妊娠期高血压疾病患者子宫蜕膜NK细胞表型分析
周 娟, 肖小敏
(暨南大学附属第一医院妇产科,广东 广州 510632)
妊娠期高血压疾病患者子宫蜕膜NK细胞表型分析
周 娟, 肖小敏△
(暨南大学附属第一医院妇产科,广东 广州 510632)
目的观察妊娠期高血压疾病患者子宫蜕膜自然杀伤细胞(dNK 细胞)的表型。方法选取2008年8月至2009年3月在广州市暨南大学附属第一医院妇产科因妊娠期高血压疾病行剖宫产的单胎妊娠孕妇20例作为妊娠期高血压组,随机选取同期在此院因社会心理因素行选择性剖宫产的正常单胎妊娠孕妇15例作为正常对照组。收集孕晚期子宫蜕膜组织,机械研磨加梯度离心法提取蜕膜内单核细胞,流式细胞技术(FCM)筛选出dNK细胞,并检测dNK细胞表面CD56及CD16的表达情况。结果(1)妊娠期高血压组与正常对照组CD56brightCD16-CD3-dNK细胞的数量均多于CD56dimCD16+CD3-dNK细胞,且差异显著(均P<0.01)。(2)妊娠期高血压组与正常对照组CD56brightCD16-CD3-dNK细胞所占比例无显著差异(P>0.05),CD56dimCD16+CD3-dNK细胞所占比例亦无显著差异(P>0.05)。(3)妊娠期高血压组与正常对照组dNK细胞表面CD16分子的表达率并无显著差异(P>0.05)。结论妊娠期高血压疾病患者与正常孕妇孕晚期子宫蜕膜内dNK细胞表型无明显改变,均以CD56brightCD16-CD3-亚型为主。
子宫蜕膜; 自然杀伤细胞; 妊娠高血压; 母-胎界面; 细胞表型
妊娠期高血压疾病是产科最常见及严重的妊娠合并症之一,研究证实此病的发生与母体免疫系统功能混乱密切相关[1]。母-胎界面是母体与胎儿成分直接接触的部位,其局部免疫微环境在妊娠的建立、维持以及临产的发动中起着重要的作用。子宫蜕膜自然杀伤细胞(decidual natural killer cells, dNK cells)是母-胎界面最主要的免疫细胞[2],在局部有重要的免疫调节作用。研究表明其主要分为CD56brightCD16-CD3-与CD56dimCD16+CD3-2个亚型:前者属分泌型细胞,可分泌各种细胞/化学因子、血管生成因子等,有利于妊娠的建立、维持[3];而后者具有细胞毒性杀伤作用,杀伤非“己”物质。两者的平衡对于正常妊娠至关重要。已知正常妊娠者体内的dNK细胞以CD56brightCD16-CD3-亚型为主[4]。而在病理性妊娠如反复自发性流产患者体内,dNK细胞以CD56dimCD16+CD3-亚型为主,造成子宫局部的免疫杀伤作用增强,从而导致胚胎发育异常、坏死以至流产[5]。有学者[6]提出:既然妊娠期高血压和反复自发性流产在病理生理学上存在某些相似之处,是否其dNK细胞同样以CD56dimCD16+CD3-亚型为主?Wilczynski等[7]报道本病患者dNK表型存在变化,CD56dimCD16+CD3-亚型增加。而Zhou等[8]则报道dNK细胞表型并未改变。考虑到母-胎界面在妊娠中的重要性等各种因素,本研究着重对妊娠期高血压疾病患者孕晚期子宫蜕膜内dNK细胞的表型进行了研究。
材 料 和 方 法
1材料
1.1标本 选取2008年8月至2009年3月在广州市暨南大学附属第一医院因妊娠期高血压疾病行剖宫产的单胎妊娠孕妇20例作为妊娠期高血压组。除妊娠期高血压疾病外, 无其它妊娠合并症及并发症。既往无高血压及肝脏疾病史;无肾脏疾病、器官移植、免疫治疗及输血史;月经规则,末次月经清楚。随机选取同期在此院因社会心理因素行选择性剖宫产的正常单胎妊娠孕妇15例作为正常对照组。2组孕妇的平均年龄、孕龄及孕产次等均无显著差异。术前取得上述剖宫产者的同意,在其胎盘娩出后,刮取胎盘附着部位宫壁的蜕膜组织。
1.2主要试剂 RPMI-1640培养液(含Hepes、L-谷氨酰胺)购于Gibco,台盼蓝购于Sigma, 小鼠抗人CD56-PE、CD16-FITC、CD3-PE-Cy5及小鼠IgG1-FITC、IgG1-PE、IgG1-PE-Cy5均购于BD PharMingen。胎牛血清(FBS)、人淋巴细胞分离液(Ficoll)和双抗(青霉素、链霉素)均购于天津灏洋生物有限公司。
2方法
2.1细胞悬液的制备 所取蜕膜组织用RPMI-1640培养液(含青霉素105U/L和链霉素100 mg/L)洗涤2次(尽量洗去组织中的血液、胎粪、胎脂等物质),放入装有RPMI-1640完全培养基(含10%胎牛血清)的小烧杯中。用眼科剪将组织反复剪碎直至大小约1 mm×1 mm×1 mm,研磨棒轻轻研磨组织, 200目滤网过滤收集滤液。将人淋巴细胞分离液(Ficoll)平铺于15 mL无菌离心管中,将收集的滤液按照1∶1的比例平铺于分离液上(形成清楚的分界面)。20 ℃下2 000 r/min离心20 min。离心后吸取淋巴细胞分离液与滤液间的云雾样絮状物层并置于另一支无菌离心管中。RPMI-1640完全培养基洗涤2次(20℃下1 500 r/min离心5 min),最后1次离心弃上清液后加入RPMI-1640完全培养基吹打混合均匀,调整细胞浓度约为109cells/L,制成细胞悬液。取少许细胞悬液,台盼蓝染液染色检测细胞活力>90%。
2.2dNK细胞表型检测 每份标本编2支试管,每管分别加入100 μL细胞悬液。每份标本的1号管中加入对照试剂(小鼠IgG1-PE、小鼠IgG1-FITC及小鼠IgG1-PE-Cy5)各20 μL,2号管中加入小鼠抗人CD56-PE、CD16-FITC及CD3-PE-Cy5各20 μL标记dNK(CD56+CD3-)细胞、CD56brightCD16-CD3-dNK细胞及CD56dimCD16+CD3-dNK细胞,室温下孵育25 min。磷酸盐缓冲溶液(PBS)洗涤2次(室温下1 500 r/min离心5 min),离心去上清后每管内加300 μL PBS重悬细胞。
上机检测:激光管预热30 min后用荧光微球(Beckman-Coulter)调整仪器,使各放大器接收信号的HCV值<2%,以1号管作为空白定标,计算10 000个细胞,记录标本的阳性细胞百分率和平均荧光强度。
3统计学处理
采用SPSS 13.0软件对所有数据进行统计学处理分析。妊娠期高血压组与正常对照组内的比较采用配对资料的秩和检验;妊娠期高血压组与正常对照组间的比较采用两样本比较的秩和检验,结果以中位数、四分位间距(QR)、最大值、最小值表示。
结 果
流式细胞技术分选出荧光抗体标记后CD56表达阳性、CD3表达阴性(CD56+CD3-)的细胞即dNK细胞。根据dNK细胞表面CD56表达水平的高低及是否表达CD16,将其分为CD56brightCD16-CD3-dNK细胞及CD56dimCD16+CD3-dNK细胞。
1妊娠期高血压组
孕晚期子宫蜕膜dNK细胞仍以CD56brightCD16-CD3-亚型为主,其数量(55.0%±20.1%)多于CD56dimCD16+CD3-亚型(9.4%±8.7%),两者差异显著差异(P<0.01),见表1。
表1妊娠期高血压组CD56brightCD16-CD3-dNK细胞与CD56dimCD16+CD3-dNK细胞的比较
Table 1. The comparison between the CD56brightCD16-CD3-dNK cells and CD56dimCD16+CD3-dNK cells in the HDCP group(n=20)

SubgroupMedianMaximumMinimumQRCD56brightCD16-62.5%82.2%7.3%21.7%CD56dimCD16+7.9%**43.5%2.4%4.8%**
**P<0.01vsCD56brightCD16-.
2正常对照组
孕晚期子宫蜕膜dNK细胞亦以CD56brightCD16-CD3-亚型为主,其数量(59.5%±17.6%)多于CD56dimCD16+CD3-亚型(8.9%±7.8%),两者差异显著(P<0.01),见表2。
表2正常对照组CD56brightCD16-CD3-dNK细胞与CD56dimCD16+CD3-dNK细胞的比较
Table 2. The comparison between the CD56brightCD16-CD3-dNK cells and CD56dimCD16+CD3-dNK cells in the normal control group(n=15)
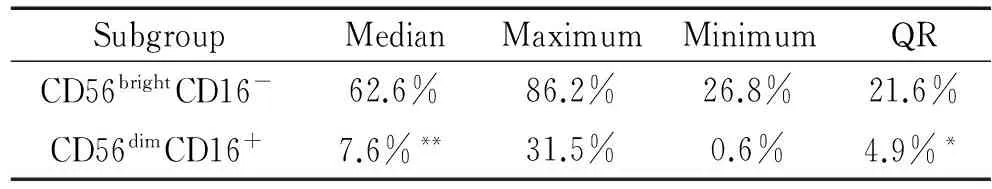
SubgroupMedianMaximumMinimumQRCD56brightCD16-62.6%86.2%26.8%21.6%CD56dimCD16+7.6%**31.5%0.6%4.9%*
**P<0.01vsCD56brightCD16-.
3妊娠期高血压组与正常对照组的比较
(1)2组dNK细胞均以CD56brightCD16-CD3-亚型为主,分别为(59.5%±17.6%)和(55.0%±20.1%),无显著差异(P>0.05),见表3、图1。
(2)2组CD56dimCD16+CD3-dNK细胞的数量分别为(8.9%±7.8%)和(9.4%±8.7%),亦无显著差异(P>0.05),见表4、图1。
(3)2组dNK细胞表面CD16的表达率分别为(13.6%±8.3%)和(12.9%±9.5%),无显著差异(P>0.05),见表5、图2。
表3妊娠期高血压组与正常对照组间CD56brightCD16-CD3-dNK细胞的比较
Table 3. CD56brightCD16-CD3-dNK cells in HDCP group and normal control group

GroupnMedianMaximumMinimumQRHDCP2062.5%82.2%7.3%21.7%Normalcontrol1562.6%86.2%26.8%21.6%

Figure 1. FCM analysis of CD56-positive and CD3-negative (CD56+CD3-) cells in uterine decidual NK(dNK) cells. According to the expression of CD56 on the surface of dNK cells and whether CD16 were expressed, we sorted out the CD56brightCD16-CD3-dNK cells (shown as the R3 domain) and the CD56dimCD16+CD3-dNK cells (shown as the R4 domain).There was no statistical difference between HDCP group and normal control group,in neither CD56brightCD16-CD3-dNK cells nor CD56dimCD16+CD3-dNK cells.
图1妊娠期高血压组与正常对照组之间CD56brightCD16-CD3-dNK细胞及CD56dimCD16+CD3-dNK细胞的比较
表4妊娠期高血压组与正常对照组间CD56dimCD16+CD3-dNK细胞的比较
Table 4. CD56dimCD16+CD3-dNK cells in HDCP group and normal control group
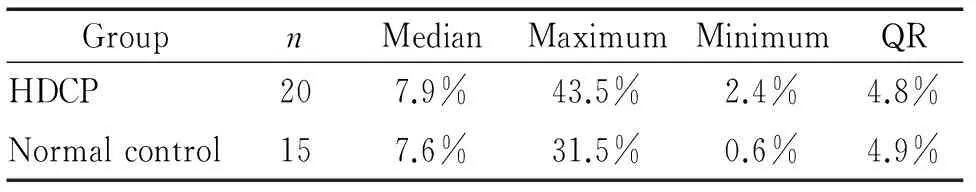
GroupnMedianMaximumMinimumQRHDCP207.9%43.5%2.4%4.8%Normalcontrol157.6%31.5%0.6%4.9%
表5妊娠期高血压组与正常对照组dNK细胞表面CD16表达情况的比较
Table 5. The expression of CD16 on the surface of dNK cells between HDCP group and normal control group

GroupnMedianMaximumMinimumQRHDCP2011.0%46.3%2.8%6.1%Normalcontrol1511.5%33.6%1.0%9.4%

Figure 2. dNK cells were screened by FCM. The A1 and A2 domains represent the expression of CD16 on the surface of dNK cells from HDCP group and normal control group, respectively. There was no significant difference in the expression of CD16 on dNK cells between the two groups.
图2妊娠期高血压组与正常对照组dNK细胞表面CD16表达情况的比较
讨 论
研究证实子宫蜕膜dNK细胞主要包括2大亚群:CD56brightCD16-CD3-dNK细胞及CD56dimCD16+CD3-dNK细胞。2种细胞在细胞表型、基因表达及功能上均存在很大差别。CD56brightCD16-CD3-dNK细胞属分泌型细胞,具有低细胞毒性的特点。通过产生各种细胞因子(如白细胞介素8、干扰素-γ)、血管源性分子(如血管内皮生长因子、胎盘生长因子及血管生成素2)等生物活性物质参与妊娠的免疫耐受[9]、子宫螺旋动脉的构建[10],调节绒毛外滋养细胞(EVT)的侵润及胎盘的发育形成[11,12]。CD56dimCD16+CD3-dNK细胞内含有大量的穿孔素、颗粒酶等杀伤性物质,主要起细胞毒性杀伤作用。它不仅可以通过直接释放预先储存的杀伤性物质来杀伤靶细胞,还可通过抗体依赖性细胞毒性作用(ADCC)来实现。其表面表达的CD16可作为低亲和力的 FcgRIII,与已结合到靶细胞上的IgG抗体的Fc段相结合,增强NK细胞的杀伤作用[13]。姓娠期高血压的发病机制尚不清楚[14]。长期以来有关病理性妊娠的大量研究表明,子宫局部免疫微环境的异常是导致妊娠失败的主要原因之一,清楚了解此处的微循环特点对了解疾病的发生机制及预防治疗有着重要的意义。本实验在对母-胎界面dNK细胞进行研究时发现妊娠期高血压疾病患者孕晚期子宫蜕膜dNK细胞的表型未发生明显改变,仍以CD56brightCD16-CD3-dNK细胞为主,妊娠期高血压疾病患者与正常孕妇dNK细胞表面CD16的表达率并无显著差异。
然而,即使是同一表型的细胞,在功能上也不一定完全一致。已知dNK细胞表面表达2种类型的受体:杀伤细胞活化性受体(killer activating receptors,KARs)和杀伤细胞抑制性受体(killer inhibiting receptors,KIRs),其与相应配体如绒毛外滋养细胞表面表达的HLA-C、HLA-E及HLA-G等相互作用后,能上调/下调dNK细胞的功能,从而产生不同的生物学效应。Hiby等[15]指出抑制dNK细胞会增加患子痫前期的可能性;Hanna等[12]认为活化的dNK细胞可通过分泌NK细胞来源的生长因子及化学因子促进滋养细胞侵润、蜕膜血管形成从而防止子痫前期的发生。不仅如此,dNK细胞本身的反应性亦可影响其生物学效应(如对于趋化信号的反应性可影响其在子宫内的募集)。因此,我们推测是否因为CD56brightCD16-CD3-dNK细胞功能异常导致了本病的发生,认为有必要对dNK细胞功能进行进一步深入研究。
综上所述,妊娠期高血压疾病患者与正常孕妇孕晚期子宫蜕膜dNK细胞的表型均以CD56brightCD16-CD3-亚型为主。推测可能dNK细胞自身功能异常与本病发生有关。
[1] Moffett A, Hiby SE. How does the maternal immune system contribute to the development of pre-eclampsia?[J]. Placenta, 2007, 28(Suppl A):S51-S56.
[2] Moffett-King A. Natural killer cells and pregnancy[J]. Nat Rev Immunol, 2002, 2(9):656-663.
[3] Lash GE, Schiessl B, Kirkley M, et al. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy[J]. J Leukoc Biol, 2006, 80 (3):572-580.
[4] Saito S, Nakashima A, Myojo-Higuma S, et al. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy[J]. J Reprod Immunol, 2008, 77(1):14-22.
[5] Lachapelle MH, Miron P, Hemmings R, et al. Endometrial T,B and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome[J]. J Immunol,1996, 156(10):4027-4034.
[6] Wilczyński JR. Immunological analogy between allograft rejection, recurrent abortion and pre-eclampsia-the same basic mechanism?[J]. Human Immunol, 2006, 67(7):492-511.
[7] Wilczyński JR, Tchórzewski H, Banasik M, et al. Lymphocyte subset distribution and cytokine secretion in third trimester decidua in normal pregnancy and preeclampsia[J]. Eur J Obstet Gynecol Reprod Biol, 2003, 109(1):8-15.
[8] 周建军,胡娅莉,郝 莎,等.子痫前期患者母-胎界面子宫自然杀伤细胞免疫表型及辅助性T淋巴细胞1、2免疫状态的研究[J].中华妇产科杂志, 2007, 42(4):244-248.
[9] Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential[J]. J Exp Med, 2003, 198(8):1201-1212.
[10]Manaster I, Mandelboim O. The unique properties of human NK cells in the uterine mucosa[J]. Placenta, 2008, 29(Suppl A):S60-S66.
[11]Hu Y, Dutz JP, MacCalman CD, et al. Decidual natural killer cells alterinvitrofirst trimester extravillous cytotrophoblast migration: a role for interferon-gamma[J]. J Immunol, 2006, 177(12):8522-8530.
[12]Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetai-maternal interface[J]. Nature Med, 2006, 12(9):1065-1074.
[13]Cooper MA, Fehniger TA. The biology of human natural killer cell subets[J]. Trends Immunol, 2001, 22(11):633-640.
[14]毛东伟,杨东霞,段志宇,等.先兆子痫胎盘的基因表达谱研究[J].中国病理生理杂志,2009,25(9):1806-1809.
[15]Hiby SE, Walker JJ, O’Shaughnessy KM, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success[J]. J Exp Med, 2004, 200(8):957-965.
PhenotypeofdecidualNKcellsinwomenwithhypertensivedisordercomplicatingpregnancy
ZHOU Juan, XIAO Xiao-min
(DepartmentofObstetricsandGynecology,TheFirstAffiliatedHospitalofJinanUniversity,Guangzhou510632,China.E-mail:hellen118@163.com)
AIM: To investigate the phenotype of uterine decidual natural killer cells (dNK cells) in women with hypertensive disorder complicating pregnancy (HDCP).METHODSAll the study subjects were collected from Department of Obstetrics and Gynecology, The First Affiliated Hospital of Jinan University, Guangzhou, China. Twenty cases of singleton pregnancy who underwent caesarean section because of HDCP were selected as HDCP group, and 15 cases of singleton pregnancy
selective caesarean section because of social-psychological concerns were also randomly selected as normal control group. The decidual tissues were sampled immediately after caesarean section. The mononuclear cells were extracted from the tissues by means of mechanic grinding and gradient centrifugation. The technique of flow cytometry was used for dNK cell sorting and the expression of CD56 and CD16 on the surface of cells was also examined.RESULTSIn HDCP group and normal control group, the proportions of CD56brightCD16-CD3-dNK cells were significantly higher than those of CD56dimCD16+CD3-dNK cells (P<0.01). Neither the CD56brightCD16-CD3-subset nor the CD56dimCD16+CD3-subset had statistical difference between HDCP group and normal control group. No significant difference of CD16 expression on the surface of dNK cells between HDCP group and normal control group was observed (P>0.05).CONCLUSIONThe phenotypes of dNK cells from the women with HDCP and from healthy pregnant women are both dominated by CD56brightCD16-CD3-subset, without significant difference.
Uterine decidua; Natural killer cells; Hypertension,pregnancy-induced; Maternal-fetal interface; Cell phenotype
R714.2; R363.2
A
10.3969/j.issn.1000-4718.2011.01.036
1000-4718(2011)01-0183-04
2010-05-26
2010-11-10
△通讯作者 Tel:020-38688660;E-mail:hellen118@163.com

