“三明治式”多层Au/Pt复合薄膜的制备及其对甲醇的电氧化
赵 静 孙 越 李永军 梁 韧
(湖南大学化学化工学院化学系,长沙410082)
“三明治式”多层Au/Pt复合薄膜的制备及其对甲醇的电氧化
赵 静 孙 越 李永军*梁 韧
(湖南大学化学化工学院化学系,长沙410082)
采用界面组装、欠电位沉积和氧化还原置换反应组合方法制备了单层Pt/Au复合薄膜,并且不需要任何有机偶联剂;组装单层Pt/Au复合薄膜为三类多层Pt/Au复合薄膜:(Pt/Au)n、Ptm/Au和(Pt3/Au)k(n、m和k分别为Pt/Au、Pt和Pt3/Au的层数).采用电子显微镜研究了Au纳米粒子单层膜和Pt/Au复合多层膜的形貌.对于所有的多层膜电极而言,其电化学活性面积随着层数的增加而增加.通过研究甲醇在每一类Pt/Au复合薄膜上的氧化电流密度,考察了其对甲醇的电催化和抗毒化性能.对于同一类复合薄膜而言,甲醇分别在(Pt/Au)3、Pt3/ Au和(Pt3/Au)2电极上均具有最大的氧化电流密度,且优于本体Pt电极.在这三种电极中,(Pt/Au)3电极无论从电流密度上还是从抗毒化能力上讲,其性能是最好的,而且其抗毒化能力也优于商业Pt/C催化剂.这种良好的催化性能源于Au和Pt之间最大化的协同效应,这取决于Pt和Au原子比率以及Pt纳米层和Au纳米层之间的排布方式.
界面组装;欠电位沉积;Au/Pt复合多层膜;电氧化;甲醇
1 Introduction
Direct methanol fuel cells(DMFCs)have received extensive attention as portable electronic devices due to their high energy density,low pollutant emission,low operating temperature, and relatively compact design.1-3Platinum,as one of the most effective anodic electrocatalysts at present,is indispensable in methanol oxidation.However,pure Pt metallic catalyst is expensive and extremely susceptible to be poisoned by the CO-like intermediate derived from the oxidation of methanol;its application is very limited.To improve the catalytic efficiency and lower the content of costly Pt in catalysts,designing Ptbased bimetallic catalysts becomes a common way.4,5The performance of this kind of composite catalysts is dependent on synergistic effects of hetero-metals and significantly different from that of either component.6
Although gold is rather inert in its bulk form,gold nanoparticles(AuNPs)exhibit good catalytic activity for CO electrooxidation,and are dependent on the synergetic effects,Au/Pt bimetallic catalyst,such as Pt decorated Au,7-10the mixture of Au and Pt nanopowder,11and Pt-based alloy,12has been reported to show better electrocatalytic activity for methanol oxidation than pure Pt.13,14However,the alloy catalysts can not maintain long-term stability due to the behavior of phase separation.
Recent researches have also found that Pt thin films deposited on a second metal can not only provide good catalytic activity,but also greatly increase the utilization efficiency of Pt.15-17It has been theoretically attributed to the shift of average d band center energy,decreasing the metal-adsorbate bonding strength.18,19The under-potential deposition(UPD)technique has been proposed to indirectly prepare Pt metallic monolayer.20,21Typically,a Cu monolayer is electrodeposited on a foreign metal substrate by UPD,and then displayed into a PtCl62-solution.Thus,a Pt monolayer is achieved through a redox replacement reaction.By this UPD-redox replacement technique, many types of Au/Pt catalysts have been prepared to exploit their electrocatalytic performance,such as Pt decorated AuNP film,22Au nanotube film,23and sponge-like Au film.24These Au/ Pt catalysts show high electrocatalytic activity and good longterm stability for methanol oxidation.
In the case of Pt decorated AuNP film catalyst,the catalytic performance depends on the particle density of pre-deposited Au film25and the coverage of Pt layer on AuNPs.26Closepacked AuNP film serving as a substrate for depositing Pt is necessary forimproving catalytic efficiency.27Generally, AuNPs were fabricated on the electrode surface by the linkerassisted method,27,28but the coverage of AuNPs on the electrode surface is very low,generally less than 40%.Therefore, the Pt content of the resulting Pt/Au composite multilayer films after UPD-redox replacement is also very low.This means that the synergistic effect between Au and Pt is not entirely shown.To increase Pt content to improve the catalytic efficiency,the multi-cycle UPD-redox replacement has been used to increase the coverage and thickness of Pt layer.29
Recently,our group developed several methods to assemble close-packed AuNP monolayers at liquid-liquid interfaces,30-33and a linker-free method to prepare multilayer nanoparticle films by repeatedly transferring Au monolayer to the same glass carbon(GC)electrode surface.34Here,the interfacial assembly of Au nanoparticles and UPD-redox replacement were combined to fabricate sandwich-like Pt/Au composite multilayer films for methanol electro-oxidation.This sandwich-like Pt/ Au composite multilayer film can fully exhibit the synergistic effect between Au and Pt because each Pt layer is located between twoAuNP monolayer films.
2 Experimental
2.1 Chemicals
Chloroauric acid tetrahydrate(HAuCl4·4H2O,≥99.9%),hexachloroplatinic(IV)acid hexahydrate(H2PtCl6·6H2O,≥99.5%) and n-butanol(C4H10O,99%)were obtained from Sinopharm Chemical Reagent Co.Ltd.(China).All chemicals were of analytical grade and were used as received.All solutions were prepared with Milli-Q water.
2.2 Synthesis and assembly of Au nanoparticles
AuNP aqueous solution was prepared according to Frensʹ method.35Typically,1%(w)sodium citrate aqueous solution was added into a boiling aqueous solution containing 0.01% (w)HAuCl4under vigorous stirring conditions,and after 40 min,AuNP aqueous solution was obtained.The AuNP size was estimated to be 18 nm according to transmission electron microscopy(TEM)images.
AuNP monolayer film was prepared as follows:32,335 mL of n-butanol was poured into a Petri dish containing 20 mL of asprepared AuNP aqueous solution,and consequently an oil/water interface was formed immediately between n-butanol and water.The above system was kept silent for 15 min to accumulate AuNPs at the interface.The resulting AuNP monolayer film was transferred onto a GC surface for morphological characterization or electrochemical measurements when most of n-butanol was removed.The detailed information about the preparation and transfer of AuNP monolayer can be found in our previous publications.32,33
2.3 Fabrication of Pt/Au composite multilayer films
To enhance the adherence force between AuNP monolayer film and GC,the resulting Au/GC electrode was firstly kept in an oven at 90°C for 15 min,which is a key step for ensuring the stability of Pt/Au composite multilayer films to be prepared;otherwise,Pt/Au composite multilayer films are apt to peel from the GC surface.The Au/GC electrode after heating treatment was subsequently inserted in a deaerated 0.1 mol·L-1H2SO4+1 mmol·L-1CuSO4solution for under-depositing Cu layer on the AuNP surface(Cu/Au/GC).A Cu UPD layer can be obtained by holding the potential at 0.05 V for 200 s in the potentiostatic mode,20and the thicker UPD layer can be obtained by repeating the UPD process several times.The Cu/Au/ GC electrode was rapidly transferred into a deaerated 5 mmol· L-1H2PtCl6solution,and was kept for 10 min.The Cu UPD layer was gradually replaced by Pt layer via the redox replacement reaction between Cu and PtCl2-6.Finally,a Pt/Au/GC electrode was obtained,as shown in Scheme 1(a-d).We designated the above combination of UPD and redox replacement process as“the UPD-redox replacement”.
Here,we adopted two methods to fabricate sandwich-like Pt/ Au composite multilayer films:
(1)The resulting Pt/Au/CG electrode was used as the substrate to deposit another AuNP monolayer film and then the UPD-redox replacement is performed,as shown in Scheme 1 (e).A Pt/Au/Pt/Au/GC electrode can be obtained,designated as(Pt/Au)2/GC.By repeating AuNP monolayer transfer and the UPD-redox replacement,(Pt/Au)3/GC,(Pt/Au)4/GC,and(Pt/ Au)n/GC electrodes can be fabricated easily.
(2)To increase Pt content in each AuNP monolayer film,the resulting Pt/Au/GC electrode was used again as a substrate to repeat the UPD-redox replacement to fabricate Pt2/Au/GC,Pt3/ Au/GC,and Ptm/Au/GC electrodes.Pt3/Au/GC was further chosen as the substrate to construct(Pt3/Au)k/GC.n,m,and k are the number of Pt/Au,Pt,and Pt3/Au units,respectively.
Surface morphologies of all above Pt/Au composite multilayer films are further examined after electrochemical experiments and no noticeable change was observed,indicating that these electrode films are stable.
2.4 Instruments and measurements
The electrochemical cell with a three-electrode configuration was used for all the electrochemical measurements on a CHI660B electrochemical workstation(Chenhua,China).The working electrode is bulk Pt(Ptbulk),(Pt/Au)n/GC,Ptm/Au/GC, or(Pt3/Au)k/GC;a Pt wire and a saturated calomel electrode (SCE)were used as the counter electrode and the reference electrode,respectively.
Scanning electron microscopy(SEM)images of Pt/Au films were obtained on Hitachi-S4800 scanning electron microscope (Hitachi,Japan)at an accelerating voltage of 3.0 kV.Transmission electron microscopy(TEM)images were recorded on JEM-3010 transmission electron microscope(JEOL,Japan)operating at 200 kV.
3 Results and discussion
3.1 Fabrication and characterization of(Pt/Au)n/GC, Ptm/Au/GC,and(Pt3/Au)k/GC electrodes
The AuNP monolayer prepared at n-butanol/water interface was transferred onto GC surface for SEM characterization and onto Cu grid coated carbon for TEM characterization,as shown in Fig.1.The entire AuNP monolayer film is not perfect due to the existence of some vacancies,but in the short range, AuNPs show a close-packed structure,similar to our previous results.33
After the Au/GC electrode was treated via a UPD replacement,the surface morphology has a slight change due to the epitaxial growth of Cu on AuNPs:36most of neighboring AuNPs connected each other and formed a chain-like structure except for the existence of some isolated AuNPs,as shown in
Fig.2(A).Electrochemical linear sweep voltammetry further confirmed the success of Cu under-potential deposition,as shown in Fig.2(B).A conspicuous anodic peak appeared at about 0.24 V,consistent with the oxidation potential of UPD Cu,37,38which indicates that UPD Cu layer has been fabricated on theAu/GC surface.
When the Cu/Au/GC electrode was rapidly immersed into the deaerated H2PtCl6solution for 10 min,the UPD Cu layer was replaced completely by a Pt layer.37,39Thus,a Pt/Au/GC electrode was obtained,as shown in Fig.3(A),which nearly has the same morphology as Cu/Au/GC(Fig.2(A)).By repeating the fabrication cycles as described in Experimental Section,sandwich-like(Au/Pt)ncomposite multilayer films can be easily created on a GC electrode surface.Compared with the increase of n(n=1,2,3,4,5),most of initial vacancies disappears in the film and the macroscopic structure become more compact and rougher due to the overlapping between Pt/Au layers.SEM images of Au/Pt multilayer composite multilayer films as n=3,5 are shown in Fig.3(B,C).This morphology change is similar to that of Ptm/Au/GC with the increase of m (m=1,2,3,4,5)and(Pt3/Au)k/GC with the increase of k(k=1, 2,3)(not shown).
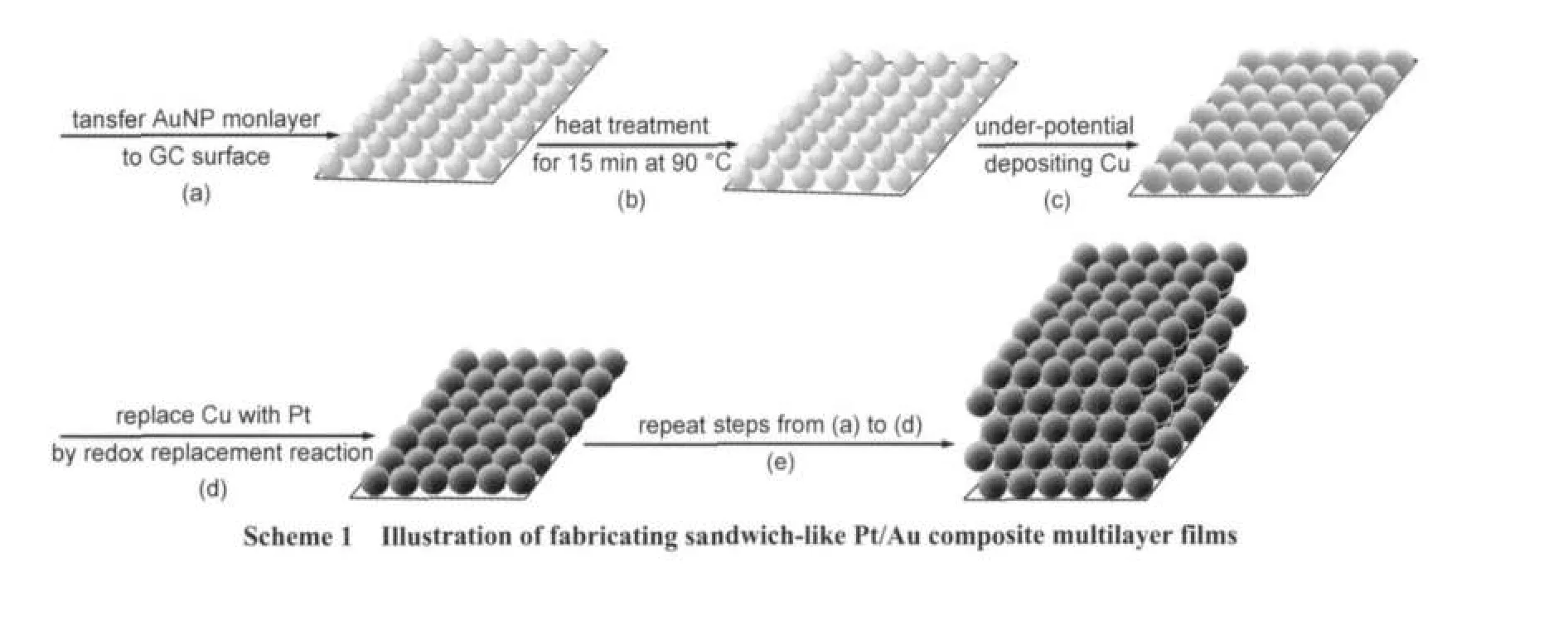
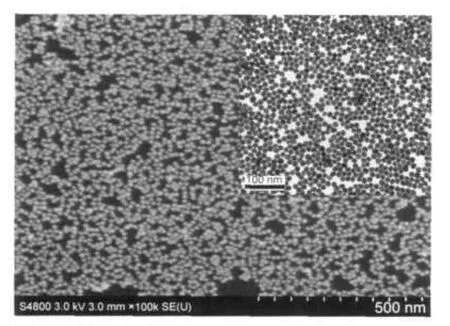
Fig.1 SEM and TEM(inset)images ofAuNPmonolayer
All resulting composite electrodes,Au/GC,(Pt/Au)n/GC,Ptm/ Au/GC,and(Pt3/Au)k/GC,were characterized in 0.5 mol·L-1H2SO4using cyclic voltammetric technique,as shown in Fig.4. The CV curve of the bareAu film(Fig.4(A))shows two characteristic redox peaks of Au species:1.1 V in the forward scan and 0.8 V in the backward scan,and a larger double-layer region below 0.7 V presenting the nanoparticle nature of the Au film.20However,the Au redox peaks can not be discerned in the CVs on various Pt/Au composite multilayer film electrodes due to the lower current in the defined potential range(Fig.4 (B,C,D)).All the resulting Pt/Au composite multilayer films have the same CV characteristics:anodic peaks at~1.0 V,cathodic peaks at 0.53 V,and pairs of redox peaks at the potential range from-0.2 to 0.2 V,which is the typical voltammetric pattern of Pt species.40-42In the case of(Pt/Au)n/GC and(Pt3/ Au)k/GC electrodes,peak currents gradually become more and more intense with the increase of n and k,respectively,indicating that this linker-free layer-by-layer assembly works very well for preparing Au/Pt multilayer composite multilayer films.For Ptm/Au/GC electrodes,the increase of peak current originates from the increase of exposed Pt surface area.Based on the redox replacementit is clear that each Pt layer just covers 50 percent of the surface of AuNPs,and is a sub-monolayer rather than a full monolayer. Thus,increasing the number of Pt deposition cycles can increase the content of Pt and surface roughness,which contributed to the increase of exposed Pt surface area.
3.2 Catalytic activity of Pt/Au composite multilayer films to electrooxidation of methanol
To explore the relationship between electrocatalytic activity and film structure,methanol electrooxidation was performed on(Pt/Au)n/GC,Ptm/Au/GC,and(Pt3/Au)k/GC electrodes.For comparison,a bulk Pt(Ptbulk)electrode was also used.Fig.5(A) shows the CVs of Ptbulkand(Pt/Au)n/GC electrodes in a N2-saturated 0.1 mol·L-1CH3OH+0.1 mol·L-1H2SO4solution.To reflect exactly the effect of AuNPs on catalytic performance of Pt,the catalytic current per unit area of Pt catalyst was adopted.The effective surface area of Pt layer was estimated from the total hydrogen desorption charge in Fig.4 according to the well-accepted monolayer hydrogen absorption charge of 210 mC·cm-2.43A primary oxidation peak at~0.65 V in the forward scan and the secondary oxidation peak at~0.43 V in the backward scan can be observed on the Ptbulkor(Pt/Au)n/GC(n=1,2, 3,4,5)electrode(Fig.5(A)).The catalytic current density on Ptbulkelectrode is far lower than those on(Pt/Au)n/GC,suggesting that those Pt/Au composite multilayer films have satisfacto-ry catalytic efficiency.It may be attributed to Pt microstructures and the synergistic effect of Au and Pt nanoparticles. With the increase of n,the oxidation potential slightly shifts positive and the catalytic current density dramatically increases,and reaches a maximum value as n=3.Subsequently,the current density decreased again.Despite that,the(Pt/Au)4/GC and(Pt/Au)5/GC electrodes still exhibit better catalytic activity than Ptbulk.



Fig.4 CVs ofAu/GC(A),(Pt/Au)n/GC(B),Ptm/Au/GC(C),and(Pt3/Au)k/GC(D)electrodes in 0.5 mol·L-1H2SO4solution scan rate(v):50 mV·s-1
In methanol electrooxidation,the ratio of the forward anodic peak current density(If)to the backward anodic peak current density(Ib),If/Ib,is commonly used to evaluate the catalyst tolerance to carbonaceous species accumulation.44A low If/Ibratio shows poor oxidation of methanol to carbon dioxide during the anodic scan and excessive accumulation of carbonaceous residues on the catalyst surface,while a high If/Ibratio indicates the converse case.As shown in Fig.5(B),(Pt/Au)1/GC,(Pt/Au)2/ GC,and(Pt/Au)3/GC electrodes show higher If/Ibratio than Ptbulk,indicating that those three film electrodes possess better CO-poisoning tolerance.
To explore the influence of Pt content on AuNP monolayer on catalytic activity,Ptm/Au/GC electrode was fabricated.Fig.6 (A)shows CVs of Ptm/Au/GC(m=1,2,3,4,5)electrodes in a N2-saturated 0.1 mol·L-1CH3OH+0.1 mol·L-1H2SO4solution. As the m value increased,the catalytic current density gradually increased and subsequently decreased.When m=3,the current density is the largest one.But the poisoning tolerance(If/ Ib)was not always improved,as shown in Fig.6(B).The If/Ibratio of Ptm/Au/GC initially weakens with the increase of m,and when m>4,the If/Ibratio of Ptm/Au/GC almost remains constant.The results indicate that increasing Pt content may increase the catalytic current,but has no significance for improving poisoning tolerance of Pt-based catalysts.

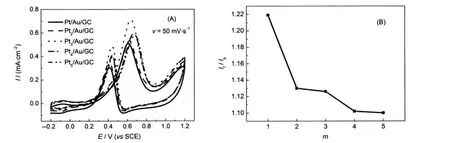
Fig.6 (A)CVs of Ptm/Au/GC electrodes in 0.1 mol·L-1CH3OH+0.1 mol·L-1H2SO4solution and(B)the dependence of If/Ibvalues and the layer number(m)

Fig.7 (A)CVs of(Pt3/Au)k/GC electrodes in 0.1 mol·L-1CH3OH+0.1 mol·L-1H2SO4solution and(B)the dependence of If/Ibvalues and the layer number(k)
To examine the structural effect of sandwich-like film catalysts,Pt3/Au/GC was also used as a substrate to fabricate(Pt3/ Au)k/GC electrodes and we found that the(Pt3/Au)2/GC electrode has the high catalytic current density among(Pt3/Au)kelectrodes,as shown in Fig.7(A),but the If/Ibratios of(Pt3/Au)2/ GC still show a decreasing tendency(Fig 7(B)).
Among various different electrodes,the composite electrodes exhibiting the largest catalytic current density in each type of composite electrodes were examined by EDX.EDX results indicate that the atomic ratios of Pt/Au in(Pt/Au)3/GC,Pt3/Au/GC, and(Pt3/Au)2/GC electrode are close to 5/6,12/1,and 5/1,respectively.The difference of Pt:Au atomic ratios indicates that the catalytic performance is not entirely determined by the content of Pt andAu.The actual structures of composites and the arrangement of Pt and Au nanoparticles are two important factors affecting Pt/Au catalytic performance.27,45-48For comparison,current densities and If/Ibvalues in CVs of methanol electrooxidation on Ptbulk,(Pt/Au)3/GC,Pt3/Au/GC,and(Pt3/Au)2/GC were summarized in Table 1.The current density on Ptbulkis the lowest among those electrodes although its If/Ibvalue is slightly larger than that on(Pt3/Au)2/GC,close to that on Pt3/Au/GC. (Pt/Au)3is the best catalyst film because whether current density or CO-poisoning tolerance is far better than the commercial Pt/C catalyst(Johnson Matthey,20%(w)).24
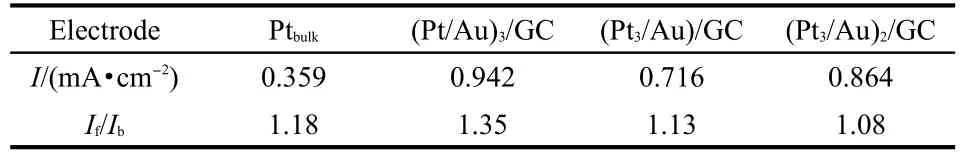
Table 1 Oxidation peak current densities and If/Ibvalues of different electrodes
4 Conclusions
Linker-free multilayer films were fabricated by combining AuNP monolayer transfer,UPD and redox-replacement.All asprepared Pt/Au multi-layer composites were used as catalyst films for study of methanol oxidation.The results indicate that the catalytic activity of the composite multilayer film depends on many factors,such as the atomic ratio of Pt/Au,the arrangement of Pt and Au nanoparticles.(Pt/Au)3films shows not only the best electrocatalytic performance,but also the atom number of Pt only accounts for 58%of the total atom number,fully showing the maximum synergistic effect between Au and Pt. Other metal(e.g.,Ru,Fe,Pd)and metal oxide(e.g.,SnO2, TiO2,ZnO)nanoparticles are now used to construct alternative multilayer composite multilayer films with Pt nanoparticles to further improve the performance of Pt-based catalysts for commercial utilization.
(1) Steele,B.C.H.;Heinzel,A.Nature 2001,414,345.
(2) Liu,H.S.;Song,C.J.;Zhang,L.;Zhang,J.J.;Wang,H.J.; Wilkinson,D.P.J.Power Sources 2006,155,95.
(3) Winter,M.;Brodd,R.J.Chem.Rev.2004,104,4245.
(4) Iwasita,T.;Hoster,H.;John-Anacker,A.;Lin,W.F.;Vielstich, W.Langmuir 1999,16,522.
(5)Liu,Z.;Reed,D.;Kwon,G.M.;Shamsuzzoha,D.;Nikles,E. J.Phys.Chem.C 2007,111,14223.
(6) Zhou,S.G.;McIlwrath,K.;Jackson,G.;Eichhorn,B.J.Am. Chem.Soc.2006,128,1780.
(7) Guo,S.J.;Zhai,J.F.;Fang,Y.X.;Dong,S.J.;Wang,E.Chem. Asian J.2008,3,1156.
(8) Zhao,D.;Xu,B.Q.Angew.Chem.Int.Edit.2006,45,4955.
(9) Kiani,A.;Fard,E.N.Electrochim.Acta 2009,54,7254.
(10) Du,Y.;Xu,J.J.;Chen,H.Y.Electrochem.Commun.2009,11, 1717.
(11) Wang,J.J.;Yin,G.P.;Wang,G.J.;Wang,Z.B.;Gao,Y.Z. Electrochem.Commun.2008,10,831.
(12) Markovic,N.M.;Ross,P.N.Surf.Sci.Rep.2002,45,117.
(13) Mott,D.;Luo,J.;Njoki,P.N.;Lin,Y.;Wang,L.Y.;Zhong,C.J. Catal.Today 2007,122,378.
(14) Zeng,J.H.;Yang,J.;Lee,J.Y.;Zhou,W.J.J.Phys.Chem.B 2006,110,24606.
(15) Zhang,J.L.;Vukmirovic,M.B.;Sasaki,K.;Nilekar,A.U.; Mavrikakis,M.;Adzic,R.R.J.Am.Chem.Soc.2005,127, 12480.
(16) Zhang,J.L.;Vukmirovic,M.B.;Xu,Y.;Mavrikakis,M.;Adzic, R.R.Angew.Chem.Int.Edit.2005,44,2132.
(17) Du,B.C.;Tong,Y.Y.J.Phys.Chem.B 2005,109,17775.
(18)Hammer,B.;Morikawa,Y.;Norskov,J.K.Phys.Rev.Lett. 1996,76,2141.
(19) Hammer,B.;Norskov,J.K.Adv.Catal.2000,45,71.
(20) Park,S.;Yang,P.;Corredor,P.;Weaver,M.J.J.Am.Chem.Soc. 2002,124,2428.
(21) Aramata,A.;Modern Aspects of Electrochemistry,Vol.31; Bockris,J.O.M.;White,R.E.;Conway,B.E.Eds.;Kluwer Academic Publishers:New York,1998;pp 181-250.
(22)Tang,H.;Chen,J.H.;Wang,M.Y.;Nie,L.H.;Kuang,Y.F.; Yao,S.Z.Appl.Catal.A 2004,275,43.
(23) Shin,T.Y.;Yoo,S.H.;Park,S.Chem.Mater.2008,20,5682.
(24)Liu,P.P.;Ge,X.B.;Wang,R.Y.;Ma,H.Y.;Ding,Y.Langmuir 2009,25,561.
(25) Park,I.S.;Lee,K.S.;Choi,J.H.;Park,H.Y.;Sung,Y.E. J.Phys.Chem.C 2007,111,19126.
(26) Ge,X.;Wang,R.;Liu,P.;Ding,Y.Chem.Mater.2007,19,5827.
(27) Kumar,S.;Zou,S.Z.Langmuir 2007,23,7365.
(28) Patra,S.;Das,J.;Yang,H.Electrochim.Acta 2009,54,3441.
(29) Huang,M.;Jin,Y.;Jiang,H.;Sun,X.;Chen,H.;Liu,B.;Wang, E.;Dong,S.J.Phys.Chem.B 2005,109,15264.
(30) Li,Y.J.;Huang,W.J.;Sun,S.G.Angew.Chem.Int.Edit.2006, 45,2537.
(31)Liu,C.;Li,Y.J.;Wang,M.H.;He,Y.;Yeung,E.S. Nanotechnology 2009,20,065604.
(32) Wang,M.H.;Li,Y.J.;Xie,Z.X.;Liu,C.;Yeung,E.S.Mater. Chem.Phys.2010,119,153.
(33)Wang,M.H.;Hu,J.W.;Li,Y.J.;Yeung,E.S.Nanotechnology 2010,21,145608.
(34)Li,Y.J.;Liu,C.;Yang,M.H.;He,Y.;Yeung,E.S. J.Electroanal.Chem.2008,622,103.
(35) Frens,G.Nat.Phys.Sci.1973,241,20.
(36) Uosaki,K.;Ye,S.;Naohara,H.;Oda,Y.;Haba,T.;Kondo,T. J.Phys.Chem.B 1997,101,7566.
(37) Kolb,D.M.Adv.Electrochem.Electrochem.Eng.1978,11,125.
(38)Mrozek,M.F.;Xie,Y.;Weaver,M.J.Anal.Chem.2001,73, 5953.
(39) Brankovic,S.R.;Wang,J.X.;Adzic,R.R.Surf.Sci.2001,474, L173.
(40) Maillard,F.;Eikerling,M.;Cherstiouk,O.V.;Schreier,S.; Savinova,E.;Stimming,U.Faraday Discuss.2004,125,357.
(41) Mayrhofer,K.J.J.;Arenz,M.;Blizanac,B.B.;Stamenkovic, V.;Ross,P.N.;Markovic,N.M.Electrochim.Acta 2005,50, 5144.
(42) Arenz,M.;Mayrhofer,K.J.J.;Stamenkovic,V.;Blizanac,B. B.;Tomoyuki,T.;Ross,P.N.;Markovic,N.M.J.Am.Chem. Soc.2005,127,6819.
(43) Biegler,T.;Rand,D.A.J.;Woods,R.J.Electroanal.Chem. 1971,29,269.
(44) Liu,Z.;Ling,X.Y.;Su,X.;Lee,J.Y.J.Phys.Chem.B 2004, 108,8234.
(45) Chang,S.C.;Ho,Y.;Weaver,M.J.Surf.Sci.1992,265,81.
(46) Park,S.;Xie,Y.;Weaver,M.J.Langmuir 2002,18,5792.
(47)Zhang,J.;Lima,F.H.B.;Shao,M.H.;Sasaki,K.;Wang,J.X.; Hanson,J.;Adzic,R.R.J.Phys.Chem.B 2005,109,22701.
(48) Zhang,J.;Mo,Y.;Vukmirovic,M.B.;Klie,R.;Sasaki,K.; Adzic,R.R.J.Phys.Chem.B 2004,108,10955.
April 11,2011;Revised:May 31,2011;Published on Web:June 8,2011.
Preparation of‘Sandwich-Like’Au/Pt Composite Multilayer Films for Methanol Electrooxidation
ZHAO Jing SUN Yue LI Yong-Jun*LIANG Ren
(Department of Chemisty,College of Chemistry and Chemical Engineering,Hunan University,Changsha 410082,P.R.China)
Pt/Au composite monolayer films were fabricated by combining interfacial assembly and under-potential deposition(UPD)with redox replacement.Based on the Pt/Au composite monolayers,an organic linker-free method was proposed for the fabrication of sandwich-like Pt/Au composite multilayer films:(Pt/Au)n,Ptm/Au,and(Pt3/Au)k(n,m,or k represents the layer number).Electron microscopy was used to characterize the morphologies of the Au monolayer films and the Pt/Au composite multilayer films. For each type of composite multilayer films,a common characteristic was that the effective electroactive areas increased with an increase in the layer number.Additionally,the electrocatalytic activities of the composite multilayer films for methanol electrooxidation are systematically discussed by examining the catalytic current densities and its tolerance toward carbonaceous species.For the same series of composite multilayer films(Pt/Au)3,Pt3/Au,and(Pt3/Au)2showed a higher catalytic current density than bulk Pt(Ptbulk).Among the three composite multilayer films,(Pt/Au)3showed the best catalytic performance in terms of the current density and tolerance toward carbonaceous species.The tolerance of(Pt/Au)3to carbonaceous species was found to be better than that of the commercial Pt/C catalyst.This better electrocatalytic activity may be attributed to the maximum synergistic effect between Au and Pt,which depends on the Pt:Au atomic ratio and also the arrangement of Pt and Au nanoparticles.
Interfacial self-assembly;Under-potential deposition;Au/Pt composite multilayer film; Electrooxidation;Methanol
O646
*Corresponding author.Email:liyongjunef@gmail.com;Tel:+86-731-88821900.
The project was supported by the National Natural Science Foundation of China(20703016),Scientific and Technological Projects of Hunan Province,China(2010FJ6030)and State Key Laboratory for Physical Chemistry of Solid Surface(Xiamen University),China.
国家自然科学基金(20703016),湖南省科技计划(2010FJ6030)及固体表面物理化学国家重点实验室(厦门大学)开放课题经费资助

