi,ITOH Hiroyuki.Synthesis of unifo rm spindle-type titania particles by the gel-solmethod[J].Journal of Colloid and Interface Science,1997(193):140-143.
[19]SUGIMOTO Tadao,ZHOU Xingping,MURAMA TSU A tsushi.Synthesis of uniform anatase TiO2nanoparticles by gel-sol method 1,solution chemistry of Ti(OH)n(4-n)+complexes[J].Journal of Colloid and Interface Science,2002(252):339-346.
[20]SUGIMOTO Tadao,ZHOU Xingping.Synthesisof uniform anatase TiO2nanoparticles by gel-solmethod 2,adsorp tion of OH-ions to Ti(OH)4gel and TiO2particles[J].Journal of Colloid and Interface Science,2002(252):347-353.
[21]SUGIMOTO Tadao,ZHOU Xingpin,MURAMA TSU A tsushi.Synthesis of uniform anatase TiO2nanoparticles by gel-sol method 3,fo rmation p rocess and size control[J].Journal of Colloid and Interface Science,2003(259):43-52.
[22]SUGIMOTO Tadao,ZHOU Xingping,MURAMA TSU A tsushi.Synthesis of unifo rm anatase TiO2nanoparticles by gel-sol method 4,shape control[J].Journal of Colloid and Interface Science,2003(259):53-61.
Preparation of mono-dispersed rhombohedra-type n ickel oxalate dehydrate by a gel-sol process
XU Yi1,L IU Zhihong2
1.Dan X ia Smelter,Shenzhen Zhong jin L ingnan N onfemet Co.L TD,Renhua 512300,China;2.College of Metallurgical Science and Engineering,Central South University,Changsha 410083,China
Monodisperse nickel oxalate dihydrate pow ders of rhom bohedron mo rphology were p repared from condensed Ni(OH)2p recurso r by the novel gel-sol p rocess.The mo rphologies and fo rmation mechanism of the particles p repared were investigated by SEM,XRD and Imagetool softw are,and the results show ed that by controlling app rop riate conditions,nickel oxalate dehydrate particles grew equably at a low er super saturation through dissolution-recrystallization p rocess.With the increase of temperature,the size of the pow ders increased,accompanied w ith the decrease of theirmono-dispersity.A t a lower temperature,the mo rphology of the pow ders was similar to spheroid.The pow ders made at 313 K were in the form ofαtype NiC2O4·2H2O w ith a size range of 0.45-0.90μm.
p reparation;nickel oxalate dehydrate;gel-sol p rocess;rhombohedron morphology
TF123.121
A
1673-9981(2011)02-0159-05
2011-05-16
徐毅(1958—),男,重庆人,高级工程师,硕士.

