不同TiO2原料制备一维钛酸盐纳米材料
李雪飞 赵 芸 矫庆泽 黎汉生 吴洪雨 刘洪博 崔文甲
(北京理工大学化工与环境学院,北京100081)
不同TiO2原料制备一维钛酸盐纳米材料
李雪飞 赵 芸 矫庆泽*黎汉生 吴洪雨 刘洪博 崔文甲
(北京理工大学化工与环境学院,北京100081)
以不同二氧化钛为原料,用水热法制备一维钛酸盐纳米材料.原料一次粒径和晶体结构对一维纳米钛酸盐的形貌和结构的影响很大.原料的一次粒径越小,反应过程中产物的形貌和晶相转变越快;纯锐钛矿相有利于钛酸盐纳米管的形成,而少量金红石相则有利于纳米管向纳米线的进一步转变和晶相转变.
钛酸盐;一维纳米材料;TiO2;水热法;形貌;晶体结构
1 Introduction
Titanium dioxide is a kind of important industrial raw materials,which is widely used in many fields such as coatings, paints,anticorrosion,plastics and so on.With the emergence of nanotechnology,through modern techniques,TiO2can be prepared into nanoparticles,nanotubes,nanorods,nanowires, nanobelts,nanofilm,etc.Because of quantum size effect,small size effect,surface effect,and quantum-tunnel effect,low dimensional TiO2-based nanostructures show novel physical and chemical properties such as unique ultramicro,effective photocatalytical activity,UV absorption properties,and have potential applications in gas sensors,1-4photocatalysts,5-8and solar cells.9-11Several different methods including anodic oxidation,12template-based approaches,13-17and hydrothermal treatment18,19were developed to synthesize TiO2-based one-dimensional nanomaterials.Among these methods,the hydrothermal reaction is a simple and cost-effective method for a large-scale production and widely employed to prepare one-dimensional titanate nanomaterials by treating TiO2powders in alkali aqueous solution.The formation mechanism of 1D titanate nanomaterials prepared using a hydrothermal reaction was examined and the sheet folding mechanism was widely accepted.20-25The ef-fects of synthetic conditions on the microstructures of the 1D titanate nanomaterials are investigated,with the emphasis on the reaction temperature,reaction time,acid washing.In addition, the microstructures of these nanomaterials were considerably affected by the crystallite size and structure of the starting material.6,26-29However,there is no general agreement for the effect of starting materials on the morphology and crystal structure of the hydrothermal product.
In the present work,the influences of the primary particle size and crystal structure of TiO2starting materials on the microstructures of the hydrothermally synthesized one-dimensional nanomaterials were investigated.
2 Experimental
TiO2-I(Xilong Chem.Co.,AR),TiO2-II(P-25 Deggussa, Germany),and TiO2-III(prepared by a sol-gel method)were used as starting materials for preparation of one-dimensional titanate nanomaterials.All other solvents and chemicals were AR grade and used as received.
TiO2-III was prepared by the following sol-gel procedure. 34.00 mL of tetrabutyl titanate(Ti(OC4H9)4)and 3.09 mL of acetylacetone were dissolved in 24.00 mL of ethanol to form solution A.7.20 mL of deionized water at pH value of 9-10 (adjusted with CH3NH2)was mixed with 11.00 mL ethanol to form solution B.Solution B was then added into the solution for a hydrolysis polycondensation reaction,where acetylacetone acted as the chelating agent to decrease the reactivity of Ti(OC4H9)4.The sol sample was dried at 60°C to remove the ethanol and then calcined at 450°C for 4 h to form pure anatase TiO2nanopowders.
For the synthesis of one-dimensional nanomaterials,0.3 g of TiO2powders(TiO2-I,TiO2-II,or TiO2-III)were hydrothermally treated with a 10 mol·L-1NaOH aqueous solution in a revolving teflonlined autoclave at 150°C for 3-48 h.The resulting samples were thoroughly washed with deionized water and naturally dried at room temperature.
The morphologies of the samples were observed on a JOEL 2010 high-resolution transmission electron microscope(HRTEM)operated at 120 kV with copper mounted holey carbon grids.The crystal structures of the samples were identified using powder X-ray diffraction(XRD)on a XʹPert Pro MPD Powder X-Ray diffractometer with Cu Kαradiation.
3 Results
The XRD patterns and TEM images of the different starting materials of TiO2powders are shown in Fig.1.The crystalline component and primary particle size of these starting materials are summarized in Table 1.TiO2-I and TiO2-II were both composed of main anatase phase and a little rutile phase,while the intensity of peaks from TiO2-I was much stronger than that of TiO2-II,which indicated the higher crystallinity of TiO2-I.The primary particle size of TiO2-I was in a large range of several nanometers to micrometers.The primary particles of TiO2-II were nearly spherical with a uniform size of about 20-40 nm. The intensity of the peaks of TiO2-III powders synthesized by sol-gel method was similar to that of TiO2-II,indicating the similar crystallinity.Moreover,TiO2-III powders were pure anatase phase with no rutile phase.TEM image showed that the size of TiO2-III was around 25 nm.
Fig.2 shows XRD patterns of the samples after the hydrothermal treatment of different TiO2powders as starting materials at 150°C for different time.As shown in Fig.2(a),the samples prepared by the hydrothermal treatment of TiO2-I for 3 to 12 h show peaks of NaxH1-xTi2-x/4□x/4O4·H2O(x~0.7,□:vacancy)as well as the peaks of anatase and rutile.After TiO2-I was hydrothermally treated formore than 18 h,peaksof NaxH1-xTi2-x/4□x/4O4·H2O and some weak peaks around 11.0° and 33.0°corresponding to NaxH2-xTi3O7were observed.Thisindicated that NaxH1-xTi2-x/4□x/4O4·H2O can transformed to NaxH2-xTi3O7with time.NaxH1-xTi2-x/4□x/4O4·H2O was completely transformed to NaxH2-xTi3O7after 48 h.
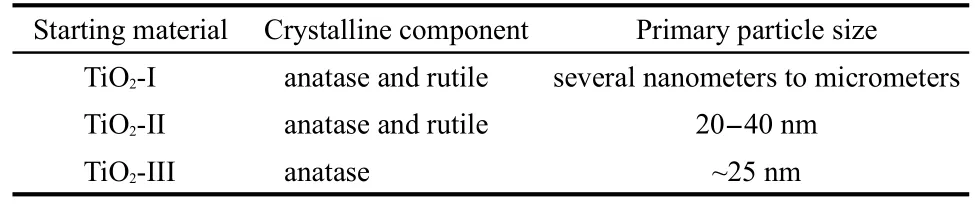
Table 1 Crystalline component and primary particle size of the different starting materials
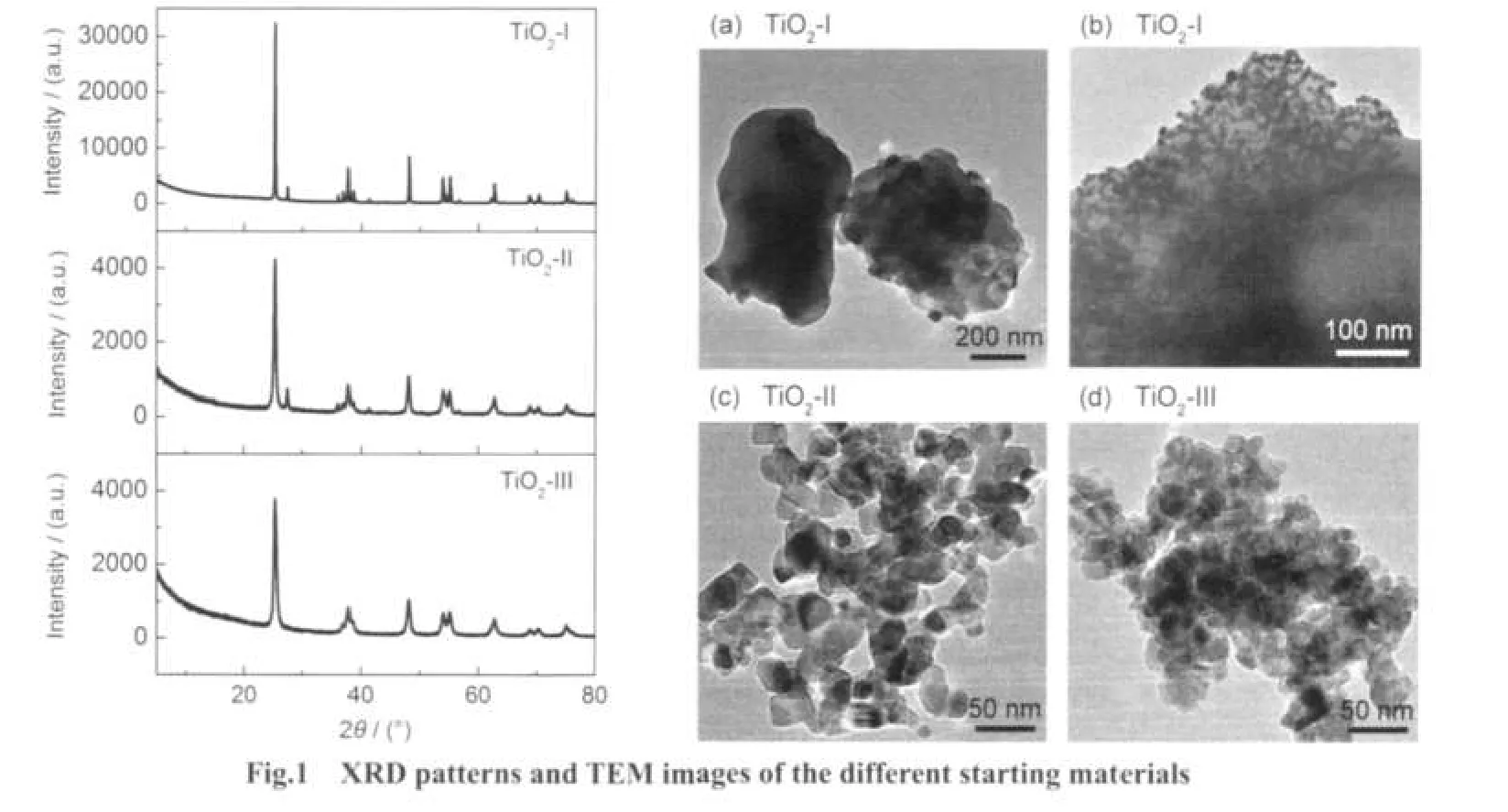
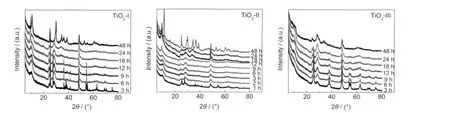
Fig.2 XRD patterns for the samples prepared from different starting materials after the hydrothermal treatment at 150°C for different time
When TiO2-II was employed as the starting materials(Fig.2 (b)),the rate of phase transformation was greatly rapid.Even after hydrothermal treatment for 3 h,neither anatase nor rutile was found and NaxH1-xTi2-x/4□x/4O4·H2O was only formed.Also, it only took 12 h for the appearance of weak peaks around 11.0°and 33.0°corresponding to NaxH2-xTi3O7.
Fig.2(c)shows the XRD patterns for the samples after the hydrothermal treatments of TiO2-III as starting materials.A very weak peak around 9.5°from NaxH1-xTi2-x/4□x/4O4·H2O was observed for samples with hydrothermal reaction time of 3-24 h.The main peak at 2θ of 25.5°of anatase decreased with the hydrothermal reaction time.After the hydrothermal treatment for 24 h,the peaks of anatase nearly diminished and a weak peak of NaxH2-xTi3O7around 33.0°appeared.The hydrothermal treatment for 48 h also resulted in the transformation from NaxH1-xTi2-x/4□x/4O4·H2O to NaxH2-xTi3O7,but the peak was much broader and its intensity was much lower,which indicated the poor crystallization of the sample.
In summary,the evolution of titanate nanostructures was found to obey“TiO2→NaxH1-xTi2-x/4□x/4O4·H2O→NaxH2-xTi3O7”transformation process.However,the rate of phase transformation was greatly affected by starting materials.Based on the above XRD results,the formation of NaxH1-xTi2-x/4□x/4O4·H2O and its transformation to NaxH2-xTi3O7from TiO2-II was much faster than TiO2-I and TiO2-III as starting materials.It might be attributed to the smaller and uniform primary particle size and the existence of rutile in TiO2-II.
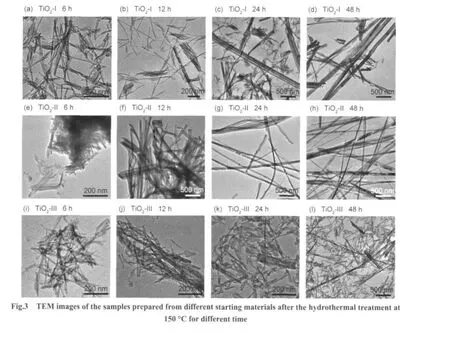
Fig.3 shows the morphologies of samples after the hydrothermal treatment of different TiO2powders as starting materials.As shown in Fig.3(a-d),for the samples with TiO2-I as the starting materials,a mixture of nanotubes together with nanosheets was obtained after hydrothermal treatment at 150°C for 6 h.Nanotube bundles were formed after 12 h,and the amount of nanotube bundles increased with increasing the hydrothermal reaction time.After 48 h,most of the nanotubes existed in the shape of bundles.When TiO2-II was used as starting materials,as shown in Fig.3(e-h),nanotubes and nanosheets coexisted in the sample after the hydrothermal treatment for 6 h.However, they aggregated in clusters,different from the sample hydrothermally treated for 6 h with TiO2-I as the starting material.In addition,the transformation from nanotubes to nanowires or nanoribbons was much easier.After 12 h,clusters composed of nanoribbons and nanotube bundles with 50-200 nm in diameter and a few tens to several hundreds of micrometers in length were obtained.With the extension of hydrothermal reaction time,the nanotube bundles converted to nanowires or nanoribbons completely.For TiO2-III,however,tubular morphology was only confirmed by TEM even after a hydrothermal treatment for 48 h,as shown in Fig.3(i-l).This difference was suggested to result from the crystalline structure of the starting TiO2materials.A mixture of anatase and a small quantity of rutile favored the formation of nanowires or nanoribbons compared to the pure anatase helpful for the formation of nanotubes.
In short,the morphology and crystal structure of titanate nanomaterials obtained by the hydrothermal treatment greatly depended on the particle size and crystal structure of starting materials besides the reaction temperature and time.
4 Discussion
At present,it was widely believed that when TiO2was hydrothermally treated with concentrated NaOH,the reaction began with the attack of Na+and OH-to the Ti―O―Ti bonds on the surface of starting materials,19causing nanosheets of layered titanates peeling off the surface of the particles and subsequently rolling into nanotubes.24,30-34The primary particles of TiO2-I were not uniform in size,and agglomerated to large secondary grains.As TiO2-I was used as the starting material,the reactive Na+and OH-species suffered non-uniform local reaction rates because of the uneven primary particle size and the restrict of penetrating into the narrowly constricted interparticle zones. On the other hand,TiO2-II primary particles had a uniform size distribution so that they could form a milky-like liquid with NaOH aqueous solution.The reaction of NaOH on the surface of the TiO2-II particles was much more thorough and uniform. Therefore,the hydrothermal reaction rate of the formation of nanotubes and their transformation to nanowires or nanoribbons was greatly increased.
The TiO2-III primary particles were similar to TiO2-II in size but they agglomerated to large secondary grains.However, when TiO2-III was used as starting materials,it was found that the morphology of the samples was uniform.Therefore,it was concluded that the morphology of the as-synthesized sample was greatly affected by the primary particle size,not the secondary grain size.The transformation process of crystalline structure of the samples prepared with TiO2-II and TiO2-III as starting materials was similar.However,after the same reaction time,the crystallinity of the samples obtained from TiO2-III was much lower than those produced with TiO2-II as starting material.Also,even after a hydrothermal treatment for 48 h,only nanotubes were obtained with TiO2-III as starting material,while nanowires and nanoribbons were obtained after a hydrothermal treatment for only 12 h with TiO2-II as starting material.Therefore,this difference is suggested to result from the crystal structure of the starting material.The existence of rutile in TiO2-II favored the formation and microstructure transformation of titanate because of the synergistic effect between anatase and rutile in the mixed-phase TiO2-II materials though the transition from rutile to titanate was more difficult than anatase.
5 Conclusions
The morphology and crystal structure of one-dimensional titanate nanomaterials formed by the hydrothermal treatment were dependent of the primary particle size and crystalline structure on the starting TiO2materials,besides the reaction temperature and time.Pure anatase TiO2was favor for the synthesis of titanate nanotubes.Smaller and deagglomerated particles of the starting materials and the existence of rutile in starting materials were helpful to quicken the formation and structure transformation of titanate.
(1) Seo,M.H.;Yuasa,M.;Kida,T.;Huh,J.S.;Shimanoe,K.; Yamazoe,N.Sens.Actuator B-Chem.2009,137(2),513.
(2) Han,C.H.;Hong,D.W.;Kim,I.J.;Gwak,J.;Han,S.D.; Singh,K.C.Sens.Actuator B-Chem.2007,128(1),320.
(3) Hong,D.U.;Han,C.H.;Park,S.H.;Kim,I.J.;Gwak,J.;Han, S.D.;Kim,H.J.Curr.Appl.Phys.2009,9(1),172.
(4) Kim,H.S.;Moon,W.T.;Jun,Y.K.;Hong,S.H.Sens.Actuator B-Chem.2006,120(1),63.
(5) Qamar,M.;Yoon,C.R.;Oh,H.J.;Lee,N.H.;Park,K.;Kim,D. H.;Lee,K.S.;Lee,W.J.;Kim,S.J.Catal.Today 2008,131 (1-4),3.
(6) Zhao,Y.;Zhao,T.Y.;Liu,Z.Y.;Nakata,K.;Nishimoto,S.; Murakami,T.;Jiang,L.;Fujishima,A.J.Mater.Chem.2010,20 (24),5095.
(7) Yu,J.G.;Yu,H.G.;Cheng,B.;Trapalis,C.J.Mol.Catal. A-Chem.2006,249(1-2),135.
(8) Li,Q.Y.;Lu,G.X.J.Power Sources 2008,185(1),577.
(9) Uchida,S.;Chiba,R.;Tomiha,M.;Masaki,N.;Shirai,M. Electrochemistry 2002,70(6),418.
(10) Hao,Y.Z.;Wang,L.G.Acta Chim.Sin.2008,66(7),757. [郝彦忠,王利刚.化学学报,2008,66(7),757.]
(11) Li,X.D.;Zhang,D.W.;Sun,Z.;Chen,Y.W.;Huang,S.M. Microelectron.J.2009,40(1),108.
(12)Gong,D.;Grimes,C.A.;Varghese,O.K.;Hu,W.C.;Singh,R. S.;Chen,Z.;Dickey,E.C.J.Mater.Res.2001,16(12),3331.
(13) Hoyer,P.Langmuir 1996,12(6),1411.
(14) Imai,H.;Takei,Y.;Shimizu,K.;Matsuda,M.;Hirashima,H. J.Mater.Chem.1999,9(12),2971.
(15) Cui,Y.T.;Wang,J.S.;Li,H.Y.;Wang,Z.Z.Chin.J.Inorg. Chem.2009,25(7),1274.[崔云涛,王金淑,李洪义,王珍珍.无机化学学报,2009,25(7),1274.]
(16)Chen,W.;Sun,X.D.;Weng,D.Mater.Lett.2006,60(29-30), 3477.
(17) Lee,J.;Ju,H.;Lee,J.K.;Kim,H.S.;Lee,J.Electrochem. Commun.2010,12(2),210.
(18) Kasuga,T.;Hiramatsu,M.;Hoson,A.;Sekino,T.;Niihara,K. Langmuir 1998,14(12),3160.
(19) Kasuga,T.;Hiramatsu,M.;Hoson,A.;Sekino,T.;Niihara,K. Adv.Mater.1999,11(15),1307.
(20)Chen,Q.;Zhou,W.Z.;Du,G.H.;Peng,L.M.Adv.Mater.2002, 14(17),1208.
(21) Horvath,E.;Kukovecz,A.;Konya,Z.;Kiricsi,I.Chem.Mat. 2007,19(4),927.
(22)Poudel,B.;Wang,W.Z.;Dames,C.;Huang,J.Y.;Kunwar,S.; Wang,D.Z.;Banerjee,D.;Chen,G.;Ren,Z.F.Nanotechnology 2005,16(9),1935.
(23)Huang,J.Q.;Cao,Y.G.;Huang,Q.F.;He,H.;Liu,Y.;Guo,W.; Hong,M.C.Cryst.Growth Des.2009,9(8),3632.
(24)Yao,B.D.;Chan,Y.F.;Zhang,X.Y.;Zhang,W.F.;Yang,Z.Y.; Wang,N.Appl.Phys.Lett.2003,82(2),281.
(25) Ma,R.Z.;Bando,Y.;Sasaki,T.Chem.Phys.Lett.2003,380 (5-6),577.
(26) Yuan,Z.Y.;Su,B.L.Colloid Surf.A-Physicochem.Eng.Asp. 2004,241(1-3),173.
(27)Afshar,S.;Hakamizadeh,M.J.Exp.Nanosci.2009,4(1),77.
(28)Poudel,B.;Wang,W.Z.;Dames,C.;Huang,J.Y.;Kunwar,S.; Wang,D.Z.;Banerjee,D.;Chen,G.;Ren,Z.F.Mater.Res.Soc. Symp.Proc.2005,836,23.
(29) Morgan,D.L.;Waclawik,E.R.;Frost,R.L.Synthesis and characterisation of titania nanotubes:Effect of phase and crystallite size on nanotube formation.InAdvanced Materials and Processing IV,4th International Conference onAdvanced Materials and Processing,Hamilton,New Zealand,Dec 10-13, 2006;Zhang,D.,Pickering,K.,Gabbitas,B.,Cao,P.,Langdon, A.,Torrens,R.,Verbeek,J.,Eds.;Trans Tech Publications LTD: Switzerland,2007;211-214.
(30)Wang,Y.Q.;Hu,G.Q.;Duan,X.F.;Sun,H.L.;Xue,Q.K. Chem.Phys.Lett.2002,365(5-6),427.
(31)Bavykin,D.V.;Parmon,V.N.;Lapkin,A.A.;Walsh,F.C. J.Mater.Chem.2004,14(22),3370.
(32) Chen,W.P.;Guo,X.Y.;Zhang,S.L.;Jin,Z.S.J.Nanopart. Res.2007,9(6),1173.
(33)Lan,Y.;Gao,X.P.;Zhu,H.Y.;Zheng,Z.F.;Yan,T.Y.;Wu,F.; Ringer,S.P.;Song,D.Y.Adv.Funct.Mater.2005,15(8),1310.
(34) Penga,H.R.;Lia,G.C.;Zhang,Z.K.Mater.Lett.2005,59
(10),1142.
March 1,2011;Revised:May 27,2011;Published on Web:June 10,2011.
Preparation of One-Dimensional Titanate Nanomaterials Using Different Titania Sources
LI Xue-Fei ZHAO Yun JIAO Qing-Ze*LI Han-Sheng WU Hong-Yu LIU Hong-Bo CUI Wen-Jia
(School of Chemical Engineering and Environment,Beijing Institute of Technology,Beijing 100081,P.R.China)
One-dimensional titanate nanomaterials were synthesized by a hydrothermal reaction using differenttitania sources.The morphologyand crystalstructure ofthe one-dimensionaltitanate nanomaterials were greatly affected by the primary particle size and crystal structure of the starting materials.The smaller initial particle size of the reactant led to a faster phase transformation of the products.The pure anatase titania favored the formation of titanate nanotubes,while the mixture of anatase titania and a small amount of rutile titania as a starting material favored the further transformation of nanotubes to nanowires or nanoribbons and promoted the phase transformation.
Titanate;One-dimensional nanomaterials;TiO2;Hydrothermal method;Morphology; Crystal structure
O649
*Corresponding author.Email:jiaoqz@bit.edu.cn;Tel:+86-10-68918979.
The project was supported by the Basic Research Foundation of Beijing Institute of Technology,China(20070542001).
北京理工大学基础研究基金(20070542001)资助项目

