La-Mg-Ni系储氢合金储氢性能研究
彭 能,苏玉长,肖方明
1.中南大学材料科学与工程学院,湖南长沙410083;2.广东省工业技术研究院(广州有色金属研究院),广东广州510650
La-Mg-Ni系储氢合金储氢性能研究
彭 能1,2,苏玉长1,肖方明2
1.中南大学材料科学与工程学院,湖南长沙410083;2.广东省工业技术研究院(广州有色金属研究院),广东广州510650
采用快淬法制备了不同镁含量的La-M g-Ni系储氢合金,并研究了La-M g-Ni系储氢合金的储氢特性.结果表明,La-Mg-Ni系储氢合金主要由LaNi5和LaNi3两相组成;随着镁含量的增加,储氢合金的吸放氢平台压力降低,吸氢量提高;与化学计量比储氢合金相比,非化学计量比的储氢合金平台压力提高;在1173 K热处理4 h后,储氢合金具有较好的吸/放氢平台性能,其吸氢量可达1.59%.
快淬法;La-M g-Ni系储氢合金;储氢性能
商品化AB5型储氢合金储氢量低、生产成本较高,储氢容量的提升空间不大,从而限制了其在燃料电池中的应用.而AB3型La-M g-Ni系合金具有储氢量大、放电容量高、活化快等优势,近年来成为各高校、科研院所及企业的研究热点[1-7].但是该合金的相关研究仍处于实验阶段,尚存在放电容量不稳定、循环寿命差等问题.我们采用常规真空负压快淬炉,通过添加覆盖剂,选择合适的熔炼工艺条件,解决了熔炼过程中镁大量挥发导致合金成分不稳定的问题,获得了成分均匀的La-M g-Ni系纳米晶储氢合金.在前期工作[8-9]的基础上,本文研究了镁含量及热处理工艺对合金储氢性能的影响.
1 实验部分
1.1 合金粉的制备
实验用原材料为La,Ce,Ni,Co,M n,A l和M g,其纯度均大于99.9%.在氩气保护的快淬炉中,通过添加覆盖剂对原材料进行熔炼,合金液保温一段时间后经冷却铜辊制得0.05~1.5 mm厚的合金片.在850~1020℃热处理后,采用机械球磨制得一系列La-M g-Ni储氢合金.
1.2 性能测试
采用日本 RIN T-1100型 X射线衍射仪(Cu Kα靶)分析储氢合金的相结构.采用上海冶金所的 YJ-1全自动 P-C-T测试仪对合金的吸放氢性能进行表征.
2 结果与讨论
2.1 合金的相组成
图1为不同M g含量La-M g-Ni系储氢合金的X-射线衍射图.图 1表明,La-M g-Ni合金主要由LaNi5和LaNi3两相组成;当镁含量增加时,LaNi5相减少,LaNi3相增加,说明镁能够促进LaNi3相的形成[10].此外,由于 M g原子半径(1.72×10-10mm)小于La原子半径(2.74×10-10mm),当 M g替代A侧的La后,导致晶轴收缩,晶胞体积变小.这一点可以由随着镁含量不断增加,合金的特征峰逐渐向高角度方向偏移的结果来证实.
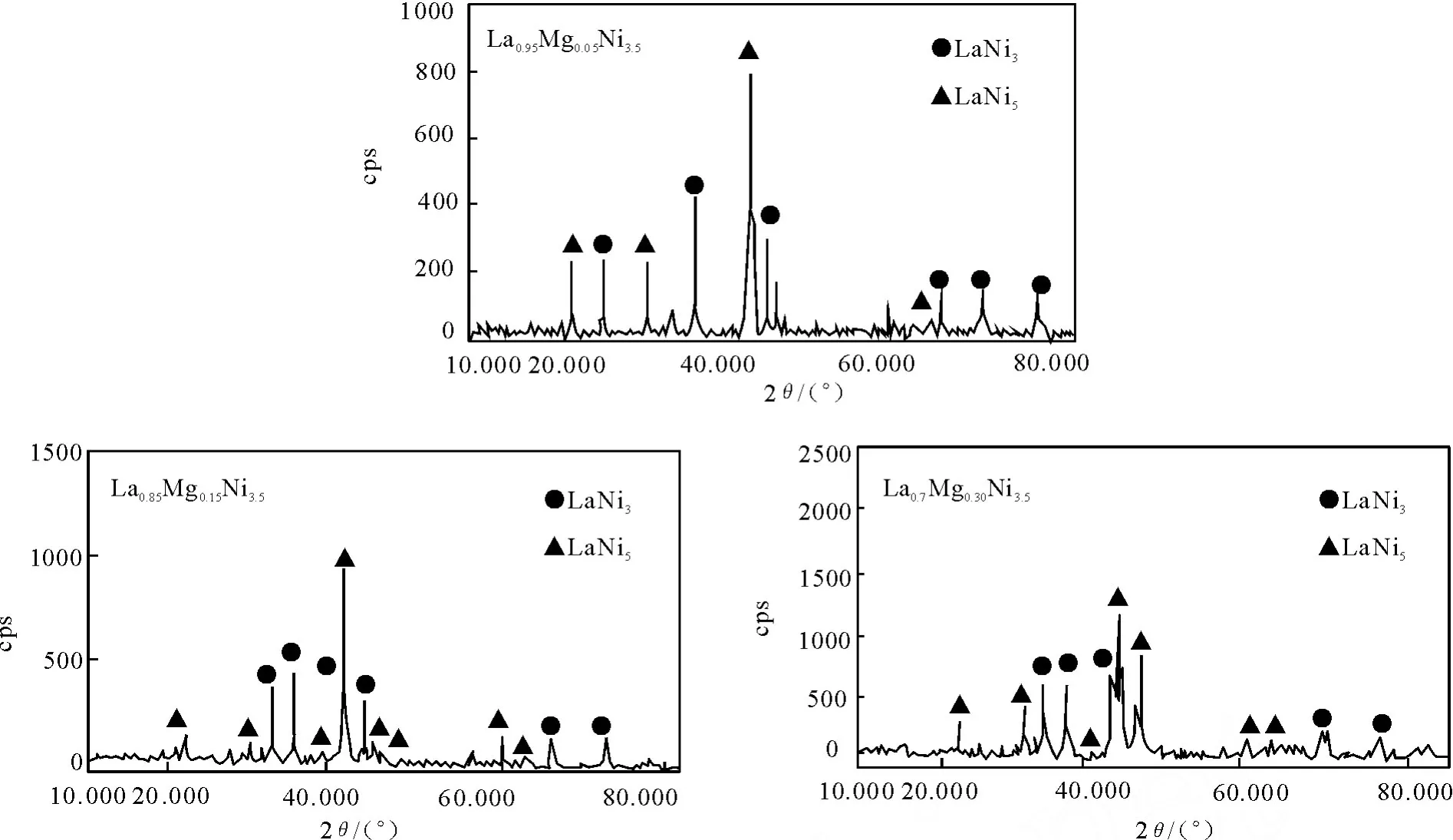
图1 储氢合金的XRD图Fig.1 XRD pattern of hydrogen sto rage alloy
2.2 La-Mg-Ni系储氢合金的储氢性能
2.2.1 镁含量对合金储氢性能的影响
不同镁含量的La-M g-Ni系储氢合金 PCT曲线如图2所示.由图2可知,随着镁含量提高,储氢合金的吸放氢平台压力降低,吸氢量提高.M g含量较高时,储氢合金平台压力较低,这是由于生成的氢化物过于稳定使部分氢无法释放出来,导致储氢合金放氢量减少,平台区域变窄.当 M g摩尔分数为0.3时,储氢容量较高,其吸氢量可达到1.54%.
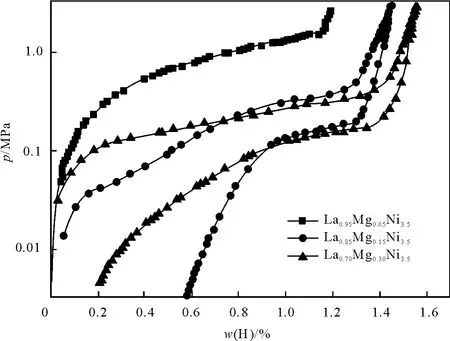
图2 在298K不同镁含量储氢合金的PCT曲线Fig.2 PCT curve of hydrogen sto rage alloys w ith different Mg content at 298K
2.2.2 非化学计量比对储氢合金储氢性能的影响
对储氢材料进行升温真空脱气后及吸放氢活化2次后,在室温(298 K)下测试其吸放氢性能,其吸放氢PCT曲线如图3所示.从图3可知,与化学计量比的La0.7M g0.3Ni3.5合金相比,两种非化学计量比的储氢合金平台压力提高.其中La0.7M g0.3Ni3.25储氢合金具有较好的平台性能,平台压力较高,吸氢量达到1.45%.
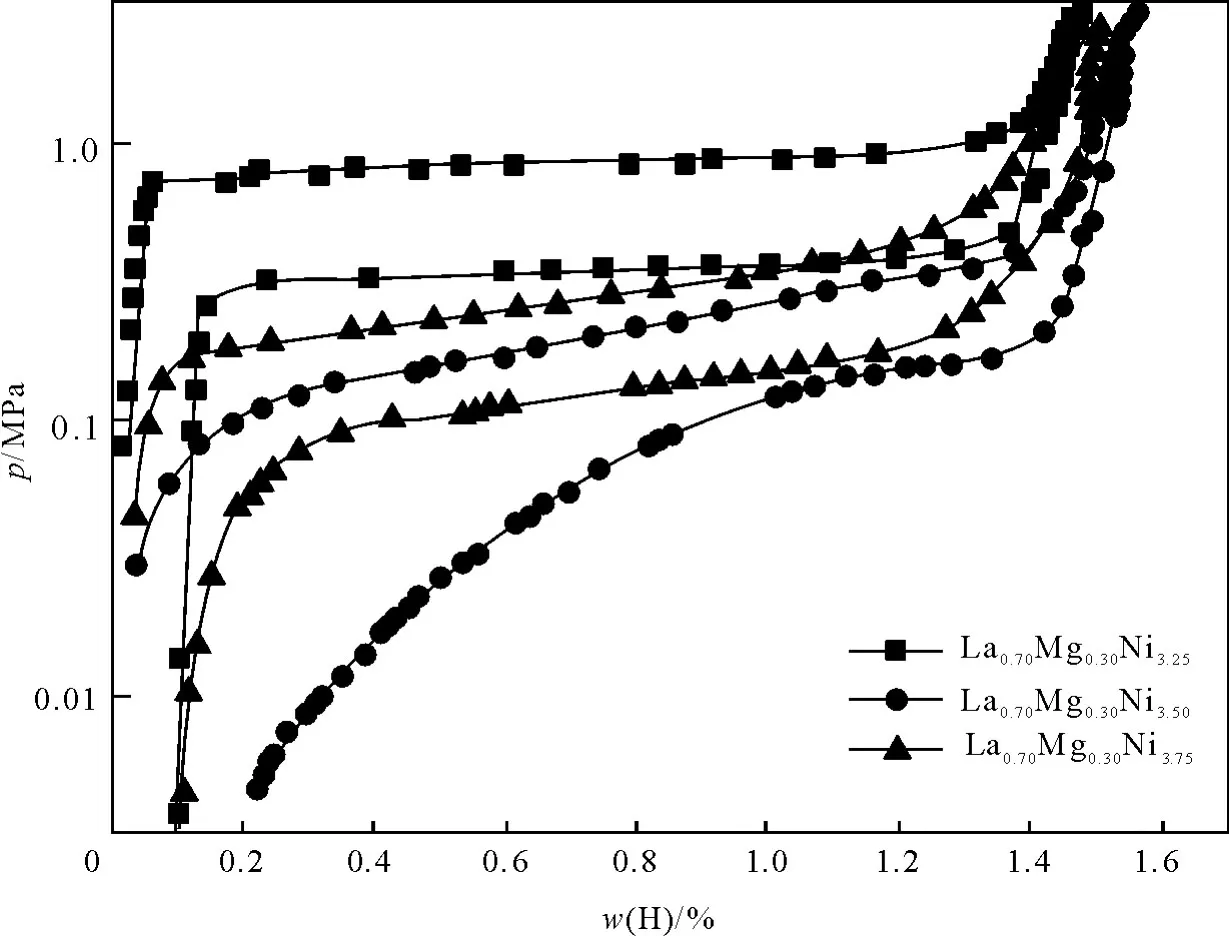
图3 在298K非化学计量比储氢合金的吸放氢性能Fig.3 The hydrogen absorp tion and desorp tion p roperties of non-stoichiometric hydrogen storage alloy at 298K
2.2.3 热处理对储氢合金储氢性能的影响
将La0.7M g0.3Ni3.5储氢合金在1173 K热处理4 h后,在室温(298 K)下测试储氢合金的吸/放氢性能,其吸放氢 PCT曲线如图4所示.由图4可知,经热处理后,La0.7M g0.3Ni3.5储氢合金的平台压力和平台性能提高,吸氢量达到1.59%.

3 结 论
采用快淬法制备了不同镁含量的La-M g-Ni系储氢合金,该合金主要由LaNi5和LaNi3两相组成.随着镁含量的增加,储氢合金的吸放氢平台压力降低,吸氢量提高.与化学计量比储氢合金La0.7M g0.3Ni3.5相比,非化学计量比的储氢合金平台压力提高.La0.7M g0.3Ni3.5储氢合金在1173 K热处理4 h后具有较好的吸/放氢平台性能,其吸氢量可达到1.59%.
[1]ZHANG Yanghuan,DONG Xiaoping,WANG Guoqing,et al.Effectsof rapid quenching onmicrostructures and electrochemical p roperties of La0.7M g0.3Ni2.55Co0.45Bx(x=0~0.2)hydrogen storage alloy[J].Transactions of Nonferrous Metals Society of China,2007,16:800-807.
[2]L IU Yongfeng,PAN Hongge,YUE Yuanjian,et al.Cycling durability and degradation behavio r of La-M g-Ni-Co type metal hydride electrodes[J].Journal of A lloys and Compounds,2005,395:291-299.
[3]PAN Hongge,CHEN Ni,GAO M ingxia,et al.Effects of annealing temperature on structure and the electrochemical p roperties of La0.7Mg0.3Ni2.45Co0.75M n0.1A l0.2hydrogen sto rage alloy[J].Journal of A lloys and Compounds,2005,397:306-312.
[4]L IU Yongfeng,PAN Hongge,GAO Mingxia,et al.Investigation on characteristicsof La0.7Mg0.3Ni2.65Mn0.1Co0.75+x(x=0~0.85)metal hydride electrode alloys fo r Ni/M H batteries PartⅡ:Electrochemical perfo rmances[J].Journal of Alloys and Compounds,2005,388:109-117.
[5]PAN Hongge,L IU Yongfeng,GAO M ingxia,et al.The structure and electrochemical p roperties of La0.7M g0.3(Ni0.85Co0.15)x(x=3.0~3.5)hydrogen sto rage alloys[J].International Journal of Hydrogen Energy,2003,28:1219-1228.
[6]ZHANG Yanghuan,L IBaowei,CA I Ying,et al.Electrochem ical perfo rmances and microstructure of the meltspun La-M g-Ni system hydrogen storage alloys[J].Rare Metal Materials and Engineering,2007,36(1):108-112.
[7]ZHANG Faliang,LUO Yongchun,WANG Dahui,et al.Structure and electrochemical p roperties of La2-xM gxNi7.0(x=0.3~0.6)hydrogen storage alloys[J].Journal of A lloys and Compounds,2007,439:181-188.
[8]WANG Ying,LU qiyun,PENG Neng,et al.Effect of heat-treatment p rocess on p roperties of rare earth M gbased system hydrogen storage alloys w ith AB3-type[J].Journal of Rare Earths,2006,24(S1):340-342.
[9]TANGRenheng,LU qiyun,XIAO Fangming,et al.Study on nanocrystalline of rare earth M g-based system hydrogen storage alloyswith AB3-type[J].Journalof Rare Earths,2006,24(S1):343-346.
[10]ZHANG Zhong,HAN Shumin,L I Yuan,et al.Electrochemical p roperties of M11-xM gxNi3.0M n0.10Co0.55A l0.10(x=0.05~0.30)hydrogen storage alloys[J].Journal of A lloys and Compounds,2007,431:208-211.
Hydrogen storage properties of La-Mg-Ni hydrogen storage alloys
PENG Neng1,2,SU Yuchang1,XIAO Fangming2
1.College of M aterial Science and Engineering,Central South University,Changsha 410083,China;2.Guangdong Genera l Research Institute of Industrial Technology(Guangzhou Research Institute of N onferrous M etals),Guangzhou 510650,China
La-M g-Ni hydrogen storage alloys w ith different M g content were p repared by rapid quenching method and hydrogen storage p ropertiesof these alloyswere investigated.XRD results showed that theses alloyswere mainly composed of LaNi5and LaNi3;w ith the increase of M g content,the hydrogen absorption/desorp tion p lateau p ressure of the alloys lowered and their hydrogen absorp tion capacity increased;p lateau p ressure of the non-stoichiometric hydrogen sto rage alloys increased compared to the stoichiometric hydrogen sto rage alloy;after 4 hoursof heat treatment at 1173 K,the hydrogen sto rage alloy had good absorp tion/desorp tion p lateau performance w ith hydrogen abso rp tion capacity up to 1.59%.
rapid quenching method;La-M g-Ni hydrogen storage alloy;hydrogen storage p roperties
TG139.7
A
1673-9981(2011)02-0121-04
2010-11-24
彭能(1980—),男,湖南娄底人,工程师,硕士研究生.

