孔状Co3O4纳米片和纳米棒的选择性合成和表征
黄庆利 陈 虎 张永才*,
(1扬州大学测试中心,扬州 225009)(2扬州大学化学化工学院,扬州 225002)
孔状Co3O4纳米片和纳米棒的选择性合成和表征
黄庆利1陈 虎2张永才*,2
(1扬州大学测试中心,扬州 225009)(2扬州大学化学化工学院,扬州 225002)
利用两步实验选择性合成孔状Co3O4纳米片和纳米棒:首先,以Co(NO3)2·6H2O,NaOH和不同量的NH4F为原料在120℃水热6 h的条件下合成了Co(OH)2-Co3O4纳米片(S1)和Co(OH)F-Co3O4纳米棒(S2);然后将所得纳米片和纳米棒在400℃时加热2 h即得到多孔的Co3O4纳米片和纳米棒。所得产物用X射线衍射(XRD)、场发射扫描电子显微镜(FE-SEM)和透射电子显微镜(TEM)进行了表征。此外电化学测试表明Co3O4纳米棒的电容量比Co3O4纳米片的更大。
半导体;纳米材料;电化学性质
Co3O4is an important transition metal oxide due to its applications as catalyst,gas sensor,magnetic material,electrode for lithium-ion batteries,electrochromic and electrochemical subassemblies,etc.,[1-5].It has been reported that the properties and practical performances of Co3O4are related to their phase,morphology,size,etc.,which in turn depend on their synthesis methods and synthesis conditions[1-5].Consequently,special attention has been paid to the synthesis of Co3O4nanomaterials with different characteristics,aiming at exploiting their potentials.
By far,various Co3O4nanostructures including nanorods[1-2],nanoplates[3],nanocubes[4],hollow spheres[5]and so forth,have been synthesized by differentmethods.Nevertheless,most of the existing methods need special instruments,complicated processes,or/and severe conditions.The precursor way has been proved to be one of the most convenient and practical techniques to prepare inorganic nanocrystals[6-18].It not only enables to avoid special instruments,complicated processes and severe preparation conditions,but also can realize the aim of controlling the morphology and crystal form of the products simply by choosing the precursor usually in the absence of any other template or surfactant[6-18].Our group previously synthesized porousnanowire ZnO by decomposing nanowire network of Zn(OH)F, which were obtained using hydrothermal methods[11].Herein,we present a simple hydrothermal route to synthesize Co(OH)2-Co3O4nanoplates and Co(OH)F-Co3O4nanorods,which are subsequently used as the precursors to obtain nanoporous Co3O4with the same morphology by calcination.
1 Experimental
All the reactants are A.R.grade and used as received.The precursors were prepared as follows:1.0 mmol Co(NO3)2·6H2O and 2.0(or 10.0)mmol NH4F were dissolved in 24 mL of distilled water and the solution was stirred at room temperature for 0.5 h.Then 2.0 mmol NaOH was added under stirring.The mixed solution was transferred into a Teflon-lined autoclave of 30 mL capacity.After being sealed and heated at 120℃for 6 h,the autoclave was cooled to room temperature naturally.The resulting pink precipitates were collected by filtration,washed with distilled water for several times,and finally dried in air at 70 ℃ for 4 h.The precursors prepared using 2.0 and 10.0 mmol NH4F were denoted as S1 and S2,respectively.
Porous black Co3O4(S3 and S4)could be yielded when S1 and S2 were heated in a muffle oven at 400℃for 2 h,respectively.
The obtained products were characterized by XRD(German Bruker AXSD8 ADVANCE X-ray diffractometer),FESEM (Japan Hitachi S-4800 FESEM),TEM(Holland Tecnai-12 TEM)and HRTEM (Holland F-30 HRTEM).Electrochemical measurements were performed using a CHI 660B electrochemical workstation.
2 Results and discussion
Fig.1(a)~(d)show the XRD patterns of S1~S4.As can be seen,the as-prepared S1 precursor comprises a mixture of hexagonal phase Co(OH)2(PDF No.30-0443)and cubic phase Co3O4,while the as-prepared S2 precursor comprises a mixture oforthorhombic structure Co(OH)F(PDF No.50-0827)and cubic phase Co3O4.In contrast,all the XRD peaks of both S3 and S4 products can be readily indexed to cubic phase of Co3O4,according to PDF No.78-1969.

Fig.1 XRD patterns of S1~S4.
The morphologies of S1~S2 were observed by SEM and TEM.The SEM (Fig.2(a))and TEM (Fig.2(b))images of S1 show that this precursor consists of nanoplates with the thickness of about 30 nm.In contrast,the S2 precursor consists of many overlapped nanorods with the diameter of about 50 nm and length of up to several micrometers,as shown by their SEM and TEM images in Fig.2(c)and(d),respectively.The aforementioned phenomena also indicated that the amount of NH4F used here played an important role in the phase and morphology of the hydrothermallyderived precursors.
The morphologies and structure of S3~S4 were observed by SEM,TEM.and HRTEM.The Co3O4products(S3 and S4)obtained after calcinations of S1 and S2 in air at 400℃for 2 h,can retain the morphologies of their precursors,as shown by the SEM and TEM images in Fig.3(a~d).But,differ from their precursors,the S3 and S4 products are porous.For example,the S3 nanoplates consist of nanoparticles(~100 nm)and nanopores (~50 nm),and the S4 nanorods consist of nanoparticles (~50 nm)and nanopores (~30 nm).The origin for the formation of porous Co3O4nanostructures may be related to the release of gaseous H2O and HF from their precursors upon thermal decomposition.
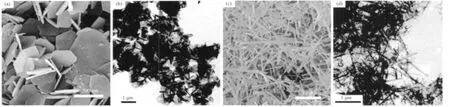
Fig.2 SEM images of(a):S1,(c)S2 and TEM images of(b):S1,(d):S2
In Fig.3(e~f),the HRTEM images showed sharp lattice fringes with 0.24 nm for Co3O4nanoplate(S3)and 0.26 nm for Co3O4nanorod (S4),all corresponding to the (311)plane of Co3O4.Most of the samples gave sharp lattice fringes with no lattice defects such as stacking faults indicating good crystallinity.
Electrochemical measurements were performed using CHI 660B electrochemical workstation.The working electrode was prepared according to the method reported in the literature[19],80wt% Co3O4powder,10wt% acetylene black and 10wt% poly(tetrafluoroethylene)(PTFE)were well mixed with a few drops of ethanol.After briefly allowing the solvent to evaporate,the resulting paste was pressed on to a nickel foam(surface 1 cm2)at 10 MPa.The electrode assembly was dried at 80℃ for 16 h.A three-electrode system was employed for cyclic voltammetry and Chronopotentiometry measurements in 2 mol·L-1KOH.Saturated calomel electrode(SCE)and Platinum were used for the reference and counter electrodes,respectively.Theirspecific capacitance was calculated according to the following equation:

Fig.3 SEM images of(a):S3,(c)S4;TEM images of(b):S3,(d):S4 and HRTEM images of(e):S3,(f):S4

where Cm(F·g-1)is the specific capacitance,I(mA)is charge-discharge current,Δt(s)is the discharging time,ΔV(V)represents the potential drop during discharge,and m(mg)is the mass of the active material within the electrode.The evaluated specific capacitances of the as-prepared Co3O4nanorods is 98 F·g-1at discharge current 4 mA whereas it is about 75 F·g-1for the asprepared Co3O4nanoplates at the same current(Fig.4).Fig.5 showed the dependence of the discharge specific capacitance on the charge-discharge cycle numbers.The discharge capacity hardly changed after 40 cycles,indicating that as-synthesized products (S3 and S4)electrode has relatively good electrochemical stability.These results indicated that the utilization rate of Co3O4nanorods is greater than Co3O4nanoplates.It is believed that the difference in electrochemical properties of S3 and S4 can be ascribed to the combining effects of their different dimensions,morphologies,microstructures and degrees of crystallinity[20].
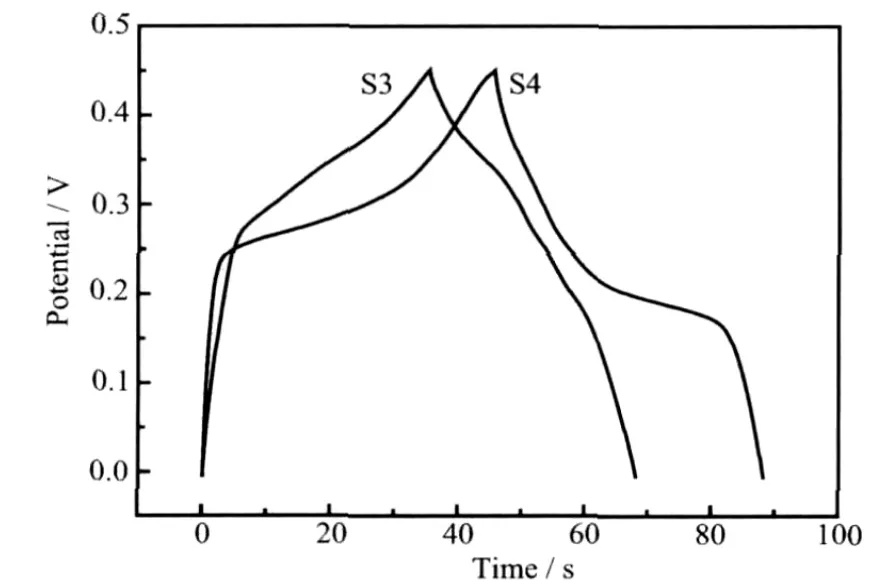
Fig.4 Charge-discharge curves at a scan rate of 4 mA for S3 and S4

Fig.5 Dependences of the discharge specific capacitance on cycle numbers
3 Conclusions
Co(OH)2-Co3O4nanoplates(S1)and Co(OH)F-Co3O4nanorods(S2)were synthesized via the hydrothermal reaction of Co(NO3)2·6H2O, NaOH and different amounts of NH4F at 120℃for 6 h,and porous Co3O4nanoplates and nanorods were obtained by thermal decomposition of S1 and S2 in air at 400℃for 2 h,respectively.It was found that the amount of NH4F used influenced both the phase and morphology of the hydrothermally-derived precursors.Electrochemical tests demonstrated the nanorods structure increased the utilization of Co3O4.The current method to selectively synthesize porous Co3O4nanorods and nanoplates was of simplicity and low cost,which may be suitable for large-scale production.
Acknowledgements:This work is supported by the University Natural Science Foundation of Jiangsu Province(No.08KJB150019).
[1]Ke X F,Cao J M,Zheng M B,et al.Mater.Lett.,2007,61:3901-3903
[2]Lai T L,Lai Y L,Lee C C,et al.Catal.Today,2008,131:105-110
[3]Li L L,Chu Y,Liu Y,et al.Mater.Lett.,2008,62:1507-1510
[4]Tripathy S K,Christy M,Park N H,et al.Mater.Lett.,2008,62:1006-1009
[5]Zhao W W,Liu Y,Li H L,et al.Mater.Lett.,2008,62:772-774
[6]Niasari M S,Mir N,Davar F.J.Phys.Chem.Solids,2009,70:847-852
[7]Ren L,Wang P P,Han Y S,et al.Chem.Phys.Lett.,2009,476:78-83
[8]Cong H P,Yu S H.Cryst.Growth Des.,2009,9:210-217
[9]ZHU Chuan-Gao(朱传高),WANG Feng-Wu(王凤武),CHU Dao-Bao(楮道葆).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23(2):335-338
[10]ZHONG Nai-Liang(钟乃良),WANG Le-Gang(王乐刚),XU Yan-Ling(徐艳玲),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(5):833-837
[11]Huang Q L,Wang M,Zhong H X,et al.Cryst.Growth Des.,2008,8:1412-1417
[12]Narayanaswamy A,Xu H,Pradhan N,et al.J.Am.Chem.Soc.,2006,128:10310-10319
[13]ZHONG Yong-Ke(钟永科),JIN Qian(金 茜),GOU Hua(勾华),et al.J.Funct.Mater.(Gongneng Cailiao),2005,36(2):276-281
[14]ZHANG Chun-Li(张春丽),ZHANG Sheng-Mao(张晟卯),LU Chun(卢 春),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2006,2(3):581-584
[15]Li B X,Xie Y,Jing M,et al.Langmuir,2006,22:9380-9385
[16]Ding H S,Chu G,Zha X J,et al.Mater.Sci.Technol.,2006,14(1):95-98
[17]LI You-Zhu(李友竹),HUANG Chao-Ming(黄昭铭),WANG Sheng-Zhang(王圣璋),et al.J.Inorg.Mater.(Wuji Cailiao Xuebao),2008,23(1):121-124
[18]HUANG Wei-Gang(黄维刚),LI Hua(林 华),TU Ming-Jing(涂铭旌).J.Funct.Mater.(Gongneng Cailiao),2006,37(3):440-445
[19]Wang Y G,Yu L,Xia Y Y.J.Electrochem.Soc.,2006,153:743-748
[20]Ji G B,Gong Z H,Zhu W X,et al.J.Alloys Compd.,2009,476:579-583
Selective Synthesis and Characterization of Porous Co3O4Nanoplates and Nanorods
HUANG Qing-Li1CHEN Hu2ZHANG Yong-Cai*,2
(1Testing Center,Yangzhou University,Yangzhou,Jiangsu 225009)(2College of Chemistry and Chemical Engineering,Yangzhou University,Yangzhou,Jiangsu 225002)
A two-step method has been proposed for the selective synthesis of porous Co3O4nanoplates and nanorods:firstly,Co(OH)2-Co3O4nanoplates(S1)and Co(OH)F-Co3O4nanorods(S2)were synthesized via the hydrothermal reaction of Co(NO3)2·6H2O,NaOH and different amounts of NH4F at 120 ℃ for 6 h;secondly,porous Co3O4nanoplates and nanorods were obtained by thermal decomposition of the S1 and S2 precursors in air at 400℃for 2 h,respectively.The obtained products were characterized by X-ray diffraction,field emission scanning electron microscopy,and transmission electron microscopy.Besides,the electrochemical tests showed that the as-prepared Co3O4nanorods have larger specific capacitances than the as-prepared Co3O4nanoplates.
semiconductors;nanomaterials;electrical properties
O614.81+2
A
1001-4861(2010)08-1394-05
2010-04-19。收修改稿日期:2010-05-18。
江苏省高校自然科学基金(No.08KJB150019)资助项目。
*通讯联系人。 E-mail:qlhuang@yzu.edu.cn
黄庆利,男,30岁,硕士,助理研究员;研究方向:功能纳米材料。
——河北省地质实验测试中心

