含三氟甲基1,2,3-三氮唑衍生物对蘑菇酪氨酸酶活性的抑制动力学
李树白 聂华丽 张海涛 薛 勇 CHRIS Branford White 朱利民,*
(1东华大学化学化工与生物工程学院,上海 201620; 2Institute for Health Research and Policy,London Metropolitan University,London N7 8DB,UK)
Tyrosinase(EC 1.14.18.1)has been identified in all forms of cellular systems[1,2].Chemical and spectroscopic studies have been shown that the active site of the enzyme consists of two copper atoms[3].Tyrosinase is a critical rate-limiting enzyme which catalyzes two distinct reactions of melanin synthesis,the hydroxylation of monophenol(monophenolase activity)and oxi-dation of o-diphenol to o-quinone(diphenolase activity)[4-6].Melanin is a phenolic biopolymer widely distributed in nature.It is the main protective pigment found in the hair,skin,and eyes of animals.Excessive melanin production or abnormal distribution can cause irregular hyperpigmentation of the skin,such as albinism,melasma,and age-related spots[7,8].
A lot of organofluorine compounds have been used as model systems for developing drugs and pesticides as the fluorine atom can modify biological activity of many organic compounds[9,10]. Within the organofluorine compounds,trifluoromethyl-containing 1,2,3-triazoles have been used as antimicrobial,antiviral,and antitumor agents and have a range of applications in pharmaceutical and agrochemical industries[11].Although a great deal of research relating to tyrosinase inhibitors have already been undertaken[12-14],there are few reports about the inhibitory effect of trifluoromethyl-containing 1,2,3-triazoles on this enzyme.
To investigate the inhibitory kinetic properties of tyrosinase in more depth,a novel trifluoromethyl-containing 1,2,3-triazole derivative(TF-TZ,Fig.1)was assayed for monophenolase and diphenolase activities.The effects of kinetic parameters and inhibition constants characterizing the system were also evaluated. The outcome from this study may provide a basis for designing and synthesizing novel tyrosinase inhibitors,new food preservatives and cosmetic additives.
1 Experimental
1.1 Reagents
Mushroom tyrosinase(EC 1.14.18.1)was obtained from Sigma (St.Louis,USA).Dimethylsulfoxide(DMSO),L-tyrosine and L-3,4-dihydroxyphenylalanine(L-DOPA)were supplied by National Pharmaceutical Group Corporation(Shanghai,China).All other chemicals and reagents were analytical grade and used without further purification.Re-distillated ion-free water was used throughout.
1.2 Kinetic assays
Monophenolase and diphenolase activities of mushroom tyrosinase were determined by measuring the rate of dopachrome formation at 475 nm(molar absorption coefficient of 3700 L· mol-1·cm-1)by using a UV-2102PC spectrophotometer(UNICO, Shanghai,China).The temperature was controlled at 30℃by a MP-5H electric heat constant temperature water box(Yiheng, Shanghai,China).This temperature was chosen to enable dopachrome stability.Steady state rate(Vss)was defined as the slope of the linear zone of dopachrome accumulation curve.One unit(U)of enzymatic activity was defined as the amount of enzyme increasing 0.001 absorbance·min-1at 475 nm in this condition[15].The lag period was determined by extrapolation of the linear portion of the dopachrome accumulation plot to the abscissa axis.All the experiments were performed in 0.1 mol·L-1sodium phosphate(pH 6.8)and the final reaction volume of the assay mixture was 3 mL.

Fig.1 Chemical structure of the trifluoromethyl-containing 1,2,3-triazole derivative(TF-TZ)
1.3 Inhibition assays
TF-TZ was dissolved in DMSO and diluted to different concentrations of this solvent and the final concentration of DMSO in the assay media being 3.3%.Controls,without the inhibitors contained 3.3%DMSO,were used.The reaction media for activity assay contained 1.5 mmol·L-1L-tyrosine or 1.0 mmol·L-1L-DOPA in 0.1 mol·L-1sodium phosphate(pH 6.8).The assay mixture,without TF-TZ and this enzyme,was incubated at 30℃for 10 min and the reaction was initiated by the addition of enzyme. zyme.The inhibition ratio(i)of tyrosinase activity(monophenlase activity and diphenolase activity)was calculated as follows[16]:

where,Vss,controland Vss,samplerepresent steady state rate for the blank and inhibitor at 475 nm,respectively.The concentration (IC50)of TF-TZ with 50%loss of enzyme activity was determined.
1.4 Kinetic analysis
The inhibition type was determined from Lineweaver-Burk plots,and the inhibition constant was estimated using a second plot where apparent Michaelis constant Kmis compared with inhibitor concentration.For the inhibitor activity,different concentrations of enzyme were added to the pre-incubated mixture as the concentrations of TF-TZ increased.
1.5 Protein determination
Protein concentration was determined according to dye-binding method of Bradford[17].
2 Results and discussion
2.1 Inhibition effect of TF-TZ on monophenolase of mushroom tyrosinase
The active site of mushroom tyrosinase contains two copper atoms and exists in three forms:Emet,Edeoxy,and Eoxy[18].Eoxyhas monophenolase activity,both Emetand Eoxyhave diphenolase activity,and Edeoxycan combine with oxygen.Emethas no monophenolase activity but has an affinity for it,binding it to form a dead-end complex,this may explain the lag period prior to attainment of a steady-state rate.The enzyme time course using L-tyrosine as substrate was shown in Fig.2.Initially,there was a slight increase in activity followed by a rapid rise,mostly linearly,giving an invariable slope.There was a marked lag period, characteristic of the monophenolase activity,which was estimated by extrapolation of the curve to the abscissa(arrows,Fig.2). The system reached the steady-state rate after lag period.The results clearly showed that TF-TZ had inhibitory effects on monophenolase activity.Both the enzyme activity and lag period were also determined(Fig.3).The relative activity(Fig.3a)decreased with increasing concentrations of TF-TZ.When the concentration of TF-TZ reached to 40 μmol·L-1,the relative enzyme activity was determined to be 41.3%.The inhibitor concentration(IC50)was 30.4 μmol·L-1.The lag period(Fig.3b)was extended with increasing concentration of TF-TZ,and was found to be 55 s without TF-TZ and 313 s in the presence of 40 μmol·L-1TF-TZ.
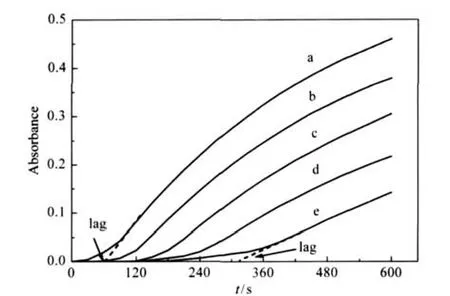
Fig.2 Progress curves for the inhibition of monophenolase activity of mushroom tyrosinase by TF-TZ at 30℃Absorbance is at 475 nm;The concentrations of TF-TZ for plots a-e were 0,10,20, 30,and 40 μmol·L-1,respectively.Assay conditions were 3 mL reaction system containing 0.1 mol·L-1sodium phosphate(pH 6.8),1.5 mmol·L-1L-tyrosine,and different concentrations of TF-TZ.Final concentrations of tyrosinase and DMSO were 7.4 μg·mL-1and 3.3%,respectively.
2.2 Inhibition effect of TF-TZ on diphenlolase of mushroom tyrosinase
The assay for diphenolase activity used L-DOPA as substrate. The time course of enzyme reaction was shown in Fig.4.The results showed that TF-TZ had inhibitory effect on the diophenolase activity of mushroom tyrosinase.The activity of enzyme decreased with increasing concentration of TF-TZ.The relative enzyme activity is given in Fig.5.When the concentration of TFTZreached 60 μmol·L-1,the relative enzyme activitywas 19.1%. The inhibitor concentration(IC50)was 34.5 μmol·L-1.
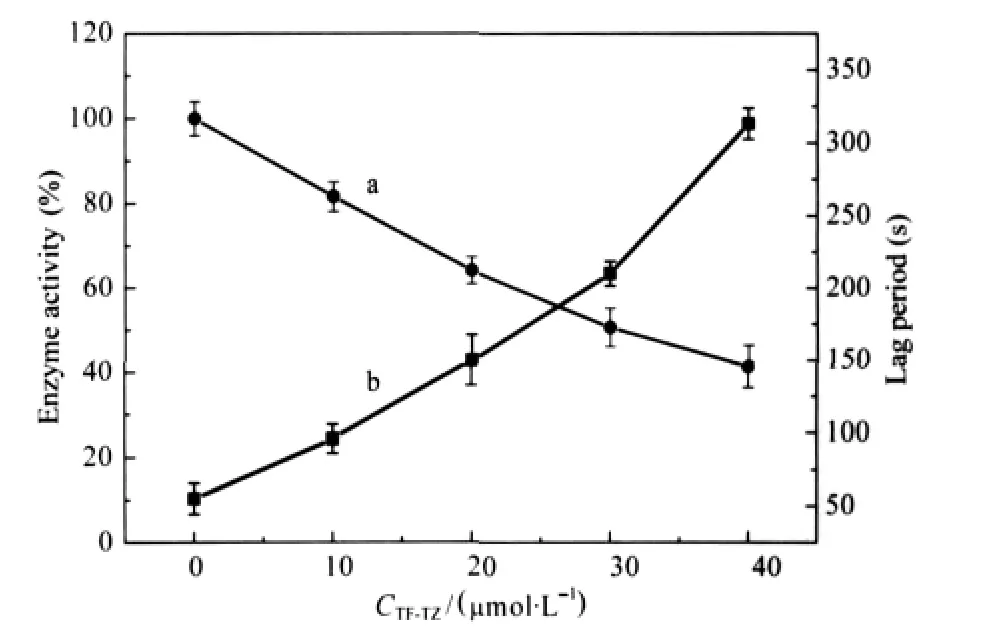
Fig.3 Effects of TF-TZ concentration on the enzyme activity (a)and the lag period(b)of monophenolase activity of mushroom tyrosinaseAssay conditions were the same as those in Fig.2.

Fig.4 Progress curve for the inhibition of diphenolase activity of mushroom tyrosinase by TF-TZ at 30℃Absorbance is at 475 nm;The concentrations of TF-TZ for plots a-e were 0,15, 30,45,and 60 μmol·L-1,respectively.Assay conditions were 3 mL reaction system containing 0.1 mol·L-1sodium phosphate(pH 6.8),1.0 mmol·L-1L-DOPA,and different concentrations of TF-TZ.Final concentrations of mushroom tyrosinaseand DMSO were 1.6 μg·mL-1and 3.3%,respectively.
2.3 Inhibition mechanism of TF-TZ on diphenolase of mushroom tyrosinase
The inhibition mechanism on tyrosinase by TF-TZ for the oxidation of L-DOPA was first studied.As shown in Fig.6,the plots of remaining enzyme activity versus the concentrations of enzyme in the presence of different concentrations of TF-TZ gave a family of straight lines,which all passed through the origin. The decrease in the slope of the line with increasing of the inhibitor concentration indicated that TF-TZ was a reversible inhibitor of this enzyme.
2.4 Determination of inhibition type of TF-TZ on diphenolase activity of mushroom tyrosinase

Fig.5 Effect of TF-TZ concentration on the enzyme activity of diphenolase activity of mushroom tyrosinaseAssay conditions were the same as those in Fig.4.
The kinetic behavior of mushroom tyrosinase during the oxidation of L-DOPA was investigated by following Michaelis-Menten kinetics.In the presence of TF-TZ,there was an increase in the Michaelis constant(Km)whereas the maximum rate of reaction(Vmax)remained unaltered(Fig.7A).Thus,TF-TZ acted as a competitive inhibitor and it was only bound with free en-zyme,not with the enzyme-substrate complex.The plot of the data(Fig.7B)by non-linear regression to this inhibition provided a parabolic profile.The profile of the Kmsuggests that both one TF-TZ molecule and two TF-TZ molecules can combine with a free tyrosinase to form inhibitor-enzyme and inhibitor-enzymeinhibitor complexes[19].This pattern indicated that TF-TZ is a parabolic-competitive inhibitor on diphenolase of the enzyme.

Fig.6 Determination of inhibition mechanism of TF-TZ on the diphenolase activity of mushroom tyrosinaseThe concentrations of TF-TZ for curves a-d were 0,14.7,29.4,and 44.1 μmol·L-1, respectively.Assay conditions were 3 mL reaction system containing 0.1 mol·L-1 sodium phosphate(pH 6.8),1.0 mmol·L-1L-DOPA,and different concentrations of TF-TZ and mushroom tyrosinase.Final concentration of DMSO was 3.3%.
The mechanism representing parabolic-competitive inhibition for the tyrosinase-catalyzed reaction is as follows:

E,S,I,P,denote enzyme(tyrosinase),substrate(L-DOPA),inhibitor(TF-TZ),and product(dopachrome),respectively.ES,EI, and EI2are the respective intermediates.k is the reaction rate constant.As it is usually the case that[S]≫[E0]and[I]≫[E0].
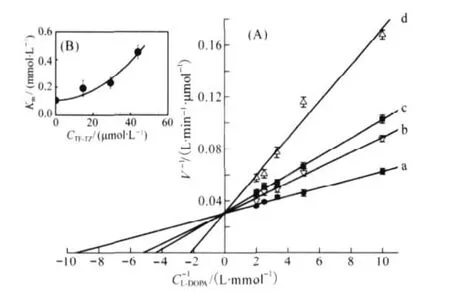
Fig.7 Lineweaver-Burk plots for the inhibition of TF-TZ on mushroom tyrosinase on the oxidation of L-DOPA at 30℃in 0.1 mol·L-1sodium phosphate(pH 6.8)The concentrations of TF-TZ for plots a-d were 0,14.7,29.4,and 44.1 μmol·L-1, respectively.The enzyme concentration was 4.8 μg·mL-1.The inset represents apparent Kmversus the TF-TZ concentration to determine the inhibition constants.
In the steady state,the rate of formation and disappearance of the complexes are identical:

The expressions for the total enzyme concentration and the reaction rate:

Insertion of the expressions for E,EI,and EI2in term of ES into the expression for the total enzyme concentration gives
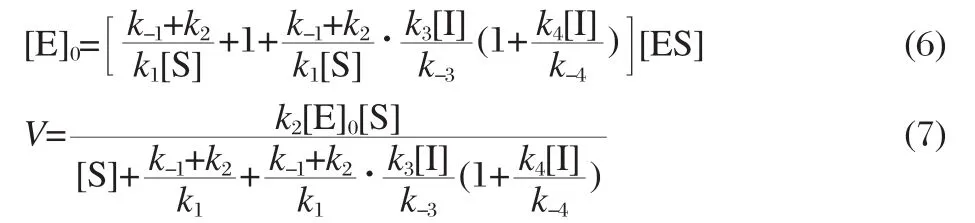
As(k-1+k2)/k1is the Michaelis constant(Km)for the reaction in the absence of inhibitor,k2[E]0is maximum reaction rate(Vmax),k-3/k3is the equilibrium constant(Ki1)for the dissociation of the complex EI into E and I,and k-4/k4is the equilibrium constant(Ki2) for the dissociation of the complex EI2into EI and I,the rate equation may be presented in the form

which in reciprocal form becomes
It is evident from Eq.(9)that the slope of Lineweaver-Burk lines is influenced by the changing[I]and[I]2.
The fitting function by the nonlinear regression(Fig.7B)is
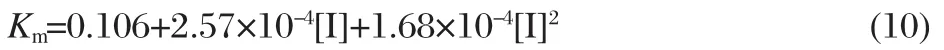
and the three coefficients of Eq.(10)correspond to the values of Km,Km/Ki1,and Km/(Ki1Ki2).So,the values of Km,Ki1,Ki2are equal to 105.7,76.9,and 9.71 μmol·L-1,respectively.
According to Eq.(8),in the absence of TF-TZ,the rate equation function is

The inhibition ratio:

When i reaches 50%,[I]=IC50,so,

The IC50value(the negative value should be removed)is
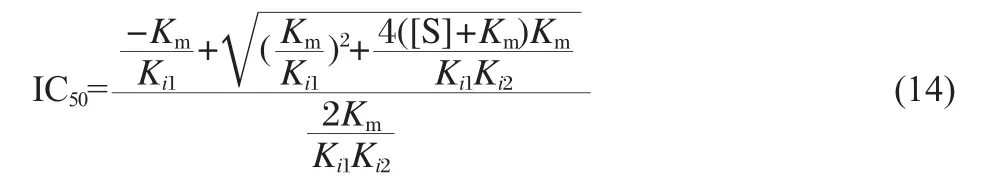
According to Eq.(14),and the values of Km,Ki1,Ki2,[S],so,in theory,the IC50value is 83.6 μmol·L-1.
Tyrosinase was stable during the time course of the activity measurements,and the stable steady rates were used as reaction velocities in the activity measurements(Figs.2 and 4).Moreover, the reaction velocity was proportional to the enzyme concentration(Fig.6).Here,specific IC50is defined as the IC50per 1 μg·mL-1tyrosinase mass.So,the specific theoretical and experimental IC50values are 17.4 and 21.7 μmol·L-1,respectively.Thus,the two values are similar,so it can be concluded that there is correlation between the theoretical and experimental results.
In this study,we report that TF-TZ is a reversible paraboliccompetitive inhibitor and it hinders the generation of dopachrome,so preventing melanin formation.Structural analysis of TF-TZ may reveal the possible reason for the mechanism.For example,TF-TZ is a large lipophilic compound which contains 1-benzyl-4-trifluoromethyl-1,2,3-triazole ring and diphenylmethanol group.It was reported that tyrosinase has two sites of combination,one for the substrate and the other for the inhibitor. The native conformation of tyrosinase is altered when the substrate is bound and some enzymes assume a similar shape so that the hydrophobic pocket becomes larger[20,21].Then the combination of L-DOPA with tyrosinase will induce a new hydrophobic pocket in the substrate-enzyme complex so that TF-TZ can just be inserted into the pocket to form inhibitor-enzyme complex.It can be concluded that the combination between two TF-TZ molecules and tyrosinase is less problematic,which means that TF-TZ could be bound to a hydrophobic pocket.We here present that TF-TZ is a strong inhibitor of tyrosinase.It also plays a key role in the interaction between enzyme active site and inhibitor.However,compared with other tyrosinase inhibitors,such as kojic acid[4],cinnamic acid and derivatives[22],and other heterocyclic compounds[23,24],differences occur,especially for the combination with free enzyme.
2.5 TF-TZ as a copper chelator to inhibit tyrosinase at its active site

Fig.8 Scan spectra for TF-TZ(a),TF-TZ with CuSO4(b), and TF-TZ with tyrosinase(c)conditions:0.1 mol·L-1sodium phosphate(pH 6.8);(a)100 μmol·L-1TF-TZ alone, (b)a+100 μmol·L-1CuSO4,(c)a+286 unit tyrosinase
To determine the inhibition of TF-TZ,chelation properties with copper ions and tyrosinase were examined.Mechanism of tyrosinase inhibition by captopril was explained previously[25]. TF-TZ forms the chelation with a copper atom in the enzyme and then reversibly inactivates the tyrosinase.In the UV-visible spectrum of TF-TZ,a characteristic shoulder peak was observed at 264 nm in the presence of copper sulphate(CuSO4),which was due to chelation for Cu2+(Fig.8).A similar peak(287 nm)due to chelation was observed in the mixture of TF-TZ and tyrosinase.This indicated that TF-TZ could not act as a substrate such as catechin because the behavior was different from that of catechin and tyrosinase[26-30].These data strongly implied that tyrosinase inhibition by TF-TZ is through the chelation process involving the active site.
3 Summary
This investigation shows that TF-TZ inhibits monophenolase and diphenolase activities of mushroom tyrosinase.Thus, TF-TZ can extend the lag period of the monophenolase activity. TF-TZ is a reversible parabolic-competitive inhibitor of the diphenolase activity.The UV spectrum of a mixture of tyrosinase and TF-TZ exhibits a characteristic shoulder peak ascribed to the chelation of TF-TZ to the active site of this enzyme.This research can shed a light for designing and synthesizing novel potent tyrosinase inhibitors,food preservatives or cosmetic additives(widely studied such as trifluoromethyl-containing 1,2,3-triazole analogues).
Acknowledgment: The authentic trifluoromethyl-containing 1,2,3-triazole was provided by Prof.QING,Feng-Ling(Key Laboratory of Organofluorine Chemistry,Shanghai Institute of Organic Chemistry, China).
1 Sánchez-Ferrer,Á.;Rodríguez-López,J.N.;García-Cánovas,F.; García-Carmona,F.Biochim.Biophys.Acta,1995,1247:1
2 Seo,S.Y.;Sharma,V.K.;Sharma,N.J.Agric.Food Chem.,2003, 51:2837
3 Fenoll,L.G.;Peñalver,M.J.;Rodríguez-López,J.N.;Varón,R.; García-Cánovas,F.;Tudela,J.Int.J.Biochem.Cell Biol.,2004, 36:235
4 Kim,H.;Choi,J.;Cho,J.K.;Kim,S.Y.;Lee,Y.S.Bioorg.Med. Chem.Lett.,2004,14:2843
5 Jiménez,M.;Chazarra,S.;Escribano,J.;Cabanes,J.;García-Carmona,F.J.Agric.Food Chem.,2001,49:4060
6 Yu,L.L.J.Agric.Food Chem.,2003,51:2344
7 Ando,H.;Kondoh,H.;Ichihashi,M.;Hearing,V.J.J.Invest. Dermatol.,2007,127:751
8 Sugumaran,M.;Nellaiappan,K.;Amaratunga,C.;Cardinale,S.; Scott,T.Arch.Biochem.Biophy.,2000,378:393
9 Qing,F.L.;Gao,W.Z.;Ying,J.W.J.Org.Chem.,2000,65:2003
10 Smart,B.E.J.Fluorine Chem.,2001,109:3
11 Zhang,C.T.;Zhang,X.G.;Qing,F.L.Tetrahedron Lett.,2008, 49:3927
12 Kubo,I.;Kinst-Hori,I.;Kubo,Y.;Yamagiwa,Y.;Kamikawa,T.; Haraguchi,H.J.Agric.Food Chem.,2000,48:1393
13 Xie,J.J.;Song,K.K.;Qiu,L.;He,Q.;Huang,H.;Chen,Q.X. Food Chem.,2007,103:1075
14 Li,S.B.;Nie,H.L.;Zhang,H.T.;Xue,Y.;Zhu,L.M.Appl. Biochem.Biotechnol.A-Enzym.Eng.Biotechnol.,2009,DOI: 10.1007/s12010-009-8760-3
15 Chen,Q.X.;Kubo,I.J.Agric.Food Chem.,2002,50:4108
16 Lee,K.H.;Aziz,F.H.A.;Syahida,A.;Abas,F.;Shaari,K.; Israf,D.A.;Lajis,N.H.Eur.J.Med.Chem.,2009,44:3195
17 Bradford,M.M.Anal.Biochem.,1976,72:248
18 García-Molina,F.;Peñalver,M.J.;Fenoll,L.G.;Rodríguez-López, J.N.;Varón,R.;García-Cánovas,F.;Tudela,J.J.Mol.Catal. B-Enzym.,2005,32:185
19 Cobos,A.;Estrada,P.Enzyme Microb.Technol.,2003,33:810
20 Huang,X.H.;Chen,Q.X.;Wang,Q.;Song,K.K.;Wang,J.;Sha, L.;Guan,X.Food Chem.,2006,94:1
21 Li,S.B.;Xue,Y.;Lv,X.Y.;Nie,H.L.;Zhu,L.M.;Zhang,H.T.; Qiu,T.;Zhou,L.M.Protein J.,2009,28:182
22 Shi,Y.;Chen,Q.X.;Wang,Q.;Song,K.K.;Qiu,L.Food Chem., 2005,92:707
23 Khan,K.M.;Mughal,U.R.;Khan,M.T.H.;Zia-Ullah;Perveenb, S.;Choudhary,M.I.Bioorg.Med.Chem.,2006,14:6027
24 Germanas,J.P.;Wang,S.;Miner,A.;Hao,W.;Ready,J.M. Bioorg.Med.Chem.Lett.,2007,17:6871
25 Espín,J.C.;Wichers,H.J.Biochim.Biophys.Acta,2001,1544: 289
26 Kim,Y.J.;Chung,J.E.;Kurisawa,M.;Uyama,H.;Kobayashi,S. Biomacromolecules,2004,5:474
27 Kermasha,S.;Bao,H.;Bisakowski,B.J.Mol.Catal.B-Enzym., 2001,11:929
28 Moridani,M.Y.;Scobie,H.;Salehi,P.;O′Brien,P.J.Chem.Res. Toxicol.,2001,14:841
29 Kubo,I.;Kinst-Hori,I.J.Agric.Food Chem.,1999,47:4121
30 Kubo,I.;Kinsy-Hori,I.;Chaudhuri,S.K.;Kubo,Y.;Sánchez,Y.; Ogura,T.Bioorg.Med.Chem.,2000,8:1749

