单核铜配合物Cu(C16H13N2OS2)2的合成、晶体结构和生物活性
仇晓阳 刘 瑛 程 艳 郑 昕 朱海亮
(1商丘师范学院化学系,商丘 476000)(2医药生物技术国家重点实验室,南京 210093)
研究简报
单核铜配合物Cu(C16H13N2OS2)2的合成、晶体结构和生物活性
仇晓阳*,1,2刘 瑛1程 艳1郑 昕1朱海亮2
(1商丘师范学院化学系,商丘 476000)(2医药生物技术国家重点实验室,南京 210093)
铜配合物;平面结构;晶体结构;生物活性
Dithiocarbazate NH2NHCS2-and its substituted derivatives remain of interest to researchers because of their dramatic variation in structure and peculiar properties[1-11].Some of these compounds have tunable electronic behavior which can be employed in nonlinear optical materials[2-5]and some show good biological activities[6-12].Furthermore,some of metal complexes derived from dithiocarbazate are well known to accelerate drug action and the efficiency of a therapeutic agent can be enhanced upon coordination with a metal ion[13].As part of our study on ligands derived from S-benzyldithiocarbazate (SBDTC),the structure of transition metal complexes with SBDTC derivative have already been reported[14].To evaluate their coordination chemistry and their potential as antitumor agents,we report here synthesis,crystal structure and bioactivity of a new planar copper complex with bidentate Schiff base derived from SBDTC.
1 Experimental
1.1 Reagent and apparatus
All chemicals were of reagent grade and were used as received.The solvents were purified using conventional methods.Elemental analyses were performed on a CHN-O-Rapid instrument.
1.2 Synthesis of the Schiff base(HL)
S-benzyldithiocarbazate(SBDTC)was prepared as previously reported[15].The Schiff base was prepared by adding a solution of SBDTC (1.99 g,0.01 mol)in absolute EtOH (20 mL)to an equimolar solution of pphthalaldehyde(1.34 g,0.01 mol)of the same solution(10 mL).The mixture was heated on a steam bath for 1 h and then cooled to 0℃in an ice bath until the Schiff base precipitated.After being filtered,washed with cold EtOH and dried in vacuo over P2O5,the yield of the product is 58%.Anal.Calc.for C16H14N2OS2(%):C,61.12;H,4.49;N,8.91.Found(%):C,61.04;H,4.45;N,8.96.
1.3 Synthesis of the copper complex Cu(L)2
Schiff base (0.16 g,0.5 mmol)dissolved in absolute ethanol was added to and ethanol solution(15 mL)of copper acetate(0.09 g,0.5 mmol).The mixture was stirred at room temperature for 0.5 h to give a black solution.Suitable rod-shaped black single crystals of the title complex for the structure determination were obtained by slow evaporation of the solution in air.Yield:61%on the basis of HL.Anal.Calc.for C32H26CuN4O2S4(%):C,55.67;H,3.80;N,8.12.Found(%):C,55.59;H,3.78;N,8.14.
1.4 X-ray crystal structure analysis
Diffraction data for the complex were collected at 298(2)K using a Bruker SMART APEXⅡCCD areadetector with Mo Kα radiation (λ=0.071 073 nm).The collected data were reduced with the SAINT[16]program,and empirical absorption correction was performed using the SADABS[17]program.The structure was solved by direct methods and refined by full-matrix leastsquares methods on F2by using the SHELXTL[18]software package.All of the non-hydrogen atoms were refined anisotropically.All hydrogen atoms were placed in geometrically idealized positions.The summary of the crystal data,experimental details and structure refinement parameters are recorded in Table 1.
CCDC:763155.
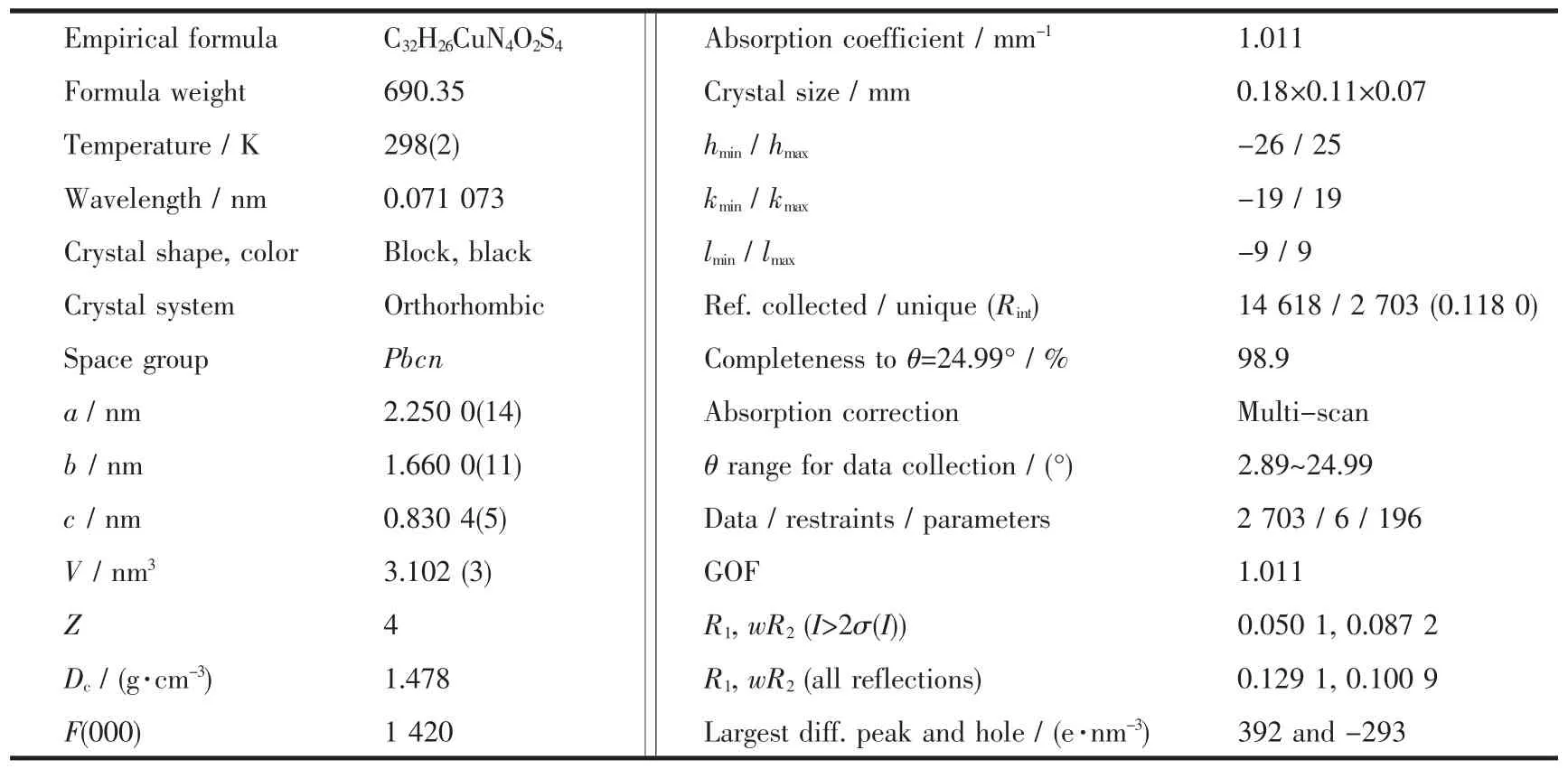
Table 1 Crystal data and structure refinement for the title complex
1.5 Biological activity assay
As a preliminary screening for antitumor activity,biological activity of the title complex was used MTT method described in our previous paper[19].The copper complex dissolved in DMF was tested against MKN45 and HEPG2.For the comparison,5-fluorouracil was also tested.
2 Results and discussion
The synthetic route of the target complex prepared in absolute ethanol solution is shown in Scheme 1.The elemental analyses are in good agreement with the chemical formulae proposed for the complex.The title complex is stable in air at room temperature,soluble in DMF,but slightly soluble in MeOH and EtOH,insolvable in water and Me2O.
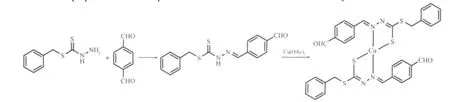
Scheme 1 Synthesis route for the title complex
2.1 Description of crystal structure
The molecular structure and the selected bond lengths and angles of the title complex are listed in Fig.2 and Table 2,respectively.As shown in Fig.2,Cucomplex exhibits central symmetry and the Cuatom sits on crystallographic inversion center.In the complex the copper atom is four-coordinate with two N atoms and two S atoms from two bidentate Schiff base ligands derived from S-benzyldithiocarbazate and forms in a slightly distorted square-planar geometry,which is similar to that of the reported work[4].The two trans angles at the coppercenter are exactly 180°,by virtue of the crystallographic symmetry (Table 2),and other angles are close to 90°,viz.83.88(12)°and 96.12(12)°,indicating a slight deviation from perfect square-planar geometry.The coordinated sulfur and nitrogen atoms in the two ligands are in opposite positions.The Cu-S and Cu-N bond length of 0.225 60(16)and 0.2011(3)nm are slightly longer than those observed in the two similar copper complexes [0.215 6(1),0.2251(1),0.1929(4)and 0.2031(2)nm][4].Two ligands coordinate with the center copperion and form two five-membered rings.The mean deviation of the fivemembered ring is 0.00695 nm.
The C(9)-S(1)bond length of 0.170 2(4)nm is intermediate 0.182 nm for C-S single bond and 0.156 nm for C=S double bond[18].It is agreement with the reported value in the similar complexes[4].The N(2)-C(9)and N(1)-C(8)bond distances of 0.128 8(5)and 0.129 1(5)nm,respectively,indicate a strong electron delocalization in the conjugation of the C-N-N-C moiety compared with the bidentate complex of zinc,[Zn(C8H9N2OS2)2][9].The N(1)-N(2)bond distances of 0.1397(4)nm are slightly shorter than the corresponding bond lengths in the copper complex(0.1409(5)nm)[4].Thus,Schiff base in the title complex behaves as the deprotonated enethiolate form and coordinates with metal ion(Fig.1).

Fig.1 Tautomeric forms of Schiff base

Table 2 Selected bond lengths(nm)and angles(°)of the title complex
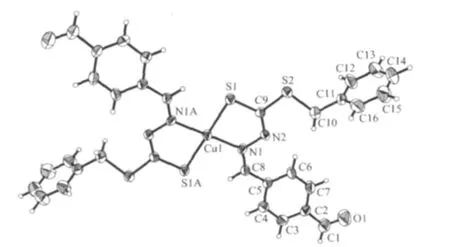
Fig.2 ORTEP view of the title complex with 30%probability displacement ellipsoids,showing the atom-labeling scheme
The crystal packing in the unit cell is shown in Fig.3.The molecular packing in the crystal is stabilized through aromatic interactions via phenyl-phenyl stacking.
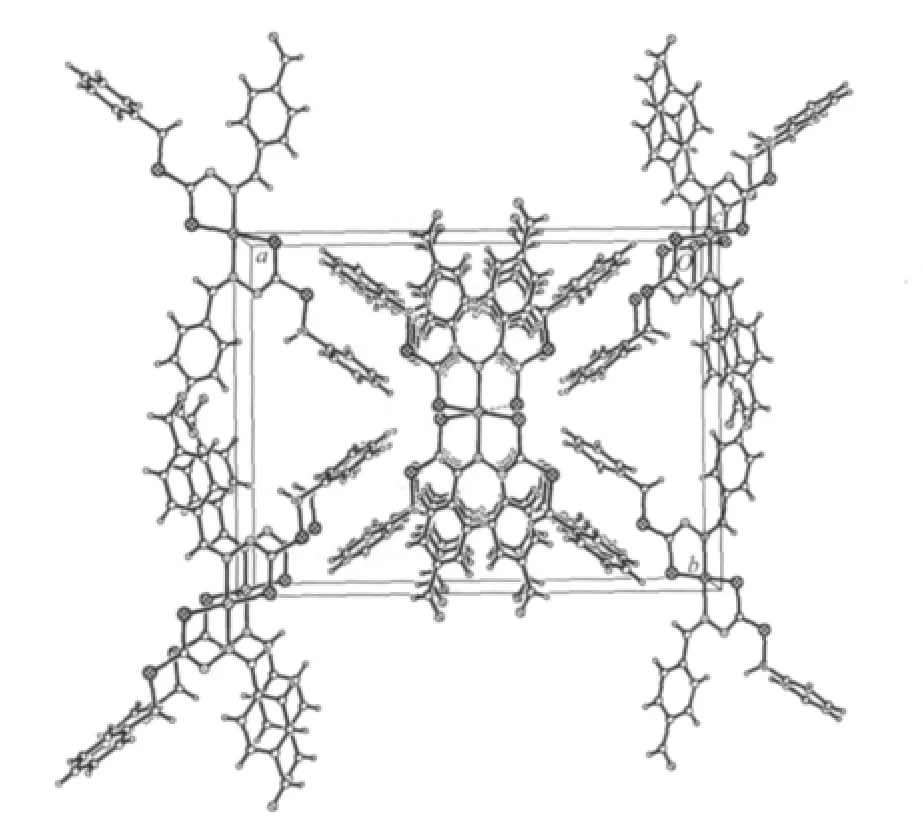
Fig.3 Packing diagram of the title complex,viewed along the c axis
2.2 Biological activity
The title complex was evaluated for its antitumor activities in vitro against HepG2 and MKN45 by MTT method.Thedatarepresentthe mean ofthree experiments performed in triplicate.IC50values of the title complex against MKN45 and HepG2,which is the concentration at which 50%survival of cells,are presented in Table 3.
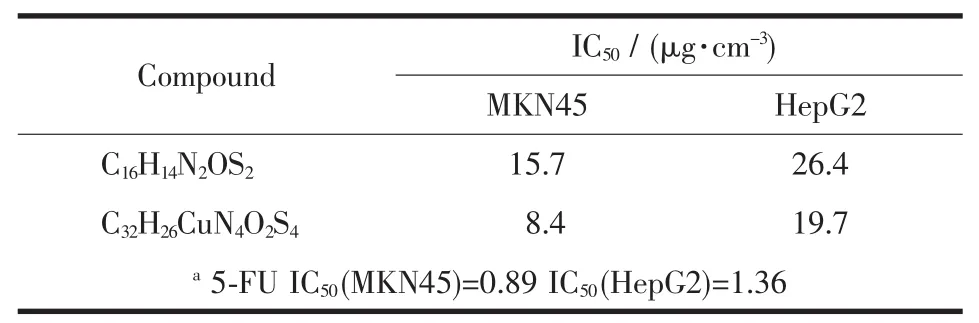
Table 3 Antitumor activity against tumor cells of the title complex and Schiff base
3 Conclusion
In this paper,the syntheses,crystal structure of Cucomplex containing two Schiff bases of S-benzyldithiocarbazate and biological activity of this Schiff base and the metal complex synthesized were investigated.The complex is central symmetry in which the copperion lies in a four-coordinate,distorted square-planar environment.The complex was evaluated for antitumor activities against two kinds of cell line by MTT method.The study indicates that the complex shows moderate activity against MKN45 cells and weak activity against HepG2 cells.
[1]Samanta S,Ghosh D,Mukhopadhyay S,et al.Inorg.Chem.,2003,42:1508-1517
[2]ZHOU Jian-Hao(周建豪),WANG Yu-Xiao(王玉晓),CHEN Xue-Tai(陈学太),et al.Chinese.J.Inorg.Chem.(Wuji Huaxue Xuebao),2002,18(5):533-536
[3]Zhou H,Li D,Wang P,et al.J.Mol.Struct.,2007,826:205-210
[4]Tian Y P,Duan C Y,You X Z,et al.Transition Met.Chem.,1998,23:17-20
[5]WU Jie-Ying(吴杰颖),TIAN Yu-Peng(田玉鹏),ZHANG Yin-Han(张银汉),et al.Acta Chim.Sin.(Huaxue Xuebao),1999,57(2):202-209
[6]SUN Gang-Chun(孙纲春),QU Jian-Qiang(曲建强),WANG Liu-Fang(王流芳),et al.Chem.Res.Appl.(Huaxue Yanjiu Yu Yingyong),2006,18(1):85-88
[7]How F N F,Crouse K A,Tahir M I M,et al.Polyhedron,2008,27:3325-3329
[8]Ali M A,Mirza A H,Butcher R J,et al.Inorg.Chim.Acta,2001,320:1-6
[9]Tarafder M T H,Chew K B,Crouse K A,et al.Polyhedron,2002,21:2683-2690
[10]Crouse K A,Chew K B,Tarafder M T H,et al.Polyhedron,2004,23:161-168
[11]Tarafder M T H,Khoo T J,Crouse K A,et al.Polyhedron,2002,21:2691-2698
[12]Thahira R B S A,Karen A,Crouse M,et al.Polyhedron,2007,26:1159-1165
[13]Tarafder M T H,Jin K T,Crouse K A,et al.Polyhedron,2002,21:2547-2554
[14]Qiu X Y,Hao F Y,Liu W S.Syn.React.Inorg.Met-Org.Nano-Met.Chem.,2006,36:595-597
[15]Ali A M,Tarafder M T H.J.Inorg.Nucl.Chem.,1977,39:1785-1791
[16]Siemens.SMART and SAINT V4 Software Reference Manual,Siemens Analytical X-ray Systems,Inc.,Madison,Wisconsin,USA,1996.
[17]Sheldrick G M.SADABS.Program for Empirical Absorption Correction of Area Detector Data,University of Göttingen,Germany,1996.
[18]Siemens.SHELXTL,Version 5 Reference Manual,Siemens Analytical X-ray Systems,Inc.,Madison,Wisconsin,USA,1996.
[19]Qiu X Y,Luo Z G,Liu W S,et al.Chin.J.Struct.Chem.,2008,27(6):707-711
[20]Sutton L E.Table of Interatomic Distances and Configurations in Molecules and Ions(Supplement).London:The Chemical Society,1965.
[21]Shier W T.Mammalian cell culture on$5 a Day:a Laboratory Manual of Low Cost Methods.Los Banos:University of the Philippines,1991.64
[22]Maccari R,Ottana R,Bottari B,et al.Bioorg.Med.Chem.Lett.,2004,14:5731-5733
Synthesis,Crystal Structure and Biological Activity of a New Mononuclear Copper Complex Cu(C16H13N2OS2)2
QIU Xiao-Yang*,1,2LIU Ying1CHENG Yan1ZHENG Xin1ZHU Hai-Liang2
(1Department of Chemistry,Shangqiu Normal University,Shangqiu,Henan 476000)(2State Key Laboratory of Pharmaceutical Biotechnology,Nanjing University,Nanjing 210093)
A planar copper complex Cu(L)2,HL=(E)-benzyl-2-(4-formylbenzylidene)-Hydrazinecarbodithioate,has been prepared via the template effect of copper ion.Crystal structure of the complex was determined by X-ray singlecrystal diffraction analysis.The crystal data for this complex:Orthorhombic,space group Pbcn,a=2.2500(14)nm,b=1.6600(11)nm,c=0.8304(5)nm,V=3.102(3)nm3,Z=4,μ=1.011 mm-1,Dc=1.478 g·cm-3,F(000)=1 420,R1=0.050 1,wR2=0.087 2(observed reflections with I>2σ(I))and R1=0.129 1,wR2=0.100 9(all reflections)GOF=1.011.In the compound,Cuatom is four-coordinated with two N atoms and two S atoms from two bidentate ligands and located in the inversion center.The preliminary bioassay indicates that the complex exhibits distinct antitumor activity.CCDC:763155.
coppercomplex;Schiff base;crystal structure;biological activity
O614.121
A
1001-4861(2010)08-1485-05
2010-03-01。收修改稿日期:2010-05-17。
河南省教育厅项目(No.2009A150020),安徽省教育厅项目(No.KJ2008B1780)资助。*
。 E-mail::qiuxiaoyang12@163.com,Tel:0370-2592844
仇晓阳,男,40岁,博士,副教授;研究方向:配位化学及生物无机化学。

