Preparation and Photoluminescence of a Novel White-Emitting Phosphor Sr1.5Ca0.5SiO4∶Eu3+,Tb3+,Eu2+
CHEN Xi,ZHAO Jun-feng,YU Li-ping,RONG Chun-ying,LI Cheng-zhi,LIAN Shi-xun
(Key Laboratory of Chemical Biology&Traditional Chinese Medicine Research(Ministry of Education); Key Laboratory of Sustainable Resources Processing and Advanced Materials of Hunan Province; College of Chemistry and Chemical Engineering,Hunan Normal University,Changsha 410081,China)
Preparation and Photoluminescence of a Novel White-Emitting Phosphor Sr1.5Ca0.5SiO4∶Eu3+,Tb3+,Eu2+
CHEN Xi,ZHAO Jun-feng,YU Li-ping,RONG Chun-ying,LI Cheng-zhi,LIAN Shi-xun*
(Key Laboratory of Chemical Biology&Traditional Chinese Medicine Research(Ministry of Education); Key Laboratory of Sustainable Resources Processing and Advanced Materials of Hunan Province; College of Chemistry and Chemical Engineering,Hunan Normal University,Changsha 410081,China)
A series of the nominal composition Sr1.5Ca0.5SiO4∶0.01 Eu3+,nTb3+(n=3.0×10-4,7.0×10-4,1.5×10-3mol)phosphors with single phase was synthesized by high temperature solid-state reaction.Upon the excitation by near-UV light(394 nm),the phosphors give blue,green and orange-red emissions which are originated from Eu2+5d→4f,Tb3+5D4→7FJand Eu3+5D0→7FJtransitions,respectively,with comparable intensities,resulting in white light emission.The results of CIE calculation show that the Sr1.5Ca0.5SiO4∶0.01 Eu3+,7.0×10-4Tb3+phosphor can provide white emission(x=0.321,y= 0.322)very close to the standard white(x=0.33,y=0.33),indicating it is a potential single-phased white phosphor for LED-based UV-chip.
Sr1.5Ca0.5SiO4;white-emitting phosphor;LED
The white light-emitting diodes(LEDs)composed of InGaN blue LEDs and a yellow YAG:Ce phosphor havebeen investigated extensively due to their applications[1].But this kind of white light has a low color rendering index(Ra<80).As the near-ultraviolet(UV)or ultraviolet chip has been applied in consumer,white light generation has been proposed to combine near-UV or ultraviolet LEDs with a single-phased white-light(SPWL)phosphor.Therefore,SPWL phosphors[2-9]have been an active research area in the study of luminescent materials.In this letter,we demonstrate that it is possible to obtain SPWL phosphor via chemical approaches with appropriate tuning of activator contents of Eu3+,Tb3+and Eu2+ions in Sr1.5Ca0.5SiO4host lattices.
A series of phosphors whose design the nominal composition is Sr1.5Ca0.5SiO4∶0.01 Eu3+,nTb3+(n=3.0× 10-4,7.0×10-4,1.5×10-3mol)was synthesized by a two-stage solid-state reaction.The well-mixed starting materials were placed in the tube furnace and calcined at 1 200℃in the air for 4 h,then,the obtained powders were fired at the same temperature in reducing atmosphere(5%H2and 95%N2)for 0.5~1.0 h in order for Eu3+ions in the host lattice to be partially reduced to Eu2+to obtain a final product of Sr1.5Ca0.5SiO4∶Eu3+,Tb3+,Eu2+(abbreviated as SCS∶ETE).The crystalline phase of the prepared samples was examined by the XRD,which indicated only a single phase belonging to Sr1.5Ca0.5SiO4.
As shown in Fig.1,under the excitation of 394 nm,the emission spectra of these SCS∶ETE phosphors exhibited three emission bands:one broad band in the blue area,a second band with sharp lines peaked in green(about 548 nm),and the third band in the orange-red region(588~720 nm).These bands are originated from Eu2+5d→4f,Tb3+5D4→7FJand Eu3+5D0→7FJtransitions,respectively,with comparable intensities,which resulted in white light emission.With the increasing of Tb3+content,both of broad Eu2+emission and sharp Eu3+emission increase.
The white emission can be further confirmed by the CIE(Commission International de I′Eclairage 1931)chromaticity coordinates for the emission spectra of SCS∶ETE.Fig.2 showed the ca.CIE chromaticity coordinates of phosphors vary from cold white to warm white(black dots a→c)with changing of Tb3+concentration(n)from 3.0× 10-4~1.5×10-3mol;when n=7.0×10-4mol,phosphor gives the pure white emission(black dot b:x=0.321,y= 0.322),which is very close to the standard white(x=0.33,y=0.33).The nominal composition Sr1.5Ca0.5SiO4∶0.01 Eu3+,7.0×10-4Tb3+is found to be the optimal composition for single-phased white-light phosphor.

Fig.1Photoluminescence spectra of Sr1.5Ca0.5SiO4∶0.01 Eu3+,n Tb3+phosphors at different Tb3+concentration excited by 394 nm(a→c:n=3.0×10-4,7.0×10-4,1.5×10-3mol).
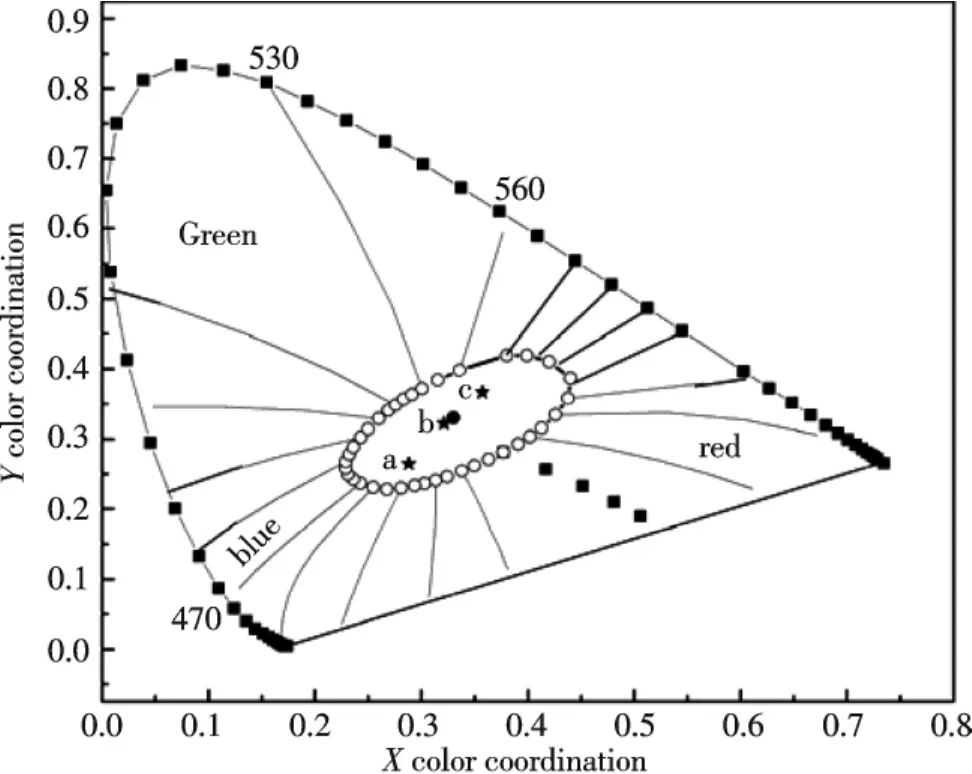
Fig.2The corresponding CIE chromaticity diagram of Sr1.5Ca0.5SiO4∶0.01 Eu3+,n Tb3+phosphors(dots a→c are agreement with n=3.0×10-4,7.0×10-4,1.5×10-3mol).
The luminescent mechanism of SCS∶ETE can be illustrated by the energy levels.The excited electrons from the7F0→5L6transition of Eu3+at 394 nm yields the emission spectrum(Fig.1),which consists of all the emission sharp lines from the5D0excited state to the7FJground state of Eu3+,i.e.,5D0→7F0(578 nm),5D0→7F1(587 nm),5D0→7F2(612 nm),5D0→7F3(650 nm),5D0→7F4(700 nm);from the5D4excited state to the7FJground state of Tb3+,i.e.,5D4→7F5(544 nm),and a broad band range of 400~540 nm centered at 462 nm from the 5d excited state to the8S7/2ground state(4f→5d)of Eu2+.The above emissions have convincingly shown that white light emission of SCS∶ETE is originated from the combination of Eu3+,Tb3+,Eu2+multicolor emitting.
On the other hand,the no-resonance energy transfer process in the system of SCS∶ETE will occur from 5d level of excited Eu2+ions to5D4level of Tb3+ion,enhancing5D4→7F5emitting.The energy transfer efficiency(ηT) increases with increasing Tb3+concentration,which is the same as Tb3+and Eu2+co-doping with(Sr,Ba)2SiO4phosphor[9].In addition,the charge transfer(CT)process from Tb3+to Eu3+has been discussed by many studies[10-13]and can be explained as the following equation:Eu3++Tb3+=Eu2++Tb4+.The CT process holds the equilibrium of the concentration of Tb3+,Eu3+and Eu2+ions in the Ca0.5Sr1.5SiO4host lattice.Therefore,we are sure that tunable white-light emission was resulted from the system of SCS∶ETE as a result of the competition and equilibrium between these two processes when the Tb3+concentration varies.
In conclusion,a series of phosphors Sr1.5Ca0.5SiO4∶Eu3+,Tb3+,Eu2+has been prepared by a two-step hightemperature solid state process.Under the excitation of 394 nm,these emission spectra consist of a broad blue band,a sharp green line and some sharp orange-red lines,which correspond to Eu2+5d→4f transitions,Tb3+5D4→7FJand Eu3+5D0→7FJtransitions,respectively.The white emission(x=0.321,y=0.322)provided by a phosphor with nominal composition Sr1.5Ca0.5SiO4∶0.01 Eu3+,7.0×10-4Tb3+is very close to the standard white,which shows it is a potential single-phased white-light phosphor for UV-light emitting diodes.
This work is partially supported by NSFC(Grant Nos.20971042,50772035),and the Science and Technology Office of Education Department of Hunan Province(No.10A070).
[1]NARENDRAN N,Gu Y M.Life of LED-based white light sources[J].J Display Technol,2005,1(1):167-171.
[2]CHOI N S,PARK K W,PARK B W,et al.Eu2+-Mn2+energy transfer in white-light-emitting T-phase(Ba,Ca)2SiO4∶Eu2+,Mn2+phosphor[J].J Lumin,2010,130(4):560-566.
[3]PARK J K,KIM C H,PARK S H,et al.Application of strontium silicate yellow phosphor for white light-emitting diodes[J]. Appl Phys Lett,2004,84(10):1647-1649.
[4]KIM J S,LIM K T,JEONG Y S,et al.Full-color Ba3MgSi2O8:Eu2+,Mn2+phosphors for white-light-emitting diodes[J]. Solid State Commun,2005,135(1-2):21-24.
[5]HE H,FU R L,SONG X F,et al.White light-emitting Mg0.1Sr1.9SiO4∶Eu2+phosphors[J].J Lumin,2008,128(3):489-493.
[6]HIRAMATSU R,ISHIDA K,AIGA F,et al.Tb3+luminescence by energy transfer from Eu2+in(Sr,Ba)SiO4phosphor[J]. J Electrochem Soc,2009,157(6):J227-J232.
[7]YANG W J,CHEN T M.White-light generation and energy transfer in SrZn2(PO4)2:Eu,Mn phosphor for ultraviolet lightemitting diodes[J].Appl Phys Lett,2006,88(10):101903-101905.
[8]ZHANG X M,WANG J,WU H,et al.One-step synthesis and double emission(Ca1+x-yEuy)Ga2S4+xphosphor for white LEDs[J].Mater Lett,2009,63(2):340-342.
[9]HUANG C H,LIU W R,CHEN T M.Single-phased white-light phosphors Ca9Gd(PO4)7:Eu2+,Mn2+under near-ultraviolet excitation[J].J Phys Chem C,2010,114:18698-18701.
[10]JIAO H,WANG J,LIAO F,et al.Cathodoluminescence of Eu3+,Tb3+,and Tb3+-Eu3+pair-activated Zn3Ta2O8[J].J Electrochem Soc,2004,151(2).
[11]JIAO H,LIAO F,TIAN S,et al.Luminescent properties of Eu3+and Tb3+activated Zn3Ta2O8[J].J Electrochem Soc,2003,150(9):H220-H224.
[12]LAWRENCE T A,MURRA K A,MAY P S.Temperature dependence of rate constants for Tb3+(5D3)cross relaxation in symmetric Tb3+pairs in Tb-doped CsCdBr3,CsMgBr3,CsMgCl3[J].J Phys Chem B,2003,107(17):4 002-4 011.
[13]DEXPERT GHYS J,CHARREIRE Y,LESKELA M,et al.Transient properties of the luminescence of Eu3+and Tb3+in oxysulfide matrices[J].J Electrochem Soc,1985,132(3):711-717.
O482.3
A
1000-2537(2011)03-0046-03
一种新型白光荧光粉Sr1.5Ca0.5SiO4∶Eu3+,Tb3+,Eu2+的合成与光致发光
陈茜,赵君风,余丽萍,荣春英,李承志,廉世勋*
(化学生物学和中药研究教育部重点实验室;资源精细化与先进材料湖南省高校重点实验室;湖南师范大学化学化工学院,中国长沙 410081)
采用高温固相法合成了名义组成为Sr1.5Ca0.5SiO4∶0.01 Eu3+,nTb3+(n=3.0×10-4,7.0×10-4,1.5× 10-3mol)的荧光粉.X射线衍射测试表明荧光粉样品为单一物相.在紫外光(394 nm)激发下,样品同时产生蓝光、绿光和红橙光发射,分别对应于Eu2+离子的5d→4f,Tb3+离子的5D4→7FJ和Eu3+离子的5D0→7FJ跃迁,表明部分Eu3+离子在还原气氛下被还原成Eu2+.红光、绿光和蓝光发射强度相当,复合得到白光.色坐标(CIE)计算结果显示,荧光粉Sr1.5Ca0.5SiO4∶0.01 Eu3+,7.0×10-4Tb3+的白色发光(CIE:x=0.321,y=0.322)接近纯白色(CIE: x=0.33,y=0.33),表明它是一种很有应用前景的基于紫外光芯片的单基白光荧光粉.
Sr1.5Ca0.5SiO4;白光发射荧光粉;发光二极管
2011-01-18
国家自然科学基金资助项目(20971042;50772035);湖南省教育厅基金资助项目(10A070)
*通讯作者,E-mail:lianshixun@yahoo.com.cn
(编辑杨春明)

